The Modification of the Gut Microbiota via Selected Specific Diets in Patients with Crohn’s Disease
Abstract
:1. Introduction
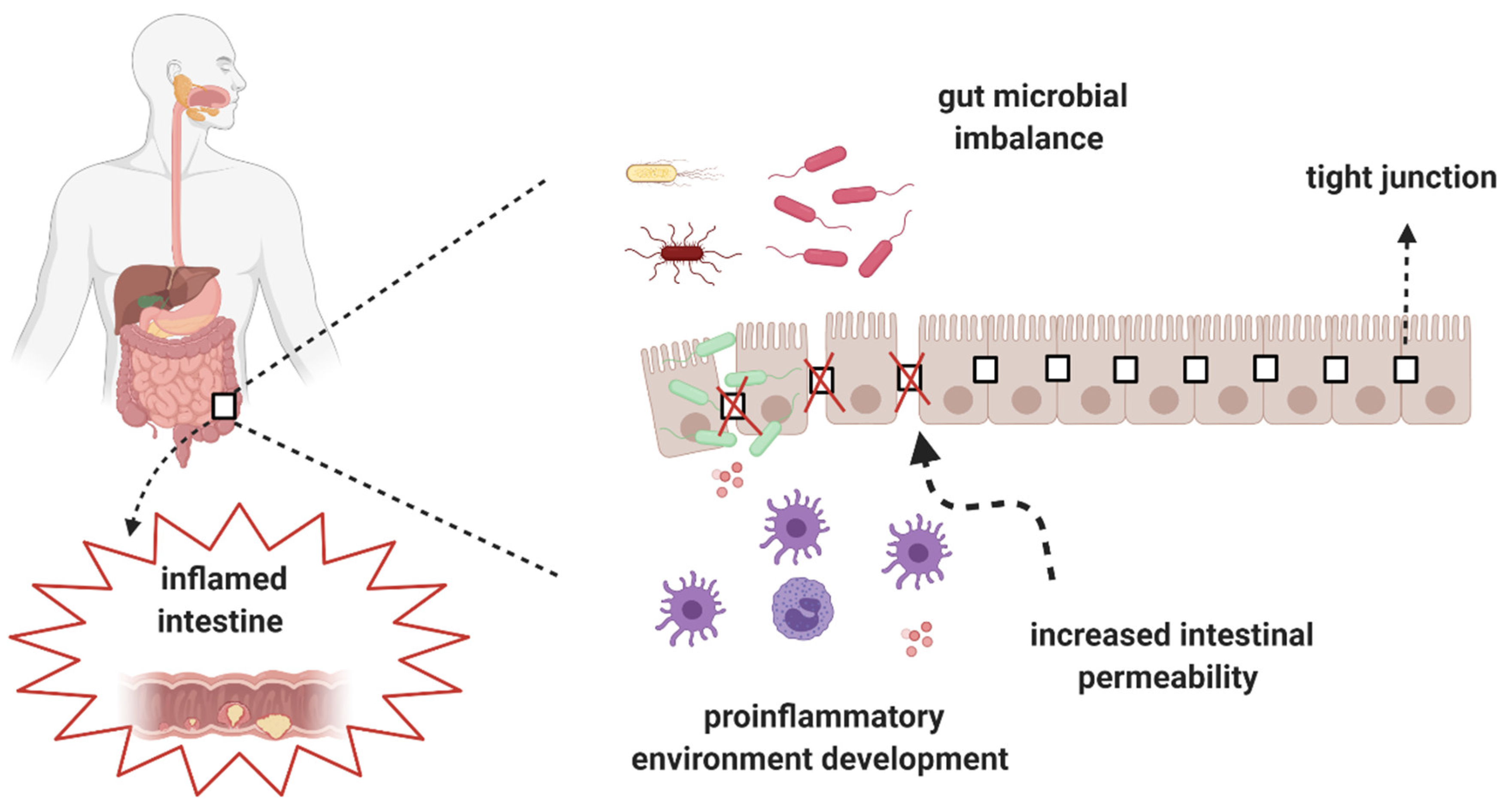
2. Alterations of the Gut Microbiota in CD
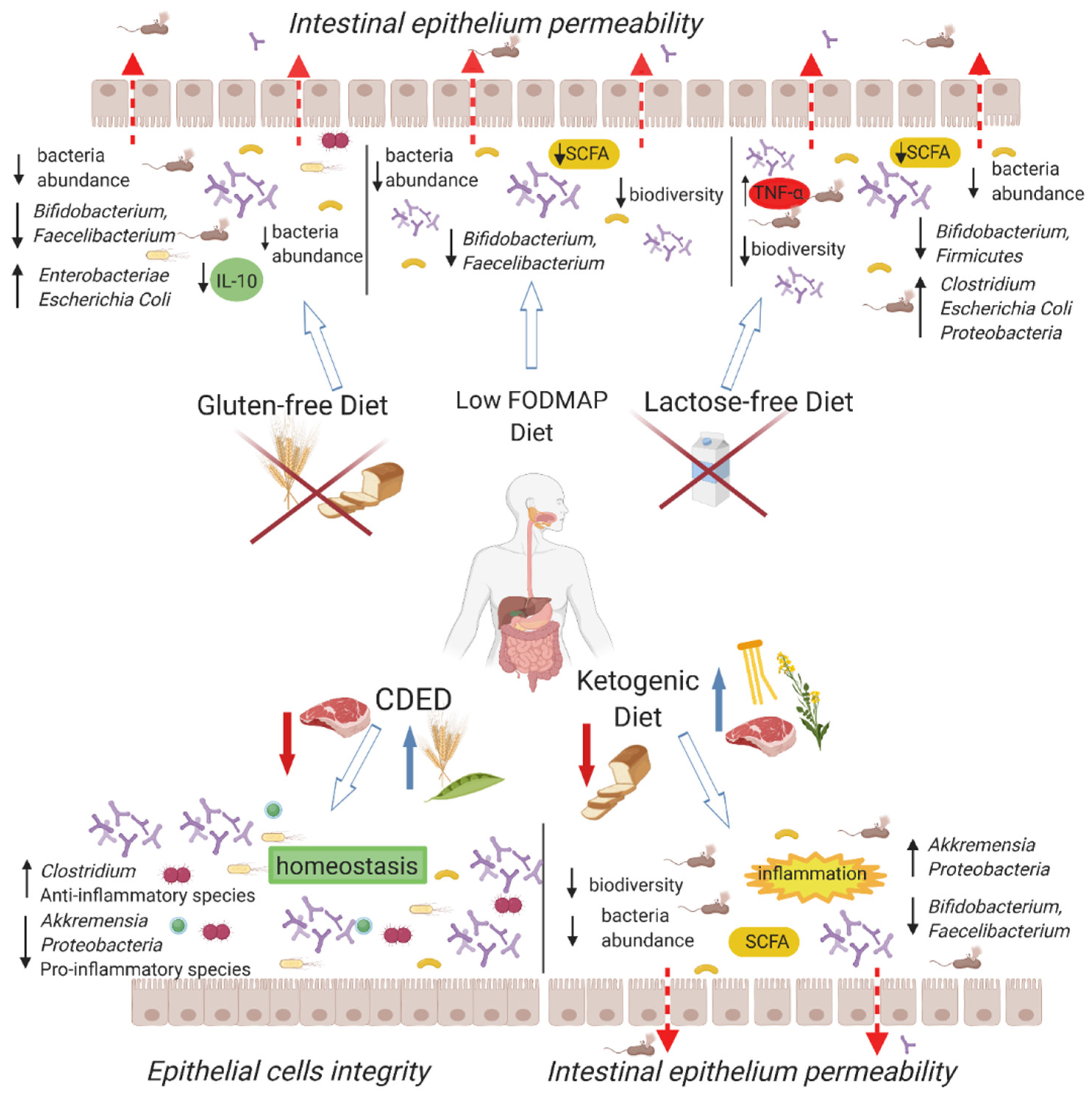
3. Low-FODMAP Diet
4. Gluten-Free Diet
5. Lactose-Free Diet
6. Ketogenic Diet
7. Specific Carbohydrate Diet (SCD)
8. Paleo Diet
9. Crohn’s Disease Exclusion Diet (CDED)
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Fölsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seksik, P.; Rigottier-Gois, L.; Gramet, G.; Sutren, M.; Pochart, P.; Marteau, P.; Jian, R.; Doré, J. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 2003, 52, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci. Hum. Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2021, 12, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Imhann, F.; Vich Vila, A.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Peterson, D.A.; Frank, D.N.; Pace, N.R.; Gordon, J.I. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 2008, 3, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The association between the gut microbiota and the inflammatory bowel disease activity: A systematic review and meta-analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahendran, V.; Riordan, S.M.; Grimm, M.C.; Tran, T.A.T.; Major, J.; Kaakoush, N.O.; Mitchell, H.; Zhang, L. Prevalence of Campylobacter species in adult Crohn’s disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS ONE 2011, 6, e25417. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Zhang, L.; Day, A.S.; Leach, S.T.; Lemberg, D.A.; Mitchell, H. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 1008–1016. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, O.Y. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 8886–8897. [Google Scholar]
- Ananthakrishnan, A.N.; Binion, D.G. Impact of Clostridium difficile on inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Barnich, N.; Sivignon, A.; Darcha, C.; Chan, C.H.F.; Stanners, C.P.; Darfeuille-Michaud, A. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J. Exp. Med. 2009, 206, 2179–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viladomiu, M.; Kivolowitz, C.; Abdulhamid, A.; Dogan, B.; Victorio, D.; Castellanos, J.G.; Woo, V.; Teng, F.; Tran, N.L.; Sczesnak, A.; et al. IgA-coated E. Coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Ma, C.; Panaccione, R.; Khanna, R.; Feagan, B.G.; Jairath, V. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn’s disease? Best Pract. Res. Clin. Gastroenterol. 2019, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Ruseler-van Embden, J.G.; Both-Patoir, H.C. Anaerobic gram-negative faecal flora in patients with Crohn’s disease and healthy subjects. Antonie Van Leeuwenhoek 1983, 49, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [Green Version]
- Rabizadeh, S.; Rhee, K.J.; Wu, S.; Huso, D.; Gan, C.M.; Golub, J.E.; Wu, X.; Zhang, M.; Sears, C.L. Enterotoxigenic Bacteroides fragilis: A potential instigator of colitis. Inflamm. Bowel Dis. 2007, 13, 1475–1483. [Google Scholar] [CrossRef] [Green Version]
- Prindiville, T.P.; Sheikh, R.A.; Cohen, S.H.; Tang, Y.J.; Cantrell, M.C.; Silva, J. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 2000, 6, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, J.R.; Holmes, E.; Khan, F.; Kochhar, S.; Scanlan, P.; Shanahan, F.; Wilson, I.D.; Wang, Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 2007, 6, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Butyrate inhibits visceral allodynia and colonic hyperpermeability in rat models of irritable bowel syndrome. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Balfour Sartor, R. Bacteria in Crohn’s disease: Mechanisms of inflammation and therapeutic implications. J. Clin. Gastroenterol. 2007, 41, 37–43. [Google Scholar] [CrossRef]
- Fong, F.L.Y.; Kirjavainen, P.; Wong, V.H.Y.; El-Nezami, H. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J. Funct. Foods 2015, 13, 71–79. [Google Scholar] [CrossRef]
- Chang, Y.L.; Rossetti, M.; Vlamakis, H.; Casero, D.; Sunga, G.; Harre, N.; Miller, S.; Humphries, R.; Stappenbeck, T.; Simpson, K.W.; et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019, 12, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Tomé, S.; Marin, A.C.; Ortega Moreno, L.; Baldan-Martin, M.; Mora-Gutiérrez, I.; Lanas-Gimeno, A.; Moreno-Monteagudo, J.A.; Santander, C.; Sánchez, B.; Chaparro, M.; et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 2605. [Google Scholar]
- Sanz, Y. Microbiome and gluten. Ann. Nutr. Metab. 2015, 67, 28–41. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [Green Version]
- Nunberg, M.Y.; Werner, L.; Kopylov, U.; Haberman, Y.; Lahad, A.; Weiss, B.; Shouval, D.S. Impaired IL-10 receptor-mediated suppression in monocyte from patients with crohn disease. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 779–784. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food composition, defining cutoff values and international application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M.; Stellingwerff, T.; Kitic, C.M.; Fell, J.W.; Ahuja, K.D.K. Low FODMAP: A preliminary strategy to reduce gastrointestinal distress in athletes. Med. Sci. Sports Exerc. 2018, 50, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP diet improves irritable bowel syndrome symptoms: A meta-analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhan, Y.; Dai, S. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin. Nutr. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and recent developments of the low-FODMAP diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo- oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of low-FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology 2020, 158, 176–188.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, A.; Bobardt, J.S.; Haβ, A.; Nichols, K.B.; Schmid, R.M.; Stein-Thoeringer, C.K. Changes in gut microbial metagenomic pathways associated with clinical outcomes after the elimination of malabsorbed sugars in an IBS cohort. Gut Microbes 2019. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Manceñido Marcos, N.; Pajares Villarroya, R.; Salinas Moreno, S.; Arribas López, M.R.; Comas Redondo, C. The association between de novo inflammatory bowel disease and celiac disease. Rev. Española Enfermedades Dig. 2019. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Sepulveda, R.; Hing, T.; Shah, N.D.; Choe, M.; Limsui, D.; Shah, S. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 147–151. [Google Scholar] [CrossRef]
- Casella, G.; Di Bella, C.; Salemme, M.; Villanacci, V.; Antonelli, E.; Baldini, V.; Bassotti, G. Celiac disease, non-celiac gluten sensitivity and inflammatory bowel disease. Minerva Gastroenterol. Dietol. 2015, 61, 267–271. [Google Scholar] [PubMed]
- Schreiner, P.; Yilmaz, B.; Rossel, J.B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef] [Green Version]
- Golfetto, L.; de Senna, F.D.; Hermes, J.; Beserra, B.T.S.; da Silva França, F.; Martinello, F. Baixa contagem de bifidobactérias em pacientes adultos com doença celíaca, em dieta isenta de glúten. Arq. Gastroenterol. 2014, 51, 139–143. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144. [Google Scholar] [CrossRef] [Green Version]
- Francavilla, R.; Piccolo, M.; Francavilla, A.; Polimeno, L.; Semeraro, F.; Cristofori, F.; Castellaneta, S.; Barone, M.; Indrio, F.; Gobbetti, M.; et al. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients with Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J. Clin. Gastroenterol. 2019, 53, E117–E125. [Google Scholar] [CrossRef] [Green Version]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Montgomery, R.K.; Krasinski, S.D.; Hirschhorn, J.N.; Grand, R.J. Lactose and lactase—Who is lactose intolerant and why? J. Pediatric Gastroenterol. Nutr. 2007, 45. [Google Scholar] [CrossRef] [PubMed]
- Scrimshaw, N.S.; Murrary, E.B. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am. J. Clin. Nutr. 1988, 48, 1083–1159. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Savaiano, D.A. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am. J. Clin. Nutr. 1996, 64, 232–236. [Google Scholar] [CrossRef]
- He, T.; Priebe, M.G.; Harmsen, H.J.M.; Stellaard, F.; Sun, X.; Welling, G.W.; Vonk, R.J. Colonic Fermentation May Play a Role in Lactose Intolerance in Humans. J. Nutr. 2006, 136, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Tirpitz, C.; Kohn, C.; Steinkamp, M.; Geerling, I.; Maier, V.; Möller, P.; Adler, G.; Reinshagen, M. Lactose intolerance in active Crohn’s disease: Clinical value of duodenal lactase analysis. J. Clin. Gastroenterol. 2002, 34, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Galiatsatos, P.; Xue, X. Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr. J. 2016, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods-A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Chang, E.B. Diet-induced expansion of pathobionts in experimental colitis: Implications for tailored therapies. Gut Microbes 2013, 4, 172–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salli, K.; Anglenius, H.; Hirvonen, J.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Tiihonen, K.; Maukonen, J.; Ouwehand, A.C. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.T.T.; Boger, M.C.L.; Dijkhuizen, L.; van Leeuwen, S.S. Stimulatory effects of novel glucosylated lactose derivatives GL34 on growth of selected gut bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, L.M.A.; Sundekilde, U.K.; Andersen, H.J.; Nielsen, D.S.; Bertram, H.C. Lactose and Bovine Milk Oligosaccharides Synergistically Stimulate B. longum subsp. longum Growth in a Simplified Model of the Infant Gut Microbiome. J. Proteome Res. 2019, 18, 3086–3098. [Google Scholar] [CrossRef]
- Almeida, C.C.; Lorena, S.L.S.; Pavan, C.R.; Akasaka, H.M.I.; Mesquita, M.A. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012, 27, 247–251. [Google Scholar] [CrossRef]
- Saltzman, J.R.; Russell, R.M.; Golner, B.; Barakat, S.; Dallal, G.E.; Goldin, B.R. A randomized trial of Lactobacillus acidophilus BG2FO4 to treat lactose intolerance. Am. J. Clin. Nutr. 1999, 69, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, W.; Wang, C.; Yu, Q.; Dai, R.; Pei, X. Lactococcus lactis expressing food-grade β-galactosidase alleviates lactose intolerance symptoms in post-weaning Balb/c mice. Appl. Microbiol. Biotechnol. 2012, 96, 1499–1506. [Google Scholar] [CrossRef]
- Ntemiri, A.; Ribière, C.; Stanton, C.; Ross, R.P.; O’Connor, E.M.; O’Toole, P.W. Retention of microbiota diversity by lactose-free milk in a mouse model of elderly gut microbiota. J. Agric. Food Chem. 2019, 67, 2098–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklas, T.A.; Qu, H.; Hughes, S.O.; He, M.; Wagner, S.E.; Foushee, H.R.; Shewchuk, R.M. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am. J. Clin. Nutr. 2011, 94, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, X.M.K.; Ronveaux, C.C.; Rust, B.M.; Newman, J.W.; Hawley, M.; Barile, D.; Mills, D.A.; Raybould, H.E. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G474–G487. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef] [Green Version]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Sampaio, L.P. de B. Ketogenic diet for epilepsy treatment. Arq. Neuropsiquiatr. 2016, 74, 842–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: Time for microbial marker of gastrointestinal disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, H.M. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominika, Ś.; Arjan, N.; Karyn, R.P.; Henryk, K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef]
- Meddah, A.T.T.; Yazourh, A.; Desmet, I.; Risbourg, B.; Verstraete, W.; Romond, M.B. The regulatory effects of whey retentate from Bifidobacteria fermented milk on the microbiota of the simulator of the human intestinal microbial ecosystem (SHIME). J. Appl. Microbiol. 2001, 91, 1110–1117. [Google Scholar] [CrossRef] [Green Version]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakodkar, S.; Farooqui, A.J.; Mikolaitis, S.L.; Mutlu, E.A. The specific carbohydrate diet for inflammatory bowel disease: A case series. J. Acad. Nutr. Diet. 2015, 115, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, S.V.; Haas, M.P. The treatment of celiac disease with the specific carbohydrate diet; report on 191 additional cases. Am. J. Gastroenterol. 1955, 23, 344–360. [Google Scholar]
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional interventions in the patient with inflammatory bowel disease. Gastroenterol. Clin. North Am. 2018, 47, 155–177. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Omura, K.; Hirano, K.; Kanehira, E.; Kaito, K.; Tamura, M.; Nishida, S.; Kawakami, K.; Watanabe, Y. Small amount of low-residue diet with parenteral nutrition can prevent decreases in intestinal mucosal integrity. Ann. Surg. 2000, 231, 112–118. [Google Scholar] [CrossRef]
- Walters, S.S. Analysis of gut microbiome and diet modification in patients with crohn’s disease. SOJ Microbiol. Infect. Dis. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovsky, A.; Kitts, C.L. Effect of the specific carbohydrate diet on the microbiome of a primary sclerosing cholangitis and ulcerative colitis patient. Cureus 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and fungi of the human gut microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Voegtlin, W.L. The Stone Age Diet: Based on In-Depth Studies of Human Ecology and the Diet of Man; Vantage Press, Incorporated: New York, NY, USA, 1975. [Google Scholar]
- Challa, H.J.; Bandlamudi, M.; Uppaluri, K.R. Paleolithic diets. In Nutrition and Cardiometabolic Health; CRC Press: Boca Raton, FL, USA, 2019; pp. 493–516. [Google Scholar]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M. Paleolithic nutrition. N. Engl. J. Med. 1985, 312, 283–289. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Hallert, C.; Björck, I.; Nyman, M.; Pousette, A.; Grännö, C.; Svensson, H. Increasing fecal butyrate in ulcerative colitis patients by diet: Controlled pilot study. Inflamm. Bowel Dis. 2003, 9, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Suga, T.; Tochihara, M.; Hibi, T.; Naganuma, M.; Homma, T.; Asakura, H.; Nakano, H.; Takahama, K.; Fujiyama, Y.; et al. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: First report of a multicenter open control trial. J. Gastroenterol. 2002, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cai, T.; Cheng, S.C.; Savova, G.; Chen, P.; Szolovits, P.; Xia, Z.; De Jager, P.L.; et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 1921–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziechciarz, P.; Horvath, A.; Shamir, R.; Szajewska, H. Meta-analysis: Enteral nutrition in active Crohn’s disease in children. Aliment. Pharmacol. Ther. 2007, 26, 795–806. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Boneh, R.S.; Shabat, C.S.; Yanai, H.; Chermesh, I.; Avraham, S.B.; Boaz, M.; Levine, A. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of Remission in children and adults failing biological therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [Green Version]
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Pediatr. 2019. [Google Scholar] [CrossRef]
- Lane, E.R.; Lee, D.; Suskind, D.L. Dietary Therapies in Pediatric Inflammatory Bowel Disease: An Evolving Inflammatory Bowel Disease Paradigm. Gastroenterol. Clin. N. Am. 2017, 46, 731–744. [Google Scholar] [CrossRef]
| Trial Name | Type of Nutrition Intervention; Population | Alternations in Microbiota |
|---|---|---|
| Staudacher HM, 2012 | Low-FODMAP; IBS | ↓ Bifidobacterium |
| Cox S.R, 2020 | Low-FODMAP; Crohn disease and Colitis ulcerosa | ↓ Bifidobacterium, in particular: Bifidobacterium adolescentis, Bifidobacterium longum, and Faecalibacterium prausnitzii |
| Schreiner P, 2019 | Gluten-free diet, Crohn disease and Colitis ulcerosa | ↓ Total amount and biodiversity |
| De Palma G, 2009 | Gluten-free diet, healthy people | ↓ Bifidobacterium, Lactobacillus, and Bifidobacterium longum ↑ Enterobacteriaceae and Escherichia coli |
| Marc J.B., 2016 | Gluten-free diet, healthy people | ↓ Veillonellaceae, Ruminococcus bromii, Roseburia faecis ↑ Clostridiacea, Coriobacteiaceae |
| Levine A, 2019 | CDED, children with Crohn disease | ↓ fecal Proteobacteria |
| Olson C.A., 2018 | Ketogenic diet; children with drug-resistant epilepsy | ↑ Parabacteroides |
| Lawrece D., 2014 | Ketogenic diet with an increased animal protein content; healthy people | ↑ Bacteroides, Alistipes, Bilophila |
| Devkota S., 2013 | Ketogenic diet with an increased saturated fatty acids content; healthy people | ↑ Bilophila wadsworthia |
| A Dubrovsky, 2018 | SCD, IBD patients and healthy control | ↑ Fusobacterium ulcerans |
| Schnorr SL, 2014 | Paleo diet, Hadza hunter–gatherers vs. Italian control | ↑ Prevotella (Bacteroidetes), Treponema (Spirochaetes), and unclassified Bacteroidetes |
| Halmos E., 2015 | Low-FODMAP; IBS | ↓ Total amount ↓ Cluster Clorstridium XIVa, Akkermansia municiphila, Ruminococcus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starz, E.; Wzorek, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Stachowska, L.; Przewłócka, K.; Stachowska, E.; Skonieczna-Żydecka, K. The Modification of the Gut Microbiota via Selected Specific Diets in Patients with Crohn’s Disease. Nutrients 2021, 13, 2125. https://doi.org/10.3390/nu13072125
Starz E, Wzorek K, Folwarski M, Kaźmierczak-Siedlecka K, Stachowska L, Przewłócka K, Stachowska E, Skonieczna-Żydecka K. The Modification of the Gut Microbiota via Selected Specific Diets in Patients with Crohn’s Disease. Nutrients. 2021; 13(7):2125. https://doi.org/10.3390/nu13072125
Chicago/Turabian StyleStarz, Eliza, Karolina Wzorek, Marcin Folwarski, Karolina Kaźmierczak-Siedlecka, Laura Stachowska, Katarzyna Przewłócka, Ewa Stachowska, and Karolina Skonieczna-Żydecka. 2021. "The Modification of the Gut Microbiota via Selected Specific Diets in Patients with Crohn’s Disease" Nutrients 13, no. 7: 2125. https://doi.org/10.3390/nu13072125
APA StyleStarz, E., Wzorek, K., Folwarski, M., Kaźmierczak-Siedlecka, K., Stachowska, L., Przewłócka, K., Stachowska, E., & Skonieczna-Żydecka, K. (2021). The Modification of the Gut Microbiota via Selected Specific Diets in Patients with Crohn’s Disease. Nutrients, 13(7), 2125. https://doi.org/10.3390/nu13072125







