1. Introduction
Type 2 diabetes (T2D) has become a global health burden [
1]. T2D refers to a metabolic glucose dysregulation resulting from insulin resistance (IR) and inadequate insulin secretion [
2], although the etiology of T2D is highly heterogeneous [
3]. T2D is preceded by insulin resistance, and ensues when the pancreas becomes unable to compensate for insulin resistance, resulting in glucose intolerance and hyperglycemia [
4]. Prolonged hyperglycemia can induce glucotoxicity, which causes beta cell dysfunction and altered beta cell mass, contributing to further deterioration of T2D [
5]. The primary pathophysiological defects in T2D are IR of the liver, muscle, and/or adipose tissue, as well as impaired pancreatic beta cell function (BCF) [
6]. The severity of IR in insulin-sensitive cells is not uniform, and may differ among various tissues [
7,
8]. The development of T2D results from the interaction of a person’s genetic makeup with their environment [
9], with risk factors being obesity, unhealthy diet and physical inactivity [
10].
While T2D (medical) treatment is aimed at preventing or delaying cardiovascular complications [
11], weight loss and lifestyle changes can reverse the pathophysiological processes underlying T2D, and remission can be achieved [
12,
13,
14].
Remission of T2D is defined as a state in which an individual previously diagnosed with T2D has normal glucose values in the absence of pharmacological therapy, either for a defined period of time or without a temporal definition [
15,
16], although great variability exists in the exact definition [
17,
18]. The most widely used definition of T2D remission was published in a consensus report in 2009, and includes: (1) the absence of glucose-lowering therapy; (2) normoglycemia; and (3) for a duration of ≥ 1 year [
16]. The various definitions make T2D remission difficult to effectively use as an outcome in clinical care [
19], while, for the individual with T2D, remission can be an important goal in striving to be freed from diabetes [
14]. Indeed, in a clinical setting, halting disease progression or improving glucose homeostasis could already be considered a clinically meaningful outcome. Therefore, we define T2D reversal by reaching target values for HbA1c and fasting plasma glucose (FPG) with reduced medication, or attaining normalized HbA1c and FPG values with unchanged medication.
Although consensus exists on the importance of lifestyle management and diabetes self-management education and support, with a chance for remission, T2D is mostly treated with medication [
11]. Lifestyle strategies that can be used to achieve remission of T2D often include weight loss, which is especially effective when the beta cells have not yet been irreversibly damaged [
14].
Due to the differences in underlying T2D pathophysiology and etiology, specific therapeutic approaches may be more or less beneficial. It has been shown that the diabetic phenotype, that is, the T2D subtype based on the location of the IR (i.e., muscle, liver, or both) and remaining BCF, may determine the response to different dietary interventions [
20]. Research shows that hepatic insulin resistance can be improved via a short, intense, and very low-calorie diet [
21,
22]. In the longer term, hepatic insulin resistance as well as BCF can be improved by a low carbohydrate diet [
23,
24]. Muscle insulin resistance can be addressed using a Mediterranean diet [
20]. By measuring glucose and insulin concentrations at 30 min intervals from baseline up to two hours in response to an oral glucose tolerance test (OGTT), various indices indicative of pancreatic BCF and muscle and hepatic IR can be determined, and the T2D subtype can be established [
7]. This provides insight into which pathophysiological defects should be addressed [
25], and provides a basis for a personalized (lifestyle) treatment that is more specific than the generic advice to eat healthy and increase physical activity.
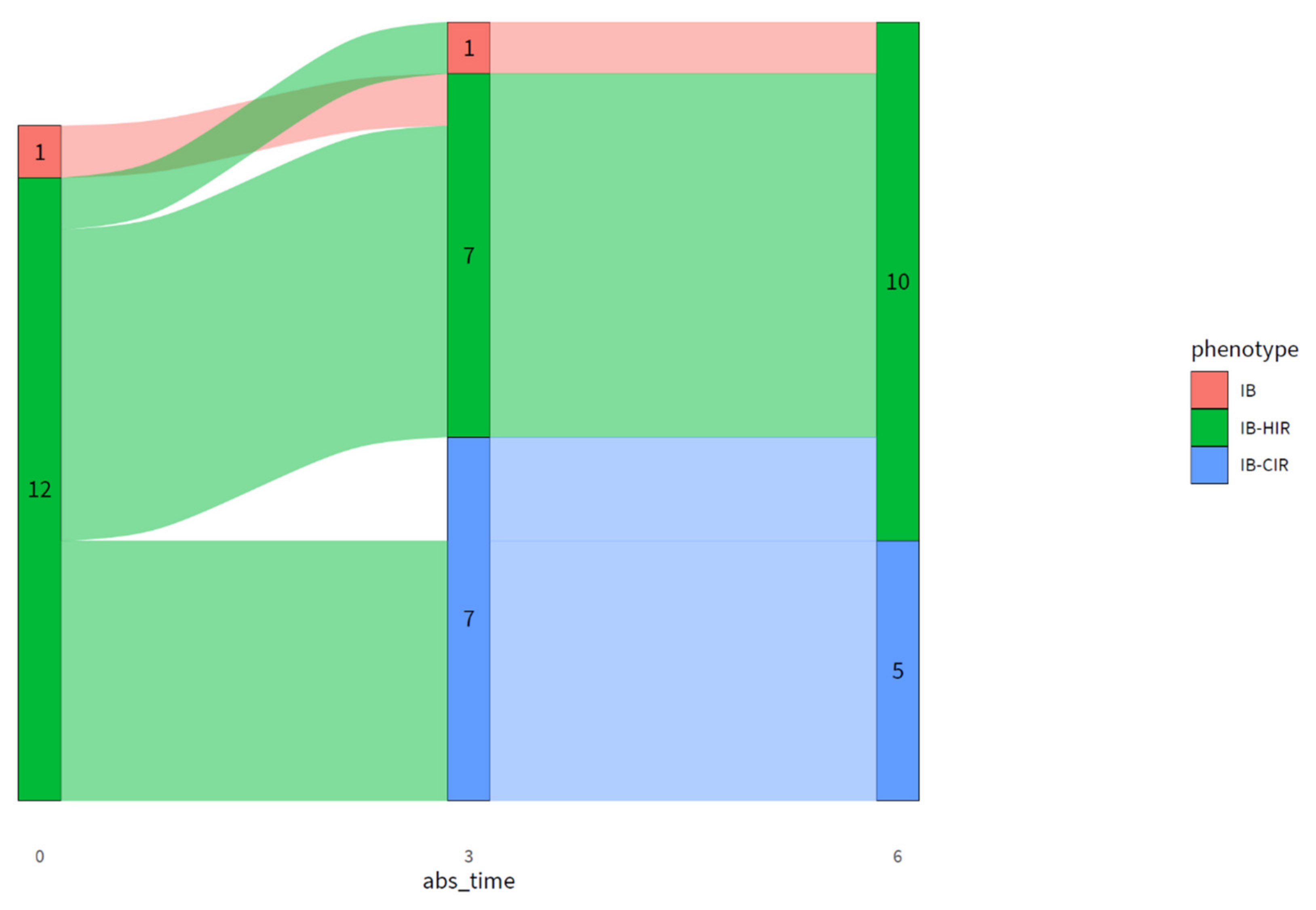
In this study, we propose the Diabetyping Lifestyle Intervention (DLI), in which the OGTT is used to determine the diabetic subtype in individuals with T2D. Information about the diabetic subtype was combined with clinical parameters and personal preferences in order to provide a personalized treatment plan, aiming for T2D remission or reversal. Although lifestyle interventions can result in diabetes remission, it is unclear to what extent T2D remission alters or improves the underlying pathophysiology of the disease. In this exploratory implementation study, in which the DLI was used in primary care, we wanted to assess whether the DLI could lead to reversal or remission of T2D, and what the effects would be on the underlying T2D pathophysiology and subtype.
4. Discussion
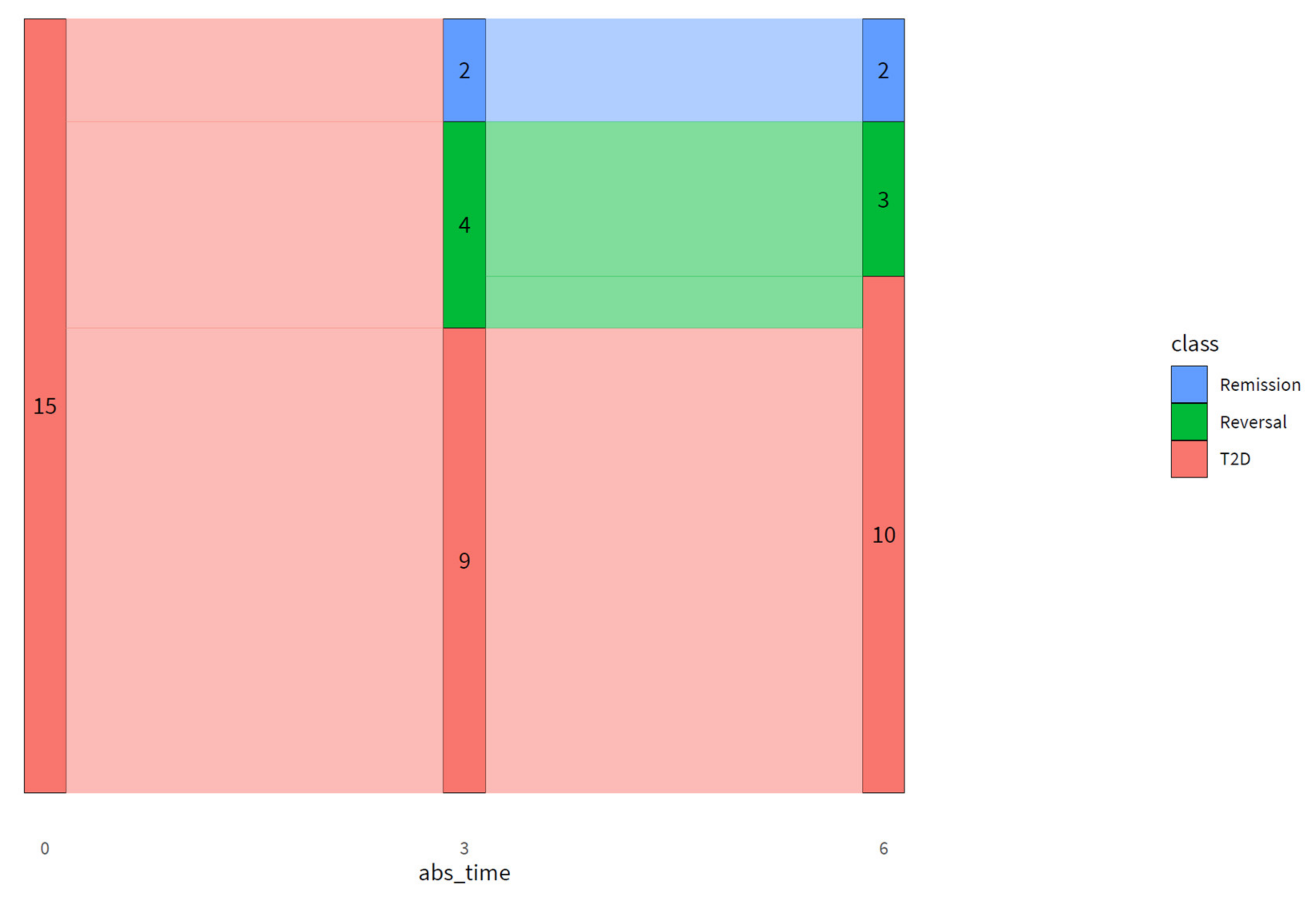
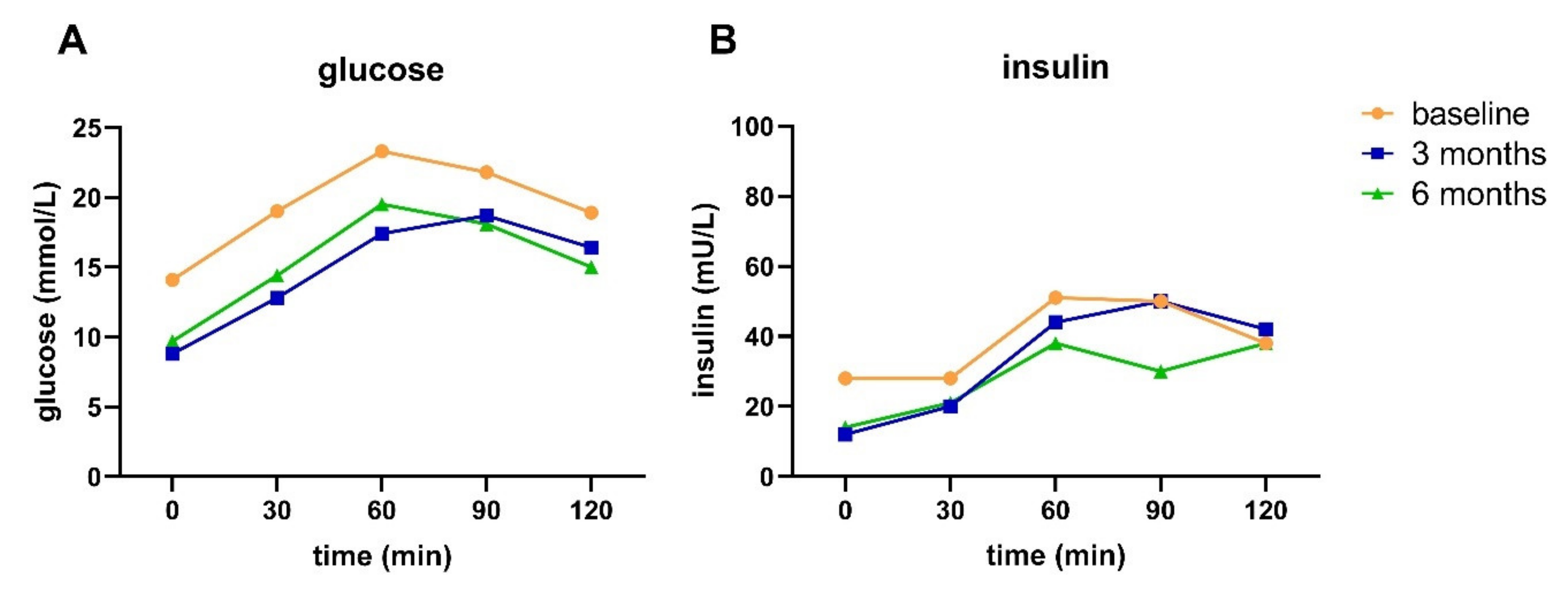
The DLI resulted in remission in two and reversal in three out of 15 participants after six months. Additionally, the DLI resulted in improved pathophysiology and glucose metabolism in people with advanced T2D, reflected by an improved fasting and 2h plasma glucose and insulin, HOMA-IR, disposition index, HIRI, and MISI. With an average weight loss of ~11 kg and an average decrease in waist circumference of ~12 cm in our study population, the improvements in glucose metabolism are probably at least partly driven by weight loss [
21].
Even though most participants achieved substantial weight loss, remission was achieved by two participants, or 13% of the study population. Higher remission numbers have been reported previously [
41,
42], although some caution should be taken in directly comparing remission numbers across studies due to differences in the definitions used for T2D remission [
18]. Additionally, our study population is relatively small compared to some landmark trials. For illustrative purposes, we compare the % remission achieved in our study with other studies. The DiRECT, U-TURN, and Look AHEAD trials achieved remission in 46, 37, and 11.5% of the study population, respectively [
41,
42,
43,
44]. Differences compared to our study were shorter disease duration, larger study population, and longer intensive intervention period. It has been shown previously that the chance of achieving (partial) T2D remission is higher among persons with a shorter disease duration, lower age, and lower baseline HbA1c, who are not using insulin, and when pharmacological treatment is not yet initiated [
43,
44]. In another primary care study with recently diagnosed T2D, 35% of the participants normalized their BCF after the lifestyle intervention [
45]. This confirms that achieving remission is especially effective in the early stages of T2D [
14]. Additionally, all participants in our study suffered from comorbidities and met the criteria for metabolic syndrome according to the International Diabetes Federation [
40]. These comorbidities may influence intervention effectiveness. Furthermore, the intensity of lifestyle treatment was recently identified as a factor influencing the chance of remission, with higher remission rates in studies using very low-calorie and longer-term diets compared to studies using moderate calorie restriction [
18]. In our study, participants followed a very low-calorie diet for only one week, followed by a six-week low-carb diet and thereafter a gradual reintroduction to a Mediterranean diet. The difference in lifestyle intervention intensity may partly explain the difference in remission rates between the aforementioned studies, although “the introduction of short-term major caloric reduction with total diet replacement and a stepped food re-introduction” has been acknowledged as an effective method for diabetes remission [
14].
Even in studies that apply longer-term intensive lifestyle interventions, non-responders are identified [
46], with non-response mainly ascribed to the lowered ability of beta cells to recover [
22]. The ability to achieve remission during an intervention consisting of a very low-calorie diet [
41,
47] or intensive physical training depended on the patient’s pancreas capacity [
42,
46]. In our study, all participants had an impaired BCF at baseline, as determined by the diabetyping algorithm. One participant had isolated impaired BCF, and 12 had impaired BCF combined with HIR. After three and six months of intervention, BCF only slightly improved as measured by an improved disposition index, while all other indices (except MISI) showed a much larger improvement. The lowered ability of BCF to recover was previously linked to disease duration [
22]. Additionally, it has been shown previously that BCF declines with age [
48,
49]. Despite the impaired BCF and higher age in our study population, two participants were able to achieve T2D remission, and three were able to achieve T2D reversal. From the data of participants A and B, it can be observed that, even though remission or reversal has been achieved, all indices except MISI were still impaired at three and six months of intervention compared to a healthy population [
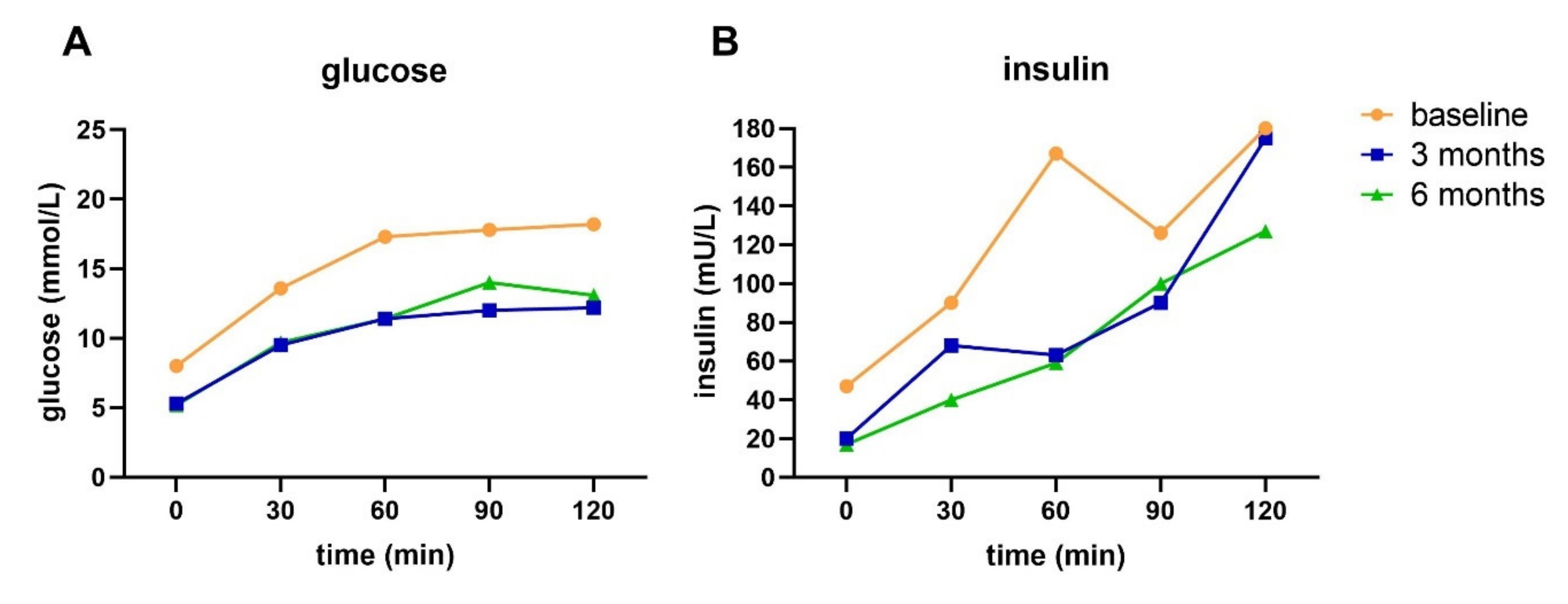
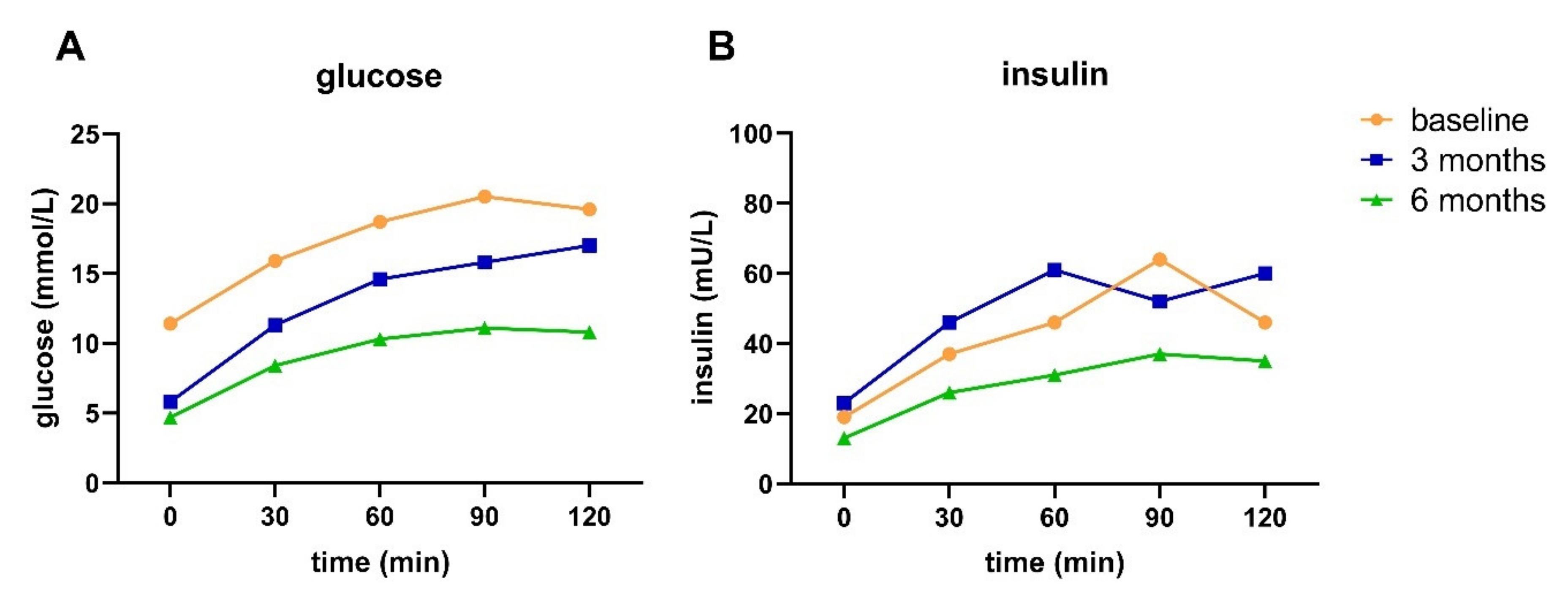
33]. This indicates that even though reversal or remission has been achieved, insulin sensitivity and BCF were not fully recovered.
Our data show that HbA1c and FPG do not provide a complete picture of the pathophysiology in people with T2D, as underlying pathophysiology—including impaired BCF and insulin resistance—can still be present in the case of normalized HbA1c and FPG and cessation of medication. Thus, achieving remission cannot be considered as a cure of T2D [
43]. We therefore suggest applying an extended OGTT to get a better understanding of the (remaining) underlying pathophysiology in people with T2D and those who are in remission. Especially in the case of remaining underlying pathophysiology, body weight regain or unhealthy lifestyle may lead to relapse [
47], implying the need for long-term adherence to a healthy lifestyle and continued monitoring of people in remission of T2D [
14]. In our study, HbA1c and FPG deteriorated from three to six months of intervention, which could be linked to a lowered compliance to the lifestyle intervention reported by participants.
In a general diabetes population, the rate of (partial) T2D remission is extremely low [
44]. In fact, most people with T2D actually show progression of the disease with rising FPG and HbA1c and increasing medication use over time [
50,
51,
52]. In that respect, in a clinical setting, halting disease progression or reversing the disease could already be considered clinically meaningful. In our study, we therefore introduced T2D reversal, meaning that a patient requires less medication for attaining target values of HbA1c and FPG or attaining normalized HbA1c and FPG values on unchanged medication. On top of the two participants achieving T2D remission, four participants achieved T2D reversal at three months, of which three were able to maintain this up to six months of intervention. Interestingly, all participants were able to lower their medication use while maintaining or lowering HbA1c and FPG values. In addition, participant C did not meet the criteria for achieving remission or reversal, but was able to attain substantially lower glucose and HbA1c levels while using less medication, which can be considered clinically relevant. As medication can induce side effects and patients experience a higher treatment burden when using multiple medications, especially insulin, a reduction in medication use can be considered a major advantage of lifestyle treatment [
51,
53,
54].
Besides improvements in glucose metabolism and body weight, improvements in overall health status were observed with improved SBP (at three months only), HDL cholesterol, and triglycerides, while overall medication use for these comorbidities was decreased. So on top of improving glucose metabolism, the lifestyle intervention also had beneficial effects in addressing the metabolic syndrome in our study population. Therefore, when assessing the effects of lifestyle interventions in T2D, overall health effects, including the impact on body weight, blood pressure, blood lipids, and medication use, should be taken into account.
The strengths of our study include that the study was conducted in a primary care setting, i.e., a real life setting. Participants showed good compliance to the advice, possibly due to the intensive and personal approach of the health care providers, and close collaboration between the different health care providers (dietician, pharmacist, practice nurse, GP). We showed that the Diabetyping Lifestyle Intervention is feasible in a Dutch primary care center.
A few limitations need to be discussed. Firstly, the study population was small, as this was the maximum number of participants that the healthcare professionals of the involved primary care center could intensively coach. Secondly, there was little diversity in terms of the T2D subtypes in our study population. This may be an artefact of current T2D diagnosis in the Netherlands. As 2h glucose is not measured as part of usual care, people with isolated impaired glucose tolerance (IGT), which often coincides with muscle IR, are easily missed [
55]. Additionally, our study population had long-standing T2D (13.4 ± 5.2 yrs), which may explain the high prevalence of impaired BCF.
Thirdly, medication was stopped at 20:00 the day before the OGTT. This was possibly not long enough, as especially long-acting insulin may still have influenced the blood glucose and insulin values. This was especially the case for the OGTT performed at baseline, since all participants stopped using insulin during the intervention period. Additionally, the OGTT was not performed after three days of unrestricted diet with at least 150 g of carbohydrates as recommended by the WHO [
27], but guidelines from standard clinical practice were followed, as the DLI was implemented as part of regular primary care. These clinical practice guidelines for the OGTT only dictate fasting of at least 10 h prior to the test. Carb load prior to the OGTT may therefore have influenced the results for participants on the low-carb diet (75 g per day) at three or six months. However, in our study, not only glucose, but also insulin is measured, allowing for interpretation of the interaction between glucose and insulin, which may be less sensitive to prior carbohydrate loading.