1. Introduction
Avoiding problematic allergens is an essential part of the management of food allergies. Moreover, patients with hen’s egg white (HEw) allergy are recommended to limit the consumption of all poultry eggs owing to the possibility of cross-reactivity [
1,
2]. In the Korean food allergen labeling system, quail eggs (QEs) are labeled as bird eggs [
3]. In the medical literature, there are reports of cross-reactivity to duck or goose eggs in patients with HEw allergy worldwide. However, it is not clear whether cross-reactivity to quail egg white (QEw) occurs in patients with HEw allergy [
1,
2,
4]. A previous study reported that the co-sensitization rate in patients with HEw allergy, which was assessed through skin prick tests (SPTs) using raw QEw, was 59.2% [
1]. The aforementioned study excluded patients with anaphylactic reaction to HEw, and the criteria for SPT readings were as follows: wheal diameter >5 mm for patients aged ≤2 years and >7 mm for patients aged >2 years. Another previous study [
4] on the characteristics of allergens in QEw in five HE-tolerant adults diagnosed with QE allergy reported that the sera of most patients cross-reacted to the protein present in the 42 kDa band (ovalbumin), whereas the sera of a few patients reacted to the 97 kDa (ovotransferrin) and 35 kDa (ovomucoid) band proteins, indicating a high degree of homology with HEw [
5].
Conversely, HEw proteins undergo changes in their antigenicity when exposed to heat [
6]. A previous study on the changes in HEw proteins after heat treatment, wherein eggs were boiled in water at 90 °C for 10 min, reported that the protein band corresponding to ovalbumin weakened progressively, whereas that corresponding to ovomucoid remained stable. In general, it is difficult to conclude whether the cross-antigenicity between the proteins is solely responsible for the clinical symptoms, especially considering the variation in the antigenicity of QEw and HEw, which is dependent on the thermal treatment, despite the high degree of homology and the corresponding co-sensitization rate [
7,
8,
9]. In the present study, we assessed the cross-antigenicity and reactivity of heat-treated QEw in a group of pediatric patients with HEw allergy, particularly in patients with a history of anaphylaxis. Additionally, we examined the antigenicity of stone-baked HEw, which is commercially available in South Korea. Commercially developed stone-baked HEs are processed on elvan stones that are heated under far-infrared light without the use of water. The process involves a gradual increase in temperature from 45 °C to 110 °C over a period of 24 h [
10]. There is no known change in the antigenicity of egg whites (EWs) cooked using this method.
In the present study, we assessed hypersensitivity in patients with HEw allergy, including those showing an anaphylactic reaction to HEw and commercially developed stone-baked HEw. We also evaluated the cross-reactivity to heat-treated QEw, which was cooked using the home-cooking process, in patients with HEw allergy. The present study aimed to provide alternative dietary choices and contribute to the improvement of the quality of life of patients with HE allergy.
3. Results
3.1. Clinical and Immunological Characteristics of Patients
The clinical and immunological characteristics of the 12 patients are shown in
Table 1. The median patient age was 2.5 years (interquartile range (IQR), 1.0–6.5 years), and the study sample comprised eight male and four female patients. Of them, seven (58.3%) had a history of immediate (less than 2 h) anaphylaxis after the consumption of boiled HEw, and five (41.7%) had a history of at least two incidents of acute urticaria. The patients were divided into two groups on the basis of their history of allergic reactions; the HEw-sIgE concentrations were significantly higher in the anaphylaxis group (26.3 kU/L; IQR, 19–34.7 kU/L) than in the urticarial group (4.3 kU/L; IQR, 3.8–4.5 kU/L) (
p < 0.05). The most frequently observed comorbidity was atopic dermatitis (
n = 11), followed by food allergies other than HEw allergy (
n = 10), allergic rhinitis (
n = 4), and bronchial asthma (
n = 3). The most commonly identified comorbid food allergies were those to cow’s milk (
n = 6) and wheat (
n = 4). None of the patients had previously consumed the QEw.
3.2. Identification of the Proteins in Processed HEw and QEw Extracts
In SDS-PAGE, the separation patterns of boiled QEw and HEw showed notable changes in the protein fractions after 15 min of boiling. The protein bands from raw HEw were identified as 14, 28, 34, 40, 52, and 69 kDa. The 52 and 69-kDa bands were stable after boiling in water. The protein bands from raw QEw were identified at 14, 18, 28, 40, and 52-kDa. The 28-kDa protein band was enhanced in the boiled extract, whereas the 52-kDa protein band was slightly diminished. Protein bands were interpreted based on the findings of a previous report [
5]. The presence of strongly stained bands for ovomucoid suggests that the antigenicity of ovomucoid of HEw and QEw remained stable during boiling. Conversely, the bands for ovalbumin became weakened by boiling. Transferrin and lysozyme were not detectable after 15 min of boiling. The commercially developed stone-baked HEw showed remarkable changes in all protein fractions. The bands for transferrin, lysozyme, ovalbumin, and ovomucoid, which remained stable despite being boiled, were barely detectable in the stone-baked HEw extracts (
Figure 1).
3.3. In Vitro Analysis of the Cross-Reactivity between HEw and QEw
Moderate cross-reactivity between boiled and raw HEw and raw and boiled QEw was observed using competitive IgE ELISA-inhibition. Raw HEw showed a wide range of inhibition rates (inhibitory concentration (IC)50, 0.820 µg) in patients with HEw-sIgE ≥ 0.35 kU/L. The inhibition rates of boiled (IC20, 0.048 µg) and raw QEw (IC20, 0.283 µg) were in the medium range (approximately 30–50%), while those of the stone-baked HEw were in the low range (<30%) (
Figure 2).
3.4. In Vivo Analysis of the Cross-Reactivity between HEw and QEw
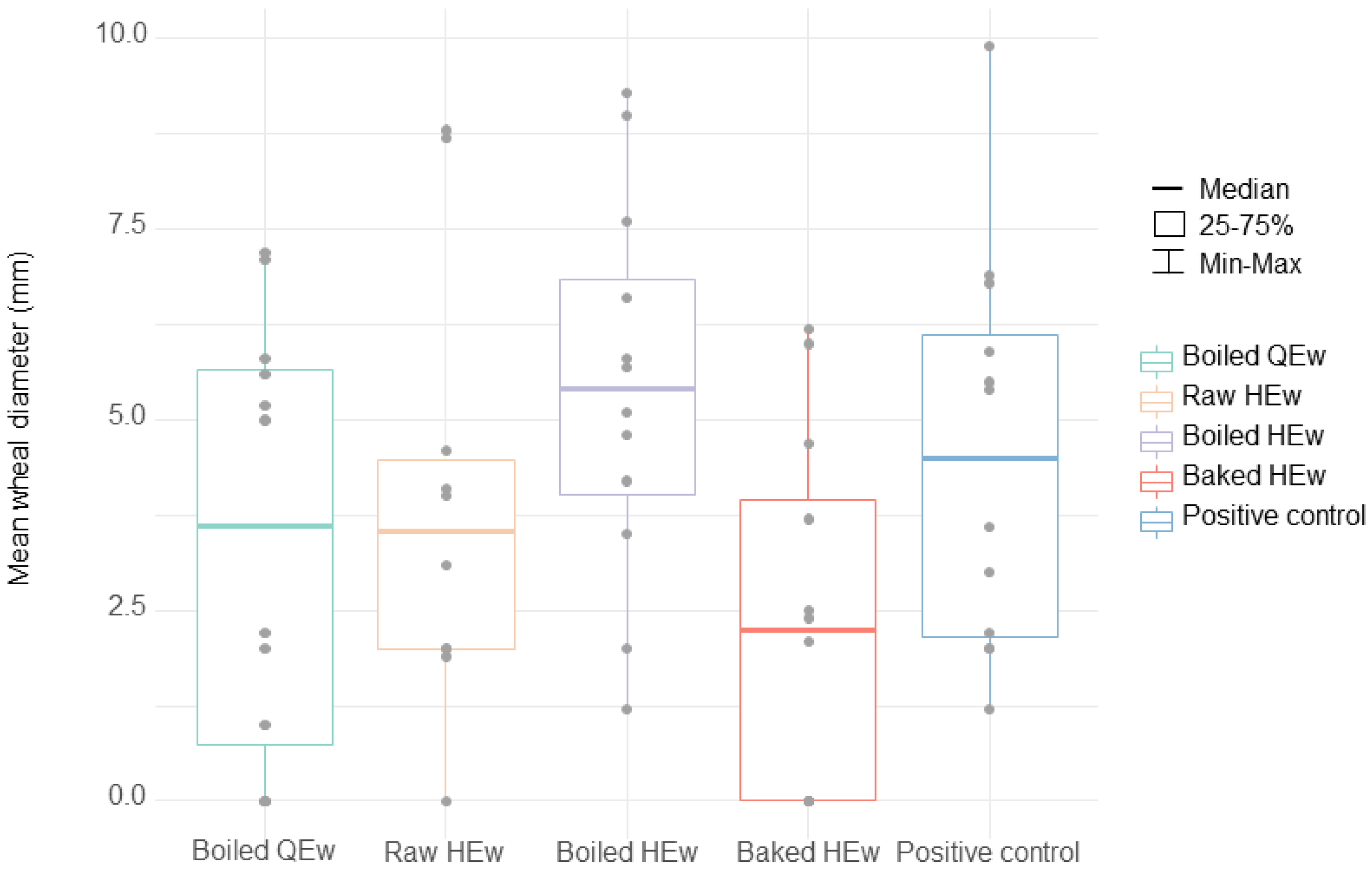
SPT results for boiled HEw indicated a relatively high median value of 5.4 mm (IQR, 0.5–5.7 mm), compared to the positive control, which displayed a median value of 4.5 mm (IQR, 2.1–6.3 mm). Three who had a history of anaphylaxis proceeded with the OFC using boiled HEw because their parents wanted to know the symptom-eliciting dose. However, two developed anaphylaxis, and one developed cough. Furthermore, one among the four patients with SPT results for boiled HEw below the cutoff value (No. 8, SPT results: 4.8 mm, HEw-sIgE concentration: 4.98 kU/L) developed anaphylaxis. Alternatively, the lowest median SPT result among all the extracts was observed with stone-baked HEw, with a value of 2.2 mm (IQR, 0.0–4.2 mm). For baked HEw, the anaphylaxis group displayed a median SPT result of 3.7 mm (IQR, 2.2–5.3 mm), which was significantly higher than that of the urticarial group (0.0 mm; IQR, 0.0–0.0 mm) (p < 0.05). Of the three patients with SPT to stone-baked HEw results above the cutoff value, two participated in the OFC because their parents wanted to know their cross-reactivity, and one (No. 5. SPT results: 6.0 mm, HEw-sIgE concentration: 22.9 kU/) passed. The SPT and/or OFC results revealed that only 16.7% (2/12) of the patients demonstrated cross-reactivity to stone-baked HEw. The median SPT value for raw HEw was 3.5 mm (IQR, 4.0–4.6 mm), and 66.7% (8/12) of the patients displayed values above the cutoff. Although the median SPT result for boiled QEw was higher than that for stone-baked HEw (3.6 mm; IQR, 0.5–5.7 mm), it was lower than that for boiled HEw. Three patients showed median SPT results above the cutoff value for boiled QEw. Moreover, one of the nine patients with SPT results for boiled QEw below the cutoff value displayed a larger mean wheal diameter than that observed with boiled HEw. Therefore, the OFC for boiled QEw was offered to eight (66.7%) patients, among whom four underwent OFC with parental/guardian consent; of them, three patients passed the challenge. The SPT and/or OFC results showed cross-reactivity in 41.7% (5/12) of the patients. The patient (No. 12) who did not pass the OFC was a 1-year-old infant who developed rashes and subjective irritability. The mean wheal diameter with regard to the SPT for boiled QEw was 0 mm.
SPTs were performed to identify patients with results that were below the values for both the positive control and boiled HEw. Patients without a history of anaphylaxis underwent the OFC and had negative predicted outcomes. However, in situations involving positive predicted outcomes, only if the parents were willing to consent for an OFC, these OFCs were conducted, and a total of 18 OFCs were performed in this study. Among the 14 cases with negative predicted outcomes, two patients (No. 8: boiled HEw and No. 12: boiled QEw) became symptomatic, and three of the four patients with positive predicted outcomes were symptomatic. Hence, the positive predictive value was 68.8% (95% confidence interval (CI), 22.7–94.3), with a negative predictive value of 89.1% (95% CI, 73.4–96.0), sensitivity of 60.0% (95% CI, 14.7–94.7), specificity of 92.3% (95% CI, 64.0–99.8), and accuracy of 85.2% (95% CI, 60.8–97.2) (
Table 1,
Figure 3).
4. Discussion
Previous studies have reported that despite the variations in the antigenicity of allergens present in HEw according to the type of heat treatment, the majority of the patients with HEw allergy displayed tolerance toward HEw cooked at high temperatures [
7,
8,
9]. Nevertheless, the aforementioned studies used a baked form of HEw treated with wheat and/or sugar powder. Consequently, they are not free from the matrix effect [
6,
12]. Additionally, there are challenges associated with home baking using a specialized form of mixed powder for patients with multiple food allergies, as they find it difficult to consume commercially available baked products. In the current study, 75.0% (9/12) of the patients were concomitantly allergic to cow’s milk or wheat. Furthermore, baking is not popular in South Korea, and the specifications for household ovens are variable. Hence, an alternative diet that involves home baking is impractical. Nevertheless, the ability to consume baked forms of HEw is useful when trying a low allergenic diet to achieve better quality of life and nutrition in patients with HEw-anaphylaxis, particularly compared to those who are unable to consume such products [
7,
8]. The present study aimed to examine whether the commercially developed, stone-baked HEw could be used as a hypoallergenic diet in HEw-anaphylaxis patients and to evaluate the cross-reactivity with QEw, which could be used as a potential alternative diet.
Although the criteria for SPT results used for the interpretation of sensitization vary in the literature [
1,
13,
14], a mean wheal diameter greater than 3 mm in relation to that of the negative control is commonly interpreted as the occurrence of sensitization [
15]. Nevertheless, in this study, it was presumed that the minimization of false-positive rates would be beneficial for a confirmative diagnosis of food allergy [
16]. Hence, patients who presented with a mean wheal diameter equivalent to or greater than that of the positive control (class 3) were considered to be positive, and patients with a mean wheal diameter smaller than that of the positive control but greater than that for boiled HEw were also considered positive; these patients were technically not eligible for OFC but were offered the option of undergoing it. In principle, patients with a history of anaphylaxis were excluded from the OFC, regardless of SPT results. These factors are believed to have increased the quality of the diagnosis in the current study. Furthermore, our method has a higher accuracy than the methods used in previous studies that examined the specificity of SPT with HEw in patients of similar age groups [
13,
14]. Although the cutoff value of SPT results was used as a criterion for performing the OFC, this criterion may not be suitable for pediatric patients and should be modified depending on the age and the guardians’ preferences, which is reflective of real-world clinical practices. Patient No. 3 was a one-year-old patient whose guardian was passive toward the OFC. Conversely, the guardian of patient No. 1, who had multiple food allergies and anaphylaxis, wanted to know the possibility of using QEs or baked HEs as an alternative to boiled HEs and to learn about the appropriate dose for boiled HEw. Moreover, it is often difficult to perform OFCs in pediatric patients aged <2 years. When administering the OFC for QEw to patient No. 12, the typical hassles associated with such situations were encountered, wherein the infant refused the food or fell asleep while crying excessively, which made it difficult to repeat or increase the dose of the food challenge, even though the symptoms were subjective. School-aged patients often demonstrate high resistance to unfamiliar foods that they have not consumed for a long time, which makes it difficult or even impossible to perform an OFC, especially in a blinded manner. Among the four patients who refused the OFC for boiled QEw, patient No. 6 refused to consume the eggs after the OFC for stone-baked HEw. Such challenges reflect the circumstances in which the diagnosis and treatment of pediatric food allergies must be individually tailored.
A review of the medical history of the patients revealed that none of them had previously consumed QEw. This is probably attributable to their adherence to the treatment guidelines for HEw allergy. A few previous studies on the cross-antigenicity between QEw and HEw have supported the broad avoidance of all bird egg types in the presence of HEw allergy. In particular, a previous study that investigated cross-antigenicity using SPTs demonstrated a co-sensitization rate of more than 50% [
1]. However, the cutoff values for the SPT results adopted in studies and the reported false-positive rates differ, depending on patient age and study design. In addition, the types and methods of thermal treatment of extracts used for SPTs have been inconsistent among previous studies [
8,
13,
14]. Furthermore, the HEw extracts used in previous studies for SPTs were raw or freeze-dried, unlike the HEw-containing products usually available in the market. In the present study, HEw and QEw extracts were boiled in a manner similar to that used for domestic cooking. The resultant protein bands in vitro were found to be weaker, and cross-reactivity in vivo was found to be lower (41.7%) than the results reported in previous studies [
1,
2]. Moreover, cross-reactivity in vivo was not correlated with the severity of previous allergic reactions to HEw or the concentration of HEw-sIgE. Two of the patients with anaphylaxis (28.6%) consumed boiled QEw as an alternative diet. Accordingly, there is a need to evaluate whether the diet restrictions imposed by the present guidelines are too broad and too uniform for patients. Cross-reactivity to stone-baked HEw was the lowest among all extracts in vitro and was reported to be only 16.7% in our in vivo study, which indicates its potential value as a hypoallergenic alternative in the majority of patients. Boiled HEw can be easily prepared at home, but the protein bands corresponding to the ovomucoid, which the majority of pediatric patients are sensitized to [
17], remained stable for boiled HEw. This is concurrent with the results reported in previous studies that demonstrated a high density of the band corresponding to ovomucoid on SDS-PAGE [
8,
18]. Taking the aforementioned facts into consideration, we can conclude that the symptoms are inevitable when boiled eggs are consumed.
The present study had certain limitations. First, the potential alternative diets for HEw in patients with QEw allergy were not explored, owing to the rarity of QEw allergy in children. Furthermore, some young patients were not asked to undergo the OFC. Therefore, interpreting the cross-reactivity of QEw or baked HEw in patients diagnosed with HEw based on this preliminary finding, which has relatively weak statistical evidence, is insufficient for clinicians. Regardless of the limitations, based on the current findings, we strongly suggest tailored alternative diets to be actively recommended for patients with HEw allergy, even those with a history of anaphylaxis. Moreover, because our methodology showed a good diagnostic value for outcomes of OFCs, the outcomes of this study could help physicians elucidate alternative diets for patients diagnosed with food allergy. If active efforts are added for young patients, in the future, statistically stronger evidence could be gathered using more valuable data. Heat-treated QEw and stone-baked HEw used in the current study can be useful constituents of a hypoallergenic alternative diet, not only for providing nutritional benefits but also for developing immune tolerance. In addition to detailed clinical history taking and measuring HEw-sIgE concentrations, performing SPTs or OFCs for stone-baked HEw and boiled QEw to propose an alternative diet may be beneficial for the diagnosis and treatment of patients with HEw allergy.