Dietary Behaviors and Incident COVID-19 in the UK Biobank
Abstract
:1. Introduction
2. Materials and Methods
2.1. UK Biobank
2.2. COVID-19 Diagnosis
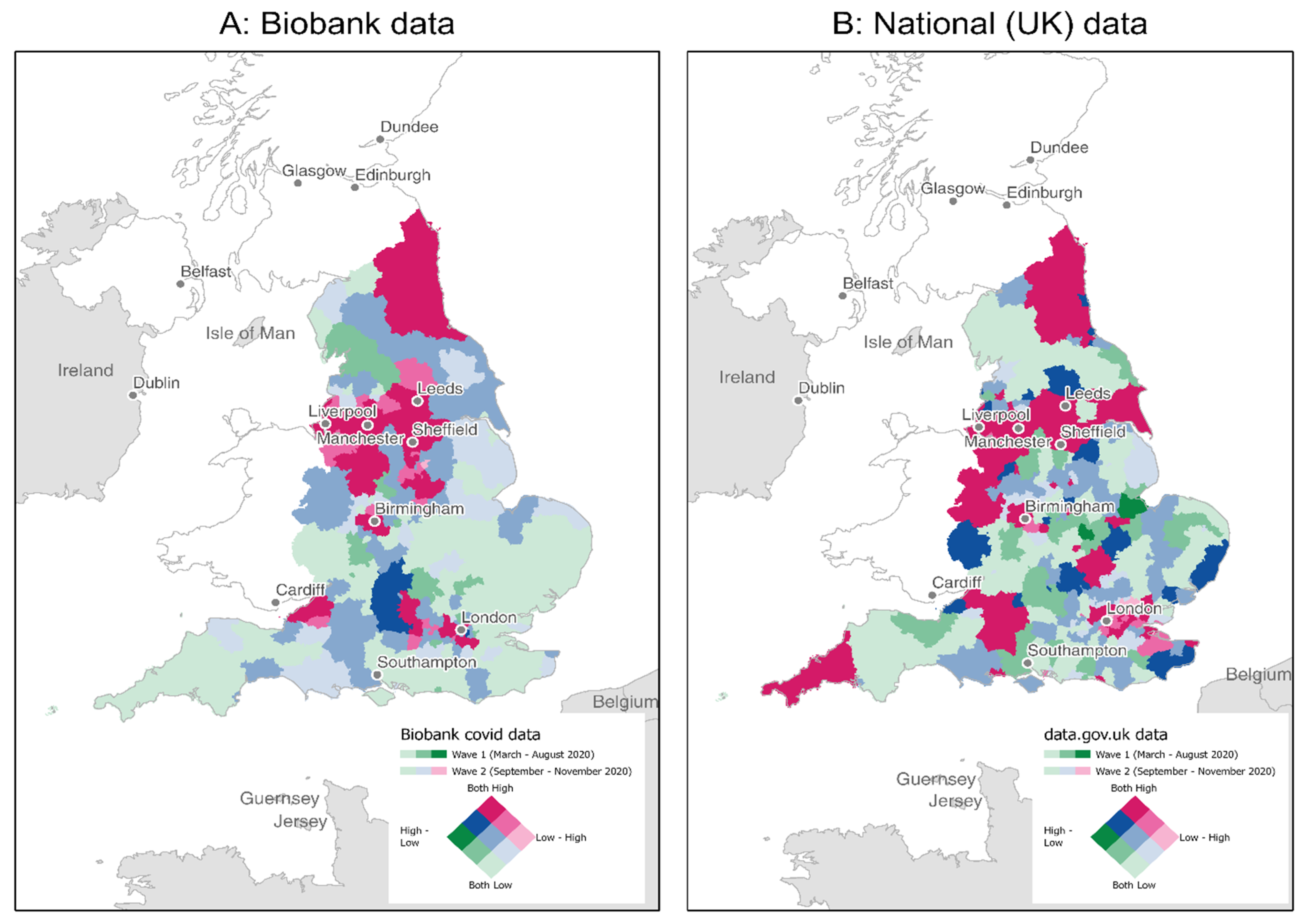
2.3. COVID-19 Exposure
2.4. Baseline Dietary Data
2.5. Other Covariates
2.6. Analysis Sample
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Nutritional Factors and COVID-19 Positivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins University Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 21 December 2020).
- NIA. Notice of Special Interest. Available online: https://grants.nih.gov/grants/guide/notice-files/NOT-AG-20-022.html (accessed on 16 March 2020).
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Frel, D.L.; Atsma, D.E.; Pijl, H.; Seidell, J.C.; Leenen, P.J.M.; Dik, W.A.; van Rossum, E.F.C. The Impact of Obesity and Lifestyle on the Immune System and Susceptibility to Infections Such as COVID-19. Front. Nutr. 2020, 7, 597600. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E.; Calder, P.C.; Miles, E.A. Diet and Immune Function. Nutrients 2019, 11, 1933. [Google Scholar] [CrossRef] [Green Version]
- Greene, M.W.; Roberts, A.P.; Fruge, A.D. Negative Association between Mediterranean Diet Adherence and COVID-19 Cases and Related Deaths in Spain and 23 OECD Countries: An Ecological Study. Front. Nutr. 2021, 8, 591964. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Rivas, I.; Romaguera, D.; Quijal-Zamorano, M.; Czarlewski, W.; Vidal, A.; Fonseca, J.; Ballester, J.; Anto, J.M.; Basagana, X.; et al. Association between consumption of vegetables and COVID-19 mortality at a country level in Europe. medRxiv 2020. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Rivas, I.; Romaguera, D.; Quijal-Zamorano, M.; Czarlewski, W.; Vidal, A.; Fonseca, J.; Ballester, J.; Anto, J.M.; Basagana, X.; et al. Association between consumption of fermented vegetables and COVID-19 mortality at a country level in Europe. medRxiv 2020. [Google Scholar] [CrossRef]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Dietary supplements during the COVID-19 pandemic: Insights from 1.4M users of the COVID Symptom Study app—A longitudinal app-based community survey. medRxiv 2020. [Google Scholar] [CrossRef]
- Boushey, C.J.; Coulston, A.M.; Rock, C.L.; Monsen, E. Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- UK Biobank. Data Showcase. Available online: https://biobank.ctsu.ox.ac.uk/crystal/browse.cgi?id=100080&cd=category (accessed on 30 December 2020).
- Armstrong, J.; Rudkin, J.K.; Allen, N.; Crook, D.W.; Wilson, D.J.; Wyllie, D.H.; O’Connell, A.M. Dynamic linkage of COVID-19 test results between Public Health England’s Second Generation Surveillance System and UK Biobank. Microb. Genom. 2020, 6. [Google Scholar] [CrossRef]
- UK Biobank. Deriving the Grid Coordinates. Available online: http://biobank.ndph.ox.ac.uk/showcase/showcase/docs/UKgrid.pdf (accessed on 30 December 2020).
- UK Summary. People Tested Positive. Available online: https://coronavirus.data.gov.uk/ (accessed on 4 April 2021).
- Maling, D.H. Coordinate Systems and Map Projections; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bradbury, K.E.; Young, H.J.; Guo, W.; Key, T.J. Dietary assessment in UK Biobank: An evaluation of the performance of the touchscreen dietary questionnaire. J. Nutr. Sci. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, A.B.; Wang, L.; Das, H.; Li, L.; Reinhold, V.N.; Bukowski, J.F. Antigens in tea-beverage prime human Vgamma 2Vdelta 2 T cells in vitro and in vivo for memory and nonmemory antibacterial cytokine responses. Proc. Natl. Acad. Sci. USA 2003, 100, 6009–6014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melamed, I.; Kark, J.D.; Spire, Z. Coffee and the immune system. Int. J. Immunopharmacol. 1990, 12, 129–134. [Google Scholar] [CrossRef]
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Serhan, C.N.; Bazinet, R.P. The need for precision nutrition, genetic variation and resolution in Covid-19 patients. Mol. Aspects Med. 2021, 77, 100943. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Cakir Edis, E. Chronic Pulmonary Diseases and COVID-19. Turk. Thorac. J. 2020, 21, 345–349. [Google Scholar] [CrossRef]
- BBC News. Covid-19 Vaccine: First Person Receives Pfizer Jab in UK. Available online: https://www.bbc.com/news/uk-55227325 (accessed on 22 March 2021).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Sun, J.; Dai, Z.; Deng, H.; Li, X.; Huang, Q.; Wu, Y.; Sun, L.; Xu, Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Virol. 2020, 127, 104371. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, F.; Sessa, F.; Valenzano, A.; Polito, R.; Monda, V.; Cibelli, G.; Villano, I.; Pisanelli, D.; Perrella, M.; Daniele, A.; et al. COVID-19: Role of Nutrition and Supplementation. Nutrients 2021, 13, 976. [Google Scholar] [CrossRef]
- James, P.T.; Ali, Z.; Armitage, A.E.; Bonell, A.; Cerami, C.; Drakesmith, H.; Jobe, M.; Jones, K.S.; Liew, Z.; Moore, S.E.; et al. Could nutrition modulate COVID-19 susceptibility and severity of disease? A systematic review. medRxiv 2020. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. A Mechanistic Link Between Selenium and Coronavirus Disease 2019 (COVID-19). Curr. Nutr. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated with Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 660420. [Google Scholar] [CrossRef] [PubMed]
- Yisak, H.; Ewunetei, A.; Kefale, B.; Mamuye, M.; Teshome, F.; Ambaw, B.; Yideg Yitbarek, G. Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Del Ser, T.; Fernandez-Blazquez, M.A.; Valenti, M.; Zea-Sevilla, M.A.; Frades, B.; Alfayate, E.; Saiz, L.; Calero, O.; Garcia-Lopez, F.J.; Rabano, A.; et al. Residence, Clinical Features, and Genetic Risk Factors Associated with Symptoms of COVID-19 in a Cohort of Older People in Madrid. Gerontology 2021, 67, 281–289. [Google Scholar] [CrossRef]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Braffett, B.H.; Simmens, S.J.; Young, H.A.; Ogden, C.L. Dietary Polyphenol Intake in US Adults and 10-Year Trends: 2007–2016. J. Acad. Nutr. Diet. 2020, 120, 1821–1833. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Jung, S.; Ko, K.S. Effects of Coffee Extracts with Different Roasting Degrees on Antioxidant and Anti-Inflammatory Systems in Mice. Nutrients 2018, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Hutachok, N.; Angkasith, P.; Chumpun, C.; Fucharoen, S.; Mackie, I.J.; Porter, J.B.; Srichairatanakool, S. Anti-Platelet Aggregation and Anti-Cyclooxygenase Activities for a Range of Coffee Extracts (Coffea arabica). Molecules 2020, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Tabrez, S.; Jabir, N.R.; Ali, M.; Kamal, M.A.; da Silva Araujo, L.; De Oliveira Santos, J.V.; Da Mata, A.; De Aguiar, R.P.S.; de Carvalho Melo Cavalcante, A.A. An Insight into the Therapeutic Potential of Major Coffee Components. Curr. Drug Metab. 2018, 19, 544–556. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Huang, W.Y.; Fu, L.; Li, C.Y.; Xu, L.P.; Zhang, L.X.; Zhang, W.M. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Shiels, M.S.; Graubard, B.I.; Katki, H.A.; Chaturvedi, A.K.; Trabert, B.; Pinto, L.A.; Kemp, T.J.; Shebl, F.M.; Mayne, S.T.; et al. Associations of Coffee Drinking with Systemic Immune and Inflammatory Markers. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1052–1060. [Google Scholar] [CrossRef] [Green Version]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Lopez, S.; Sarria, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, D.; Kvaerner, A.S.; Ma, W.; Hu, Y.; Tabung, F.K.; Nan, H.; Hu, Z.; Shen, H.; Mucci, L.A.; Chan, A.T.; et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am. J. Clin. Nutr. 2019, 109, 635–647. [Google Scholar] [CrossRef]
- Paiva, C.; Beserra, B.; Reis, C.; Dorea, J.G.; Da Costa, T.; Amato, A.A. Consumption of coffee or caffeine and serum concentration of inflammatory markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Dong, H.; Xia, S.Q.; Huang, Y.Z.; Wang, D.; Zhao, Y.; Liu, W.; Tu, S.; Zhang, M.; Wang, Q.; et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Suzuki, K.; Washio, M.; Ohfuji, S.; Adachi, S.; Kan, S.; Imai, S.; Yoshimura, K.; Miyashita, N.; Fujisawa, N.; et al. Association between coffee and green tea intake and pneumonia among the Japanese elderly: A case-control study. Sci. Rep. 2021, 11, 5570. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (Suppl. 1), A18–A25. [Google Scholar] [CrossRef]
- Gibson, A.; Edgar, J.D.; Neville, C.E.; Gilchrist, S.E.; McKinley, M.C.; Patterson, C.C.; Young, I.S.; Woodside, J.V. Effect of fruit and vegetable consumption on immune function in older people: A randomized controlled trial. Am. J. Clin. Nutr 2012, 96, 1429–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.H.; Finley, J. Potential cell culture models for antioxidant research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Iaccarino, G.; Czarlewski, W.; Haahtela, T.; Anto, A.; Akdis, C.A.; Blain, H.; Canonica, G.W.; Cardona, V.; et al. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy 2020, 10, 16. [Google Scholar] [CrossRef]
- Lange, K.W. Food science and COVID-19. Food Sci. Hum. Wellness 2020, 10, 1–5. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat. Lyon (FR): International Agency for Research on Cancer; (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 114.) 1. EXPOSURE DATA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507973/ (accessed on 28 April 2021).
- Hobbs-Grimmer, D.A.; Givens, D.I.; Lovegrove, J.A. Associations between red meat, processed red meat and total red and processed red meat consumption, nutritional adequacy and markers of health and cardio-metabolic diseases in British adults: A cross-sectional analysis using data from UK National Diet and Nutrition Survey. Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Linseisen, J.; Rohrmann, S.; Norat, T.; Gonzalez, C.A.; Dorronsoro Iraeta, M.; Morote Gomez, P.; Chirlaque, M.D.; Pozo, B.G.; Ardanaz, E.; Mattisson, I.; et al. Dietary intake of different types and characteristics of processed meat which might be associated with cancer risk--results from the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2006, 9, 449–464. [Google Scholar] [CrossRef] [Green Version]
- Linseisen, J.; Kesse, E.; Slimani, N.; Bueno-De-Mesquita, H.B.; Ocke, M.C.; Skeie, G.; Kumle, M.; Dorronsoro Iraeta, M.; Morote Gomez, P.; Janzon, L.; et al. Meat consumption in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts: Results from 24-hour dietary recalls. Public Health Nutr. 2002, 5, 1243–1258. [Google Scholar] [CrossRef] [Green Version]
- Bener, A.; Ehlayel, M.S.; Alsowaidi, S.; Sabbah, A. Role of breast feeding in primary prevention of asthma and allergic diseases in a traditional society. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 337–343. [Google Scholar]
- Hatmal, M.M.; Issa, N.N.; Alshaer, W.; Al-Ameer, H.J.; Abuyaman, O.; Tayyem, R.; Hijjawi, N.S. Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene. Medicina 2019, 55, 535. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, F.P.; Loret de Mola, C.; Davies, N.M.; Victora, C.G.; Relton, C.L. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS ONE 2017, 12, e0173070. [Google Scholar] [CrossRef] [Green Version]
- UK Biobank. Available online: https://www.ukbiobank.ac.uk/enable-your-research/publications (accessed on 28 April 2021).
- Xu, B.; Gutierrez, B.; Mekaru, S.; Sewalk, K.; Goodwin, L.; Loskill, A.; Cohn, E.L.; Hswen, Y.; Hill, S.C.; Cobo, M.M.; et al. Epidemiological data from the COVID-19 outbreak, real-time case information. Sci. Data 2020, 7, 106. [Google Scholar] [CrossRef]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
| Baseline Characteristics * | Analysis Sample | Full UKB Cohort |
|---|---|---|
| Number of Persons | 37,988 | 502,633 |
| Age, yr, mean(sd) | 57.36 (8.23) | 56.53 (8.10) |
| Female | 20,026 (52.72) | 273,453 (54.41) |
| Townsend-deprivation index, mean(sd) | −1.21 (3.12) | −1.29 (3.10) |
| White/British | 35,793 (94.22) | 472,801 (94.06) |
| Household Income, £ < 18,000 | 8264 (21.75) | 97,220 (19.34) |
| College or university degree | 11,000 (28.96) | 161,198 (32.07) |
| Currently employed | 20,354 (53.58) | 287,210 (57.14) |
| Homeowner | 34,295 (90.28) | 447,795 (89.36) |
| Number of co-habitants ≥ 4 | 6821 (17.96) | 93,987 (18.87) |
| Current smoker | 4205 (11.07) | 55,954 (11.13) |
| BMI (kg/m2), mean(sd) | 27.93 (4.95) | 27.43 (4.80) |
| Physical activity, minutes/day, mean(sd) | 75.50 (97.52) | 74.23 (96.19) |
| Poor overall health rating | 2412 (6.35) | 22,780 (4.56) |
| Using cholesterol medication | 8195 (21.57) | 86,907 (17.29) |
| Using blood pressure medication | 9610 (25.30) | 104,024 (20.70) |
| Diabetes | 2680 (7.05) | 26,552 (5.28) |
| Heart diseases | 3003 (7.91) | 29,166 (5.80) |
| Breastfed as baby | 21,051 (55.41) | 277,596 (55.39) |
| Coffee consumption, cups/day, mean(sd) | 2.02 (2.06) | 2.01 (2.02) |
| Tea consumption, cups/day, mean(sd) | 3.43 (2.73) | 3.40 (2.69) |
| Oily fish consumption, servings/day, mean(sd) | 0.16 (0.15) | 0.16 (0.15) |
| Processed meat, servings/day, mean(sd) | 0.22 (0.20) | 0.21 (0.20) |
| Red meat, servings/day, mean(sd) | 0.30 (0.21) | 0.30 (0.21) |
| Fruit (fresh/dried), servings/day, mean(sd) | 3.04 (2.60) | 3.05 (2.62) |
| Vegetables (cooked/raw), servings/day, mean(sd) | 0.82 (0.55) | 0.81 (0.56) |
| Nutritional Factor | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p ** | OR (95%CI) | p ** | OR (95%CI) | p ** | |
| Coffee, Cups/Day | ||||||
| None or <1 cup | Reference | Reference | Reference | |||
| 1 cup | 0.90 (0.83, 0.97) | 0.007 | 0.90 (0.83, 0.98) | 0.015 | 0.93 (0.86, 1.01) | 0.106 |
| 2–3 cups | 0.89 (0.83, 0.95) | 0.001 | 0.90 (0.83, 0.96) | 0.003 | 0.92 (0.85, 0.99) | 0.021 |
| ≥4 cups | 0.92 (0.85, 0.996) | 0.040 | 0.92 (0.84, 0.999) | 0.047 | 0.91 (0.83, 0.99) | 0.025 |
| Tea, cups/day | ||||||
| None or <1 cup | Reference | Reference | Reference | |||
| 1 cup | 0.92 (0.82, 1.03) | 0.157 | 0.93 (0.82, 1.04) | 0.204 | 0.94 (0.84, 1.06) | 0.319 |
| 2–3 cups | 0.93 (0.85, 1.01) | 0.074 | 0.93 (0.85, 1.01) | 0.078 | 0.92 (0.84, 1.01) | 0.069 |
| ≥4 cups | 1.00 (0.92, 1.08) | 0.941 | 0.98 (0.90, 1.06) | 0.543 | 0.96 (0.88, 1.05) | 0.347 |
| Oily Fish, Servings/Day | ||||||
| Quartile 1 (0–<0.07) | Reference | Reference | Reference | |||
| Quartile 2 (0.07–<0.14) | 0.93 (0.85, 1.02) | 0.102 | 0.94 (0.86, 1.03) | 0.183 | 0.97 (0.88, 1.06) | 0.482 |
| Quartiles 3 and 4 (≥0.14) | 0.95 (0.87, 1.04) | 0.244 | 0.98 (0.90, 1.07) | 0.654 | 1.00 (0.91, 1.09) | 0.967 |
| Processed Meat, Servings/Day | ||||||
| Quartile 1 (0–<0.07) | Reference | Reference | Reference | |||
| Quartile 2 (0.07–<0.14) | 1.02 (0.91, 1.14) | 0.699 | 1.05 (0.93, 1.19) | 0.410 | 1.04 (0.92, 1. 17) | 0.547 |
| Quartile 3 (0.14–<0.43) | 1.07 (0.96, 1.20) | 0.248 | 1.09 (0.97, 1.24) | 0.155 | 1.07 (0.94, 1.21) | 0.314 |
| Quartile 4 (≥0.43) | 1.12 (1.00, 1.25) | 0.053 | 1.14 (1.01, 1.29) | 0.036 | 1.12 (0.98, 1.26) | 0.091 |
| Red Meat, Servings/Day | ||||||
| Quartile 1 (0–<0.21) | Reference | Reference | Reference | |||
| Quartile 2 (0.21–<0.28) | 0.96 (0.89, 1.04) | 0.344 | 0.95 (0.87, 1.04) | 0.236 | 0.99 (0.90, 1.08) | 0.811 |
| Quartile 3 (0.28–<0.35) | 1.01 (0.93, 1.11) | 0.771 | 1.00 (0.90, 1.10) | 0.948 | 1.01 (0.91, 1.11) | 0.903 |
| Quartile 4 (≥0.35) | 0.99 (0.92, 1.08) | 0.878 | 0.98 (0.89, 1.07) | 0.600 | 0.98 (0.90, 1.08) | 0.697 |
| Fruit (Fresh/Dried), Servings/Day | ||||||
| Quartile 1 (0–<1.00) | Reference | Reference | Reference | |||
| Quartile 2 (1.00–<2.25) | 1.02 (0.92, 1.12) | 0.778 | 1.05 (0.95, 1.16) | 0.376 | 1.06 (0.95, 1.17) | 0.300 |
| Quartile 3 (2.25–<4.00) | 0.97 (0.87, 1.09) | 0.622 | 1.02 (0.91, 1.14) | 0.762 | 1.03 (0.91, 1.15) | 0.679 |
| Quartile 4 (≥4.00) | 0.97 (0.88, 1.09) | 0.635 | 1.03 (0.92, 1.15) | 0.660 | 1.03 (0.92, 1.16) | 0.596 |
| Vegetables (Cooked/Raw), Servings/Day | ||||||
| Quartile 1 (0–<0.50) | Reference | Reference | Reference | |||
| Quartile 2 (0.50–<0.67) | 0.92 (0.85, 0.99) | 0.034 | 0.93 (0.85, 1.00) | 0.060 | 0.96 (0.88, 1.04) | 0.271 |
| Quartile 3 (0.67–<1.00) | 0.87 (0.79, 0.96) | 0.006 | 0.88 (0.80, 0.98) | 0.015 | 0.93 (0.84, 1.03) | 0.186 |
| Quartile 4 (≥1.00) | 0.90 (0.83, 0.98) | 0.015 | 0.92 (0.84, 0.998) | 0.046 | 0.96 (0.88, 1.05) | 0.337 |
| Breastfed as a Baby | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 0.91 (0.85, 0.98) | 0.010 | 0.91 (0.85, 0.98) | 0.013 | 0.95 (0.88, 1.02) | 0.125 |
| Don’t know | 0.97 (0.91, 1.07) | 0.750 | 0.98 (0.90, 1.07) | 0.696 | 0.99 (0.91, 1.08) | 0.789 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.-H.T.; Rydland, K.J.; Achenbach, C.J.; Van Horn, L.; Cornelis, M.C. Dietary Behaviors and Incident COVID-19 in the UK Biobank. Nutrients 2021, 13, 2114. https://doi.org/10.3390/nu13062114
Vu T-HT, Rydland KJ, Achenbach CJ, Van Horn L, Cornelis MC. Dietary Behaviors and Incident COVID-19 in the UK Biobank. Nutrients. 2021; 13(6):2114. https://doi.org/10.3390/nu13062114
Chicago/Turabian StyleVu, Thanh-Huyen T., Kelsey J. Rydland, Chad J. Achenbach, Linda Van Horn, and Marilyn C. Cornelis. 2021. "Dietary Behaviors and Incident COVID-19 in the UK Biobank" Nutrients 13, no. 6: 2114. https://doi.org/10.3390/nu13062114
APA StyleVu, T.-H. T., Rydland, K. J., Achenbach, C. J., Van Horn, L., & Cornelis, M. C. (2021). Dietary Behaviors and Incident COVID-19 in the UK Biobank. Nutrients, 13(6), 2114. https://doi.org/10.3390/nu13062114







