Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
Statistics
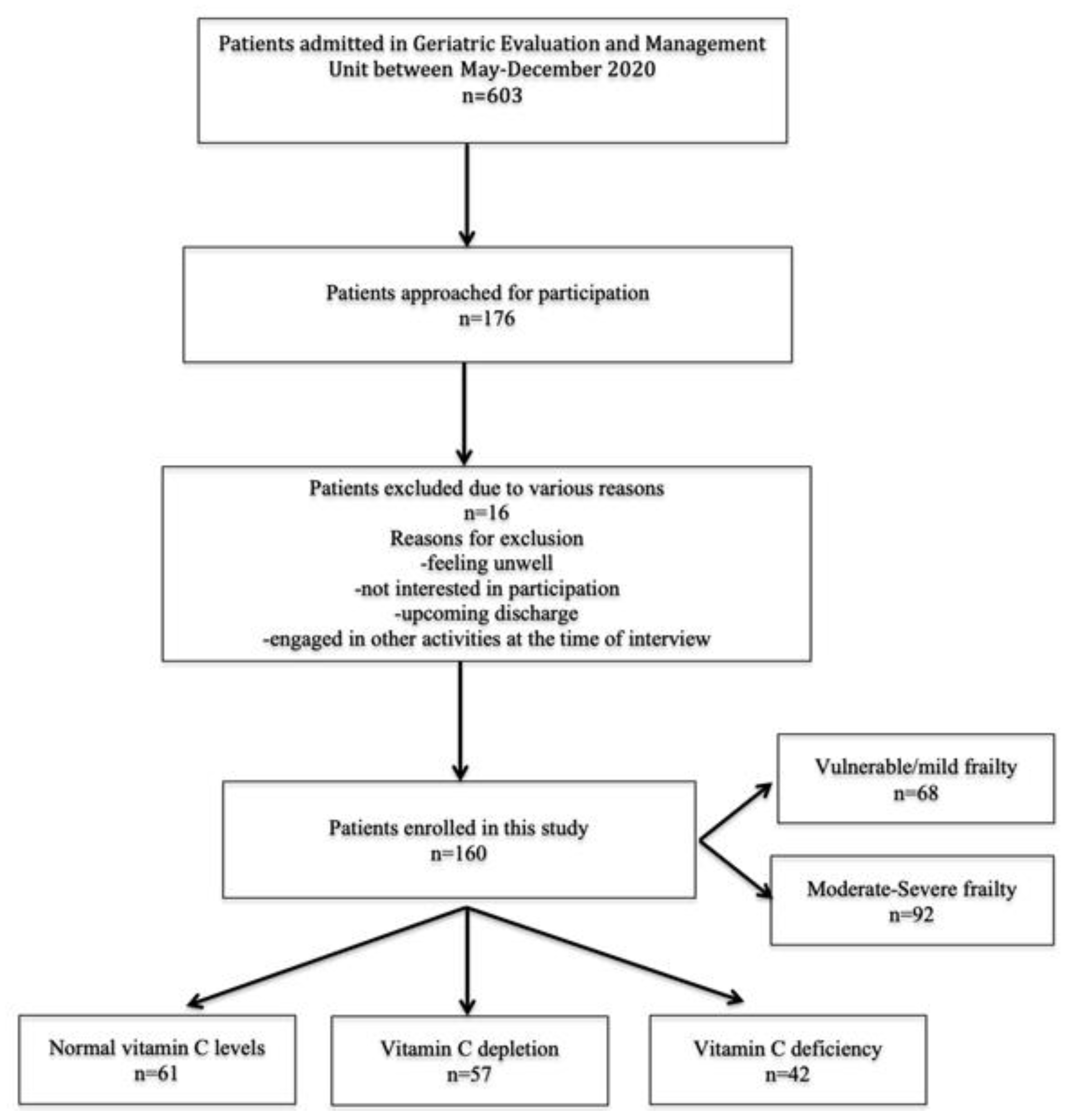
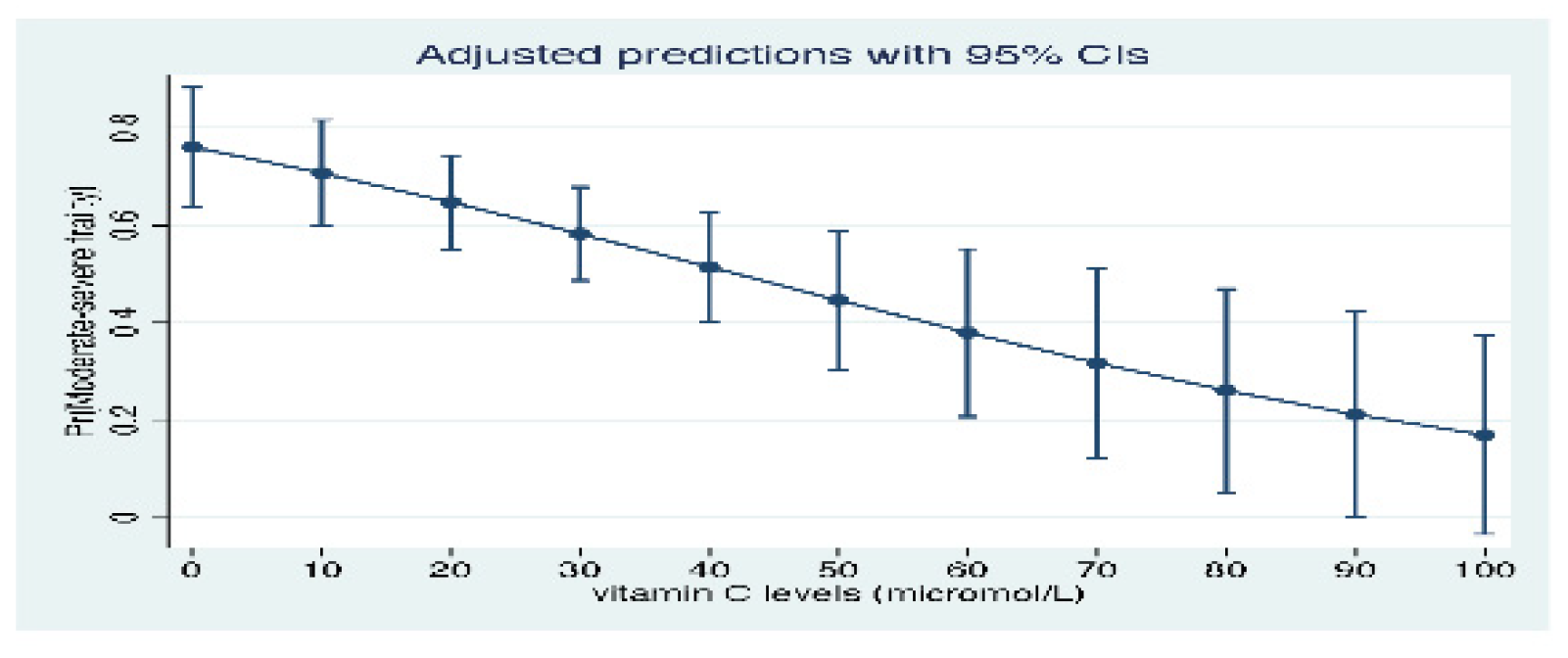
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Morley, J.E.; Vellas, B.; Van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Hammami, S.; Zarrouk, A.; Piron, C.; Almas, I.; Sakly, N.; Latteur, V. Prevalence and factors associated with frailty in hospitalized older patients. BMC Geriatr. 2020, 20, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Howson, F.F.A.; Culliford, D.J.; Sayer, A.A.; Roberts, H.C. The feasibility of assessing frailty and sarcopenia in hospitalised older people: A comparison of commonly used tools. BMC Geriatr. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Artaza-Artabe, I.; Sáez-López, P.; Sánchez-Hernández, N.; Fernández-Gutierrez, N.; Malafarina, V. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Matur. 2016, 93, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Raynaud-Simon, A.; Lesourd, B. Dénutrition du sujet âgé. Conséquences cliniques [Malnutrition in the elderly. Clinical conse-quences]. Presse Med. 2000, 29, 2183–2190. [Google Scholar]
- Semba, R.D.; Bartali, B.; Zhou, J.; Blaum, C.; Ko, C.-W.; Fried, L.P. Low Serum Micronutrient Concentrations Predict Frailty Among Older Women Living in the Community. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2006, 61, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Yokoyama, T.; Yoshida, H.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Shimizu, Y.; Kondo, Y.; Handa, S.; et al. A Significant Relationship between Plasma Vitamin C Concentration and Physical Performance among Japanese Elderly Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 295–301. [Google Scholar] [CrossRef]
- Scholz, S.S.; Borgstedt, R.; Ebeling, N.; Menzel, L.C.; Jansen, G.; Rehberg, S. Mortality in septic patients treated with vitamin C: A systematic meta-analysis. Crit. Care 2021, 25, 1–10. [Google Scholar] [CrossRef]
- Perna, S.; Francis, M.D.; Bologna, C.; Moncaglieri, F.; Riva, A.; Morazzoni, P.; Allegrini, P.; Isu, A.; Vigo, B.; Guerriero, F.; et al. Performance of Edmonton Frail Scale on frailty assessment: Its association with multi-dimensional geriatric conditions assessed with specific screening tools. BMC Geriatr. 2017, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenkel, W.J.; Jongerius, E.J.; Uitert, M.J.M.-V.; Van Munster, B.C.; De Rooij, S.E. Validation of the Charlson Comorbidity Index in Acutely Hospitalized Elderly Adults: A Prospective Cohort Study. J. Am. Geriatr. Soc. 2014, 62, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.B.; Bates, D.W.; Ngo, L.; Ufberg, J.W.; Shapiro, N.I. Charlson Index Is Associated with One-year Mortality in Emergency Department Patients with Suspected Infection. Acad. Emerg. Med. 2006, 13, 530–536. [Google Scholar] [CrossRef]
- De Villar, L.O.-P.; Martínez-Olmos, F.J.; Junqué-Jiménez, A.; Amer-Cuenca, J.J.; Gramage, J.M.; Mercer, T.; Segura-Ortí, E. Test-retest reliability and minimal detectable change scores for the short physical performance battery, one-legged standing test and timed up and go test in patients undergoing hemodialysis. PLoS ONE 2018, 13, e0201035. [Google Scholar] [CrossRef] [Green Version]
- Larsson, P.; Borge, C.R.; Nygren-Bonnier, M.; Lerdal, A.; Edvardsen, A. An evaluation of the short physical performance battery following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. BMC Res. Notes 2018, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, C.J.; Mitchell, S.N.; Butterfield, S.A. Validation of the Timed Up and Go Test for Assessing Balance Variables in Adults Aged 65 and Older. J. Aging Phys. Act. 2019, 27, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Robins, L.N.; Helzer, J.E. The Mini-Mental State Examination. Arch. Gen. Psychiatry 1983, 40, 812. [Google Scholar] [CrossRef]
- Bellón, J.Á.; Lardelli, P.; Luna, J.D.D.; Delgado, A. Validity of self reported utilisation of primary health care services in an urban population in Spain. J. Epidemiol. Community Health 2000, 54, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Park, H.; Makizako, H.; Doi, T.; Lee, S.; Suzuki, T. Depressive symptoms and cognitive performance in older adults. J. Psychiatr. Res. 2014, 57, 149–156. [Google Scholar] [CrossRef]
- Dennis, M.; Kadri, A.; Coffey, J. Depression in older people in the general hospital: A systematic review of screening instruments. Age Ageing 2012, 41, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, Y.; Thompson, C.; Kaambwa, B.; Shahi, R.; Miller, M. Validity of the Malnutrition Universal Screening Tool (MUST) in Australian hospitalized acutely unwell elderly patients. Asia Pac. J. Clin. Nutr. 2017, 26, 994–1000. [Google Scholar] [PubMed]
- Balestroni, G.; Bertolotti, G. EuroQol-5D (EQ-5D): An instrument for measuring quality of life. Monaldi Arch. Chest Dis. 2012, 78, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.K.; Montgomery, J.; Yan, Y.; Mecchella, J.N.; Bartels, S.J.; Masutani, R.; Batsis, J.A. Association Between Hospital Admission Risk Profile Score and Skilled Nursing or Acute Rehabilitation Facility Discharges in Hospitalized Older Adults. J. Am. Geriatr. Soc. 2016, 64, 2095–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessier, F.; Birlouez-Aragon, I.; Tjani, C.; Guilland, J.C. Validation of a micromethod for determining oxidized and reduced vitamin C in plasma by HPLC-fluorescence. Int. J. Vitam. Nutr. Res. 1996, 66, 166–170. [Google Scholar]
- Mitmesser, S.H.; Ye, Q.; Evans, M.; Combs, M. Determination of plasma and leukocyte vitamin C concentrations in a randomized, double-blind, placebo-controlled trial with Ester-C®. SpringerPlus 2016, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Johnston, C.S.; Corte, C. People with Marginal Vitamin C Status are at High Risk of Developing Vitamin C Deficiency. J. Am. Diet. Assoc. 1999, 99, 854–856. [Google Scholar] [CrossRef]
- Nemes, S.; Jonasson, J.M.; Genell, A.; Steineck, G. Bias in odds ratios by logistic regression modelling and sample size. BMC Med. Res. Methodol. 2009, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, Y.; Miller, M.; Shahi, R.; Doyle, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Vitamin C deficiency in Australian hospitalized patients: An observational study. Intern. Med. J. 2018. [Google Scholar] [CrossRef]
- Ravindran, P.; Wiltshire, S.; Das, K.; Wilson, R.B. Vitamin C deficiency in an Australian cohort of metropolitan surgical patients. Pathology 2018, 50, 654–658. [Google Scholar] [CrossRef] [PubMed]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T.; Myint, P.K. Plasma Vitamin C Levels: Risk Factors for Deficiency and Association with Self-Reported Functional Health in the European Prospective Investigation into Cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Jacques, P.F.; Felson, D.T.; Tucker, K.L.; Mahnken, B.; Wilson, P.W.; Rosenberg, I.H.; Rush, D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am. J. Clin. Nutr. 1997, 66, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Maras, J.E.; Bermudez, O.I.; Qiao, N.; Bakun, P.J.; Boody-Alter, E.L.; Tucker, K.L. Intake of α-tocopherol is limited among US adults. J. Am. Diet. Assoc. 2004, 104, 567–575. [Google Scholar] [CrossRef]
- Wright, J.D.; Bialostosky, K.; Gunter, E.W.; Carroll, M.D.; Najjar, M.F.; A Bowman, B.; Johnson, C.L. Blood folate and vitamin B12: United States, 1988-94. Vital-Health Stat. Ser. 11 Data Natl. Health Surv. 1998, 11, 1–78. [Google Scholar]
- Nowjack-Raymer, R.; Sheiham, A. Association of edentulism and diet and nutrition in US adults. J. Dent. Res. 2003, 82, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Michelon, E.; Blaum, C.; Semba, R.D.; Xue, Q.L.; Ricks, M.O.; Fried, L.P. Vitamin and carotenoid status in older women: Associations with the frailty syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Sanford, A.M. Anorexia of aging and its role for frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 54–60. [Google Scholar] [CrossRef]
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.G.A.; Luiking, Y.C.; De Groot, L.C.P.G.M. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Saum, K.-U.; Dieffenbach, A.K.; Jansen, E.H.; Schöttker, B.; Holleczek, B.; Hauer, K.; Brenner, H. Association between Oxidative Stress and Frailty in an Elderly German Population: Results from the ESTHER Cohort Study. Gerontology 2015, 61, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef]
- Arbogast, S.; Reid, M.B. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am. J. Physiol. Integr. Comp. Physiol. 2004, 287, R698–R705. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Kondo, H.; Nakagaki, I.; Sasaki, S.; Hori, S.; Itokawa, Y. Mechanism of oxidative stress in skeletal muscle atrophied by immobilization. Am. J. Physiol. Metab. 1993, 265, E839–E844. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, Á.; Cordero, M.D.; Salviati, L.; Artuch, R.; Pineda, M.; Briones, P.; Izquierdo, L.G.; Cotán, D.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 2009, 5, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Siu, P.M.; Pistilli, E.E.; Alway, S.E. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J. Appl. Physiol. 2008, 105, 1695–1705. [Google Scholar] [CrossRef] [Green Version]
- Lewis, L.N.; Hayhoe, R.P.G.; A Mulligan, A.; Luben, R.N.; Khaw, K.-T.; A Welch, A. Lower Dietary and Circulating Vitamin C in Middle- and Older-Aged Men and Women Are Associated with Lower Estimated Skeletal Muscle Mass. J. Nutr. 2020, 150, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; de la Puente, B.; Busquets, S.; López-Soriano, F.J.; Gea, J.; Argiles, J.M. Both oxidative and nitrosative stress are associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2005, 579, 1646–1652. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.; Sayer, A.A.; Jameson, K.; Syddall, H.; Dennison, E.M.; Cooper, C.; Robinson, S. Does diet influence physical performance in community-dwelling older people? Findings from the Hertfordshire Cohort Study. Age Ageing 2011, 40, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.; Stuijk, E.; Rodriguez-Artalejo, F.; Hagan, K.; Hu, F.; Lopez-Garcia, E. Fruits and Vegetables Intake and Risk of Frailty in Women Aged 60 and Older (OR33-08-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, N.R. A Randomized Factorial Trial of Vitamins C and E and Beta Carotene in the Secondary Prevention of Cardiovascular Events in Women. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Loria, C.M.; Klag, M.J.; E Caulfield, L.; Whelton, P.K. Vitamin C status and mortality in US adults. Am. J. Clin. Nutr. 2000, 72, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Teterina, A.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R.; Gramlich, L.; Payette, H.; Bernier, P.; Davidson, B.; et al. Factors associated with nutritional decline in hospitalised medical and surgical patients admitted for 7 d or more: A prospective cohort study. Br. J. Nutr. 2015, 114, 1612–1622. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.; Teixeira, P.P.; Eckert, I.D.C.; Burgel, C.F.; Silva, F.M. Decline of nutritional status in the first week of hospitalisation predicts longer length of stay and hospital readmission during 6-month follow-up. Br. J. Nutr. 2021, 125, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
| Variable | Non-Frail/Vulnerable/Mild Frailty | Moderate–Severe Frailty | p = Value |
|---|---|---|---|
| Number (%) | 68 (42.5) | 92 (57.5) | |
| Age, mean (SD) | 82.8 (5.7) | 85.7 (6.7) | 0.004 |
| Sex female, n (%) | 39 (57.4) | 57 (61.9) | 0.557 |
| Charlson index mean (SD) | 7.8 (2.5) | 8.8 (2.6) | 0.001 |
| Medications mean (SD) | 6.9 (3.9) | 7.9 (3.3) | 0.070 |
| Residence home alone n (%) | 32 (47.1) | 50 (54.4) | 0.362 |
| Education diploma/university n (%) | 31 (45.6) | 34 (36.9) | 0.272 |
| Income <40k/year | 34 (50.8) | 60 (65.2) | 0.067 |
| Medications mean (SD) | 6.9 (3.9) | 7.9 (3.3) | 0.070 |
| MMSE mean (SD) | 25.8 (3.4) | 23.8 (3.2) | <0.001 |
| Smokers n (%) | 41 (60.3) | 51 (55.4) | 0.539 |
| GDS mean (SD) | 3.5 (2.1) | 5.2 (3.1) | <0.001 |
| MUST score mean (SD) | 0.86 (1.1) | 0.99 (1.2) | 0.511 |
| Fruits/Vegetable intake/day mean (SD) | 1.3 (0.6) | 1.2 (0.6) | 0.187 |
| HARP score mean (SD) | 2.4 (1.2) | 3.5 (0.6) | <0.001 |
| TUG score in seconds mean (SD) | 25.3 (15.4) | 40.3 (20.5) | <0.001 |
| Vitamin C μmol/L mean (SD) | 31.8 (24.4) | 22.9 (21.4) | 0.015 |
| Hypovitaminosis C n (%) | 37 (54.4) | 62 (67.4) | 0.095 |
| Vitamin C deficient n (%) | 11 (16.2) | 31 (33.7) | 0.013 |
| Vitamin D nmol/L mean (SD) | 62.9 (27.3) | 72.4 (33.4) | 0.058 |
| Vitamin B12 pmol/L mean (SD) | 442.9 (300.7) | 502.5 (351.8) | 0.262 |
| Albumin g/L mean (SD) | 35.6 (30.0) | 31.3 (5.2) | 0.180 |
| EFS scores mean (SD) | 7.7 (1.1) | 11.3 (1.3) | <0.001 |
| SPPB scores total mean (SD) | 5.5 (2.8) | 2.7 (2.3) | <0.001 |
| EQ5D index mean (SD) | 0.78 (0.13) | 0.68 (0.16) | <0.001 |
| LOS median (IQR) | 11.5 (14) | 22.7 (17) | 0.004 |
| In-hospital mortality n (%) | 0 | 7 (7.6) | 0.02 |
| 30-day readmissions n (%) | 15 (22.1) | 20 (21.7) | 0.961 |
| Variable. | aOR | 95% CI | p Value |
|---|---|---|---|
| Vitamin C deficiency | 4.30 | 1.33–13.86 | 0.015 |
| Vitamin C depletion | 1.83 | 0.66–5.08 | 0.243 |
| Age | 0.94 | 0.87–1.02 | 0.158 |
| Sex male | 0.95 | 0.34–2.673 | 0.924 |
| Charlson index | 1.03 | 0.85–1.27 | 0.715 |
| Smokers | 0.68 | 0.28–1.66 | 0.395 |
| MUST score | 1.17 | 0.77–1.78 | 0.463 |
| MMSE score | 0.89 | 0.78–1.02 | 0.104 |
| GDS score | 1.33 | 1.09–1.61 | 0.004 |
| Fruits/Vegetable intake | 0.94 | 0.84–1.06 | 0.350 |
| HARP score | 4.78 | 2.43–9.40 | <0.001 |
| Income <40k/year | 1.67 | 0.63–4.44 | 0.302 |
| Creatinine | 0.99 | 0.98–1.00 | 0.069 |
| Vitamin D levels | 1.01 | 0.99–1.02 | 0.281 |
| Vitamin B12 levels | 1.00 | 0.99–1.00 | 0.344 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study. Nutrients 2021, 13, 2117. https://doi.org/10.3390/nu13062117
Sharma Y, Popescu A, Horwood C, Hakendorf P, Thompson C. Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study. Nutrients. 2021; 13(6):2117. https://doi.org/10.3390/nu13062117
Chicago/Turabian StyleSharma, Yogesh, Alexandra Popescu, Chris Horwood, Paul Hakendorf, and Campbell Thompson. 2021. "Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study" Nutrients 13, no. 6: 2117. https://doi.org/10.3390/nu13062117
APA StyleSharma, Y., Popescu, A., Horwood, C., Hakendorf, P., & Thompson, C. (2021). Prevalence of Hypovitaminosis C and its Relationship with Frailty in Older Hospitalised Patients: A Cross-Sectional Study. Nutrients, 13(6), 2117. https://doi.org/10.3390/nu13062117






