Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection and Patient Samples
2.2. Vitamin A Measurement
2.3. Laboratory Measurements and Validation of the Clinical Status
2.4. Data Analysis/Statistics
3. Results
3.1. Cohort Characteristics
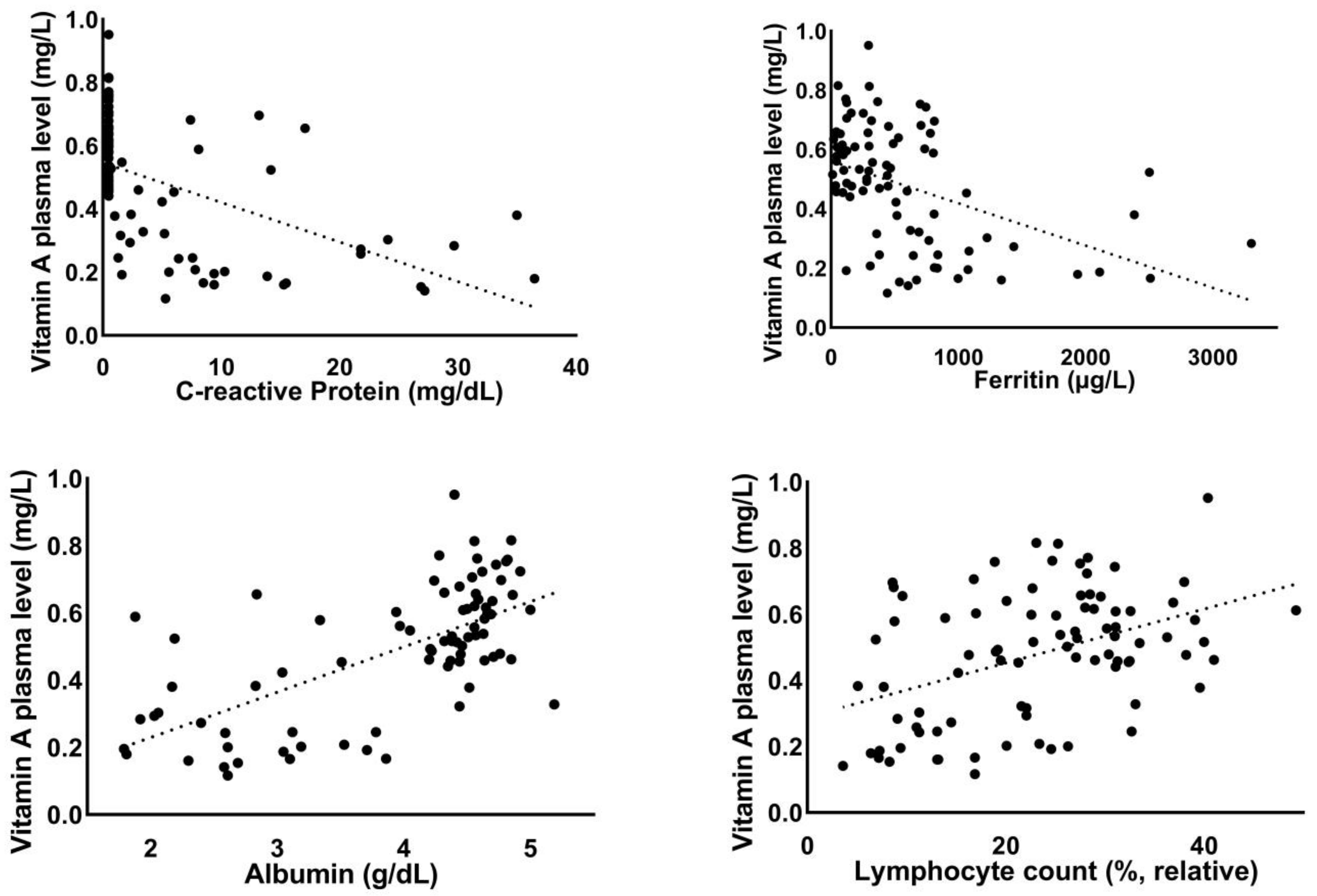
3.2. Correlation of Vitamin A Plasma Levels with Laboratory Parameters
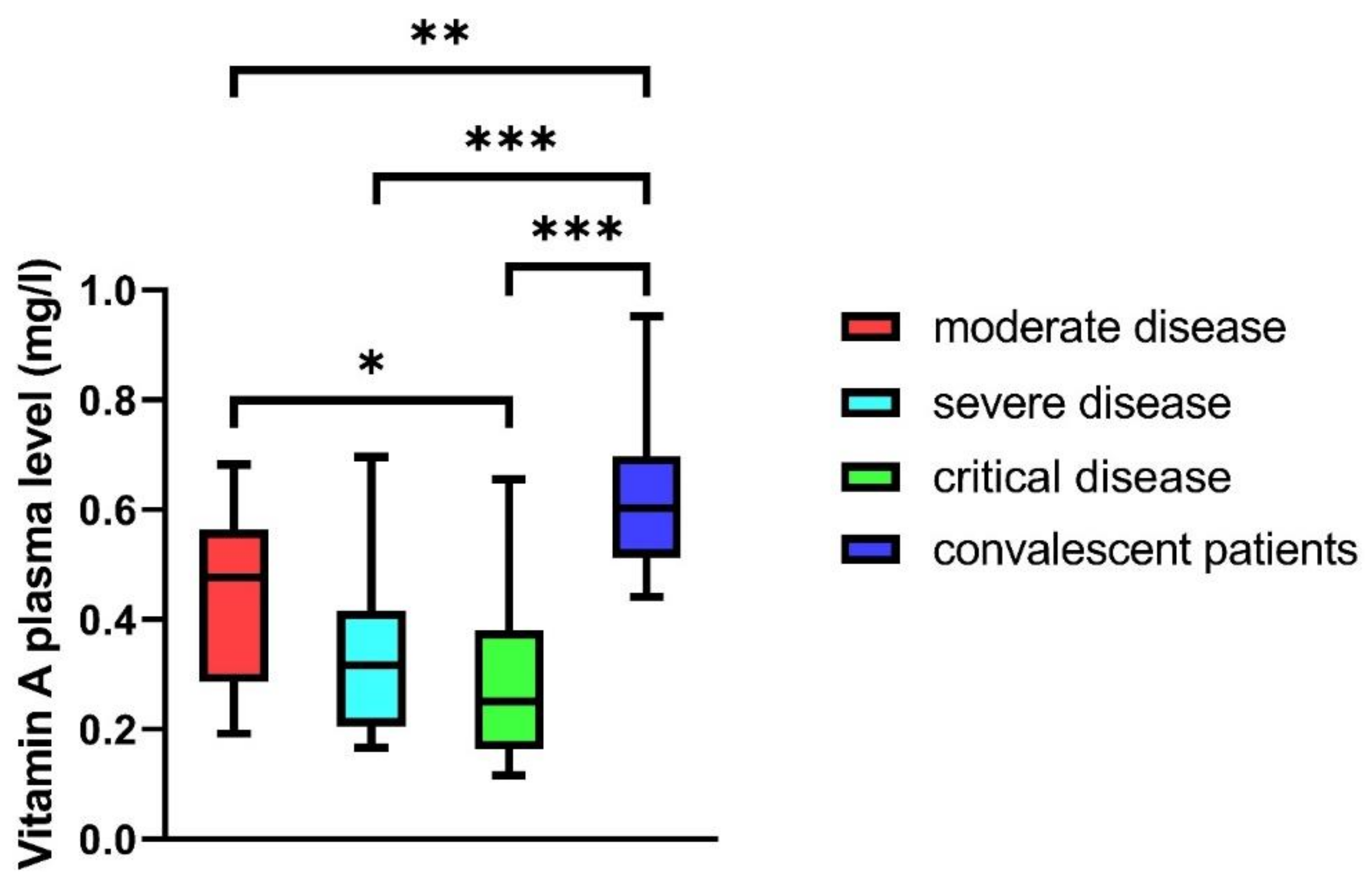
3.3. Vitamin A Plasma Levels in COVID-19 Patients
3.4. Association of Severely Reduced Vitamin A Plasma Levels with the Development of ARDS and Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Timoneda, J.; Rodriguez-Fernandez, L.; Zaragoza, R.; Marin, M.P.; Cabezuelo, M.T.; Torres, L.; Vina, J.R.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [Green Version]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raverdeau, M.; Mills, K.H. Modulation of T cell and innate immune responses by retinoic Acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef]
- Surman, S.L.; Rudraraju, R.; Sealy, R.; Jones, B.; Hurwitz, J.L. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. 2012, 25, 341–344. [Google Scholar] [CrossRef]
- Glasziou, P.P.; Mackerras, D.E. Vitamin A supplementation in infectious diseases: A meta-analysis. BMJ 1993, 306, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Hussey, G.D.; Klein, M. A randomized, controlled trial of vitamin A in children with severe measles. N. Engl. J. Med. 1990, 323, 160–164. [Google Scholar] [CrossRef]
- Penkert, R.R.; Smith, A.P.; Hrincius, E.R.; McCullers, J.A.; Vogel, P.; Smith, A.M.; Hurwitz, J.L. Vitamin A deficiency dysregulates immune responses toward influenza virus and increases mortality after bacterial coinfections. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Gieng, S.H.; Green, M.H.; Green, J.B.; Rosales, F.J. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J. Lipid Res. 2007, 48, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Aklamati, E.K.; Mulenga, M.; Dueker, S.R.; Buchholz, B.A.; Peerson, J.M.; Kafwembe, E.; Brown, K.H.; Haskell, M.J. Accelerator mass spectrometry can be used to assess vitamin A metabolism quantitatively in boys in a community setting. J. Nutr. 2010, 140, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. In WHO Global Database on Vitamin A Deficiency; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Lucena, J.F.; Alegre, F.; Martinez-Urbistondo, D.; Landecho, M.F.; Huerta, A.; Garcia-Mouriz, A.; Garcia, N.; Quiroga, J. Performance of SAPS II and SAPS 3 in intermediate care. PLoS ONE 2013, 8, e77229. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Aziz, M.; Fatima, R.; Lee-Smith, W.; Assaly, R. The association of low serum albumin level with severe COVID-19: A systematic review and meta-analysis. Crit. Care 2020, 24, 255. [Google Scholar] [CrossRef]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin. Infect. Dis. 2020, 71, 2199–2206. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Sommer, A.; Tarwotjo, I.; Hussaini, G.; Susanto, D. Increased mortality in children with mild vitamin A deficiency. Lancet 1983, 2, 585–588. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Nohr, D. Importance of vitamin-A for lung function and development. Mol. Aspects Med. 2003, 24, 431–440. [Google Scholar] [CrossRef]
- Sommer, A. Vitamin a deficiency and clinical disease: An historical overview. J. Nutr. 2008, 138, 1835–1839. [Google Scholar] [CrossRef] [Green Version]
- Manicassamy, S.; Ravindran, R.; Deng, J.; Oluoch, H.; Denning, T.L.; Kasturi, S.P.; Rosenthal, K.M.; Evavold, B.D.; Pulendran, B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med. 2009, 15, 401–409. [Google Scholar] [CrossRef]
- Ruane, D.; Brane, L.; Reis, B.S.; Cheong, C.; Poles, J.; Do, Y.; Zhu, H.; Velinzon, K.; Choi, J.H.; Studt, N.; et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J. Exp. Med. 2013, 210, 1871–1888. [Google Scholar] [CrossRef] [Green Version]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Cannons, J.L.; Grainger, J.R.; Dos Santos, L.M.; Hand, T.W.; Naik, S.; Wohlfert, E.A.; Chou, D.B.; Oldenhove, G.; Robinson, M.; et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 2011, 34, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Ross, A.C. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell Immunol. 2007, 249, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Chen, Q.; Ross, A.C. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J. Immunol. 2005, 174, 7961–7969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.G.; Lim, H.W.; Andrisani, O.M.; Broxmeyer, H.E.; Kim, C.H. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 2007, 179, 3724–3733. [Google Scholar] [CrossRef] [Green Version]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Biolo, G.; Toigo, G.; Ciocchi, B.; Situlin, R.; Iscra, F.; Gullo, A.; Guarnieri, G. Metabolic response to injury and sepsis: Changes in protein metabolism. Nutrition 1997, 13, 52S–57S. [Google Scholar] [CrossRef]

| Moderate Disease (n = 9) | Severe Disease (n = 9) | Critical Disease (n = 22) | Convalescent Patients (n = 47) | p-Value | |
|---|---|---|---|---|---|
| Age, median (min–max) | 54 (30–81) | 50 (39–73) | 58 (41–82) | 54 (41–70) | 0.24 |
| Gender, male (%) | 77.8 | 100.0 | 90.9 | 97.9 | 0.29 |
| BMI, median (IQR) | 24 (23–26) | 24 (23–26) | 27 (24–30) | 26 (24–28) | 0.05 |
| Interval from first symptom to acquisition of blood sample in days, median (IQR) | 52 (40–75) | ||||
| 8 (6–14) | 13 (8.5–17) | 12 (10–22) | 0.15 | ||
| Cardiovascular disease (abs.) | 1 | 0 | 4 | 0 | |
| Respiratory disease abs.) | 0 | 0 | 2 | 0 | |
| Kidney insufficiency (abs.) | 0 | 0 | 0 | 0 | |
| Metastatic neoplasm (abs.) | 0 | 0 | 0 | 0 | |
| Diabetes (abs.) | 0 | 0 | 1 | 0 | |
| Hematologic malignancy (abs.) | 2 | 0 | 4 | 0 | |
| Death (abs.) | 0 | 0 | 9 | 0 | |
| SAPS II, median (IQR) | 15 (13–25) | 19 (13–22) | 54 (35–72) | n.d. | <0.001 |
| Leukocytes × 109/L, median (IQR) | 4.4 (3.4–6.4) | 5.4 (3.8–7.6) | 9.2 (5.8–11) | 5.4 (4.9–6.8) | 0.21 |
| Lymphocytes (rel., %), median (IQR) | 19.5 (12.6–29.9) | 21.3 (15.0–22.8) | 10.3 (7.3–14.0) | 29.0 (25.3–32.5) | 0.003 |
| D-Dimer (mg/L), median (IQR) | 0.75 (0.32–2.31) | 0.76 (0.55–2.02) | 2.56 (1.42–7.42) | n.d. | 0.005 |
| Creatinine (mg/dL), median (IQR) | 1.0 (0.9–1.2) | 0.8 (0.8–1.0) | 1 (0.6–1.7) | 1 (0.9–1) | 0.36 |
| Ferritin (µg/L), median (IQR) | 449 (200–665) | 692 (370–938) | 917 (665–1560) | 188 (89–325) | 0.003 |
| Interleukin-6 (pg/mL), median (IQR) | 16 (10–30) | 30 (17–70) | 107 (39–239) | 2 (2–2) | <0.001 |
| Procalcitonin (ng/mL), median (IQR) | 0.11 (0.07–0.18) | 0.08 (0.07–0.12) | 0.64 (0.18–2.04) | 0.05 (0.04–0.07) | <0.001 |
| C-reactive protein (mg/dL), median (IQR) | 1.6 (0.5–3.2) | 6 (3.3–9.4) | 14.8 (6.2–24.8) | 0.5 (0.5–0.5) | <0.001 |
| PCHe (U/L), median (IQR) | 7746 (5524–9193) | 6960 (6173–8321) | 3668 (2749–4788) | 8699 (7754–10082) | <0.001 |
| Gamma-GT (U/L), median (IQR) | 43 (30–126) | 40 (30–60) | 113 (54–185) | 29 (21–47) | <0.001 |
| ALT (U/L), median (IQR) | 26 (22–46) | 33 (29–58) | 41 (29–67) | 29 (24–40) | 0.079 |
| Albumin (g/dL), median (IQR) | 3.9 (3.3–4.5) | 3.8 (3.5–4.4) | 2.3 (3.0–2.7) | 4.6 (4.4–4.7) | <0.001 |
| Vitamin A (mg/L), median (IQR) | 0.48 (0.29–0.56) | 0.32 (0.21–0.42) | 0.25 (0.16–0.38) | 0.60 (0.51–0.69) | <0.001 |
| Vitamin A < 2 mg/L (n = 11) | Vitamin A ≥ 2 mg/L (n = 29) | p-Value | |
|---|---|---|---|
| Age, median (min–max) | 52.6 (30–66) | 57.1 (33–82) | 0.52 |
| Gender, male (%) | 91 | 90 | 0.56 |
| BMI, median (IQR) | 25 (24–28) | 25 (23–28) | 0.51 |
| Interval from first symptom to acquisition of blood sample in days, median (IQR) | 11 (9–12) | 13 (8–17.5) | 0.42 |
| Preexisting disease (%) | 45 | 48 | 0.45 |
| ARDS (abs.) | 9 | 13 | 0.038 |
| Death (abs.) | 5 | 4 | 0.047 |
| SAPS II score, median (IQR) | 43 (22–61) | 28 (15.5–55.5) | 0.39 |
| Leukocytes × 109/L, median (IQR) | 5.99 (2.67–11.9) | 6.97 (4.56–9.89) | 0.56 |
| Lymphocytes (rel., %), median (IQR) | 9.4 (7.2–16.9) | 15.2 (9.35–22.1) | 0.049 |
| Lymphocytes (abs., Tsd/µL), median (IQR) | 0.69 (0.47–1.01) | 1.13 (0.79–1.38) | 0.01 |
| D-Dimer (mg/L), median (IQR) | 2.01 (0.78–4.25) | 1.78 (0.65–3.57) | 0.84 |
| Creatinine mg/dL, median (IQR) | 0.15 | ||
| Ferritin (µg/L), median (IQR) | 917 (554–1788) | 738 (465–1008) | 0.39 |
| Interleukin-6 (pg/mL), median (IQR) | 88 (37–199) | 33 (16–95) | 0.05 |
| Procalcitonin (ng/mL), median (IQR) | 0.31 (0.12–0.80) | 0.13 (0.08–0.7) | 0.3 |
| C-reactive protein (mg/dL), median (IQR) | 13.9 (8.5–26.9) | 6 (1.95–13.7) | 0.03 |
| PCHe (U/L), median (IQR) | 4082 (3012–5643) | 5555 (3566–7814) | 0.15 |
| Gamma-GT (U/L), median (IQR) | 116 (40–211) | 55 (34–130) | 0.13 |
| ALT (U/L), median (IQR) | 44 (39–70) | 31 (26–48) | 0.11 |
| Albumin g/dL, median (IQR) | 2.7 (2.2–3.3) | 3.1 (2.2–4.0) | 0.25 |
| Vitamin A (mg/L), median (IQR) | 0.17 (0.16–0.19) | 0.38 (0.28–0.52) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepasse, P.-R.; Vollenberg, R.; Fobker, M.; Kabar, I.; Schmidt, H.; Meier, J.A.; Nowacki, T.; Hüsing-Kabar, A. Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients 2021, 13, 2173. https://doi.org/10.3390/nu13072173
Tepasse P-R, Vollenberg R, Fobker M, Kabar I, Schmidt H, Meier JA, Nowacki T, Hüsing-Kabar A. Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients. 2021; 13(7):2173. https://doi.org/10.3390/nu13072173
Chicago/Turabian StyleTepasse, Phil-Robin, Richard Vollenberg, Manfred Fobker, Iyad Kabar, Hartmut Schmidt, Jörn Arne Meier, Tobias Nowacki, and Anna Hüsing-Kabar. 2021. "Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis" Nutrients 13, no. 7: 2173. https://doi.org/10.3390/nu13072173
APA StyleTepasse, P.-R., Vollenberg, R., Fobker, M., Kabar, I., Schmidt, H., Meier, J. A., Nowacki, T., & Hüsing-Kabar, A. (2021). Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Nutrients, 13(7), 2173. https://doi.org/10.3390/nu13072173






