Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome
Abstract
1. Introduction
2. Effects of Probiotics in IBS Pathophysiology
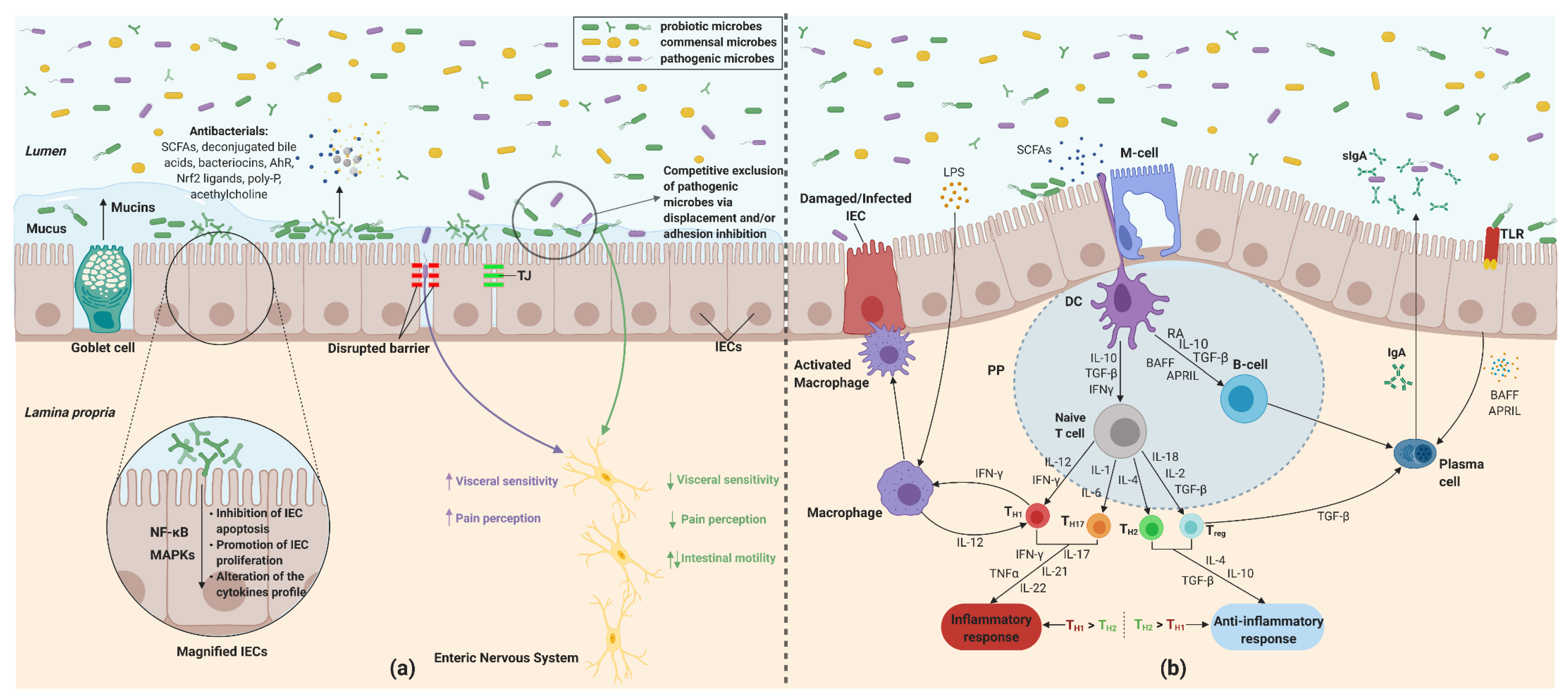
2.1. Modulation of Gut Microbiota
2.2. Alterations in the Gut Barrier Function
2.3. Intestinal Immunologic Modulation
2.3.1. Dendritic Cells
2.3.2. Macrophage and Monocytes
2.3.3. Immunoglobulin A (IgA)
2.3.4. Toll-Like Receptors
2.3.5. Th1/Th2 Cell Differentiation
2.4. Visceral Hypersensitivity and Gastrointestinal Dysmotility
2.4.1. Probiotics and Visceral Hypersensitivity
2.4.2. GI Dysmotility
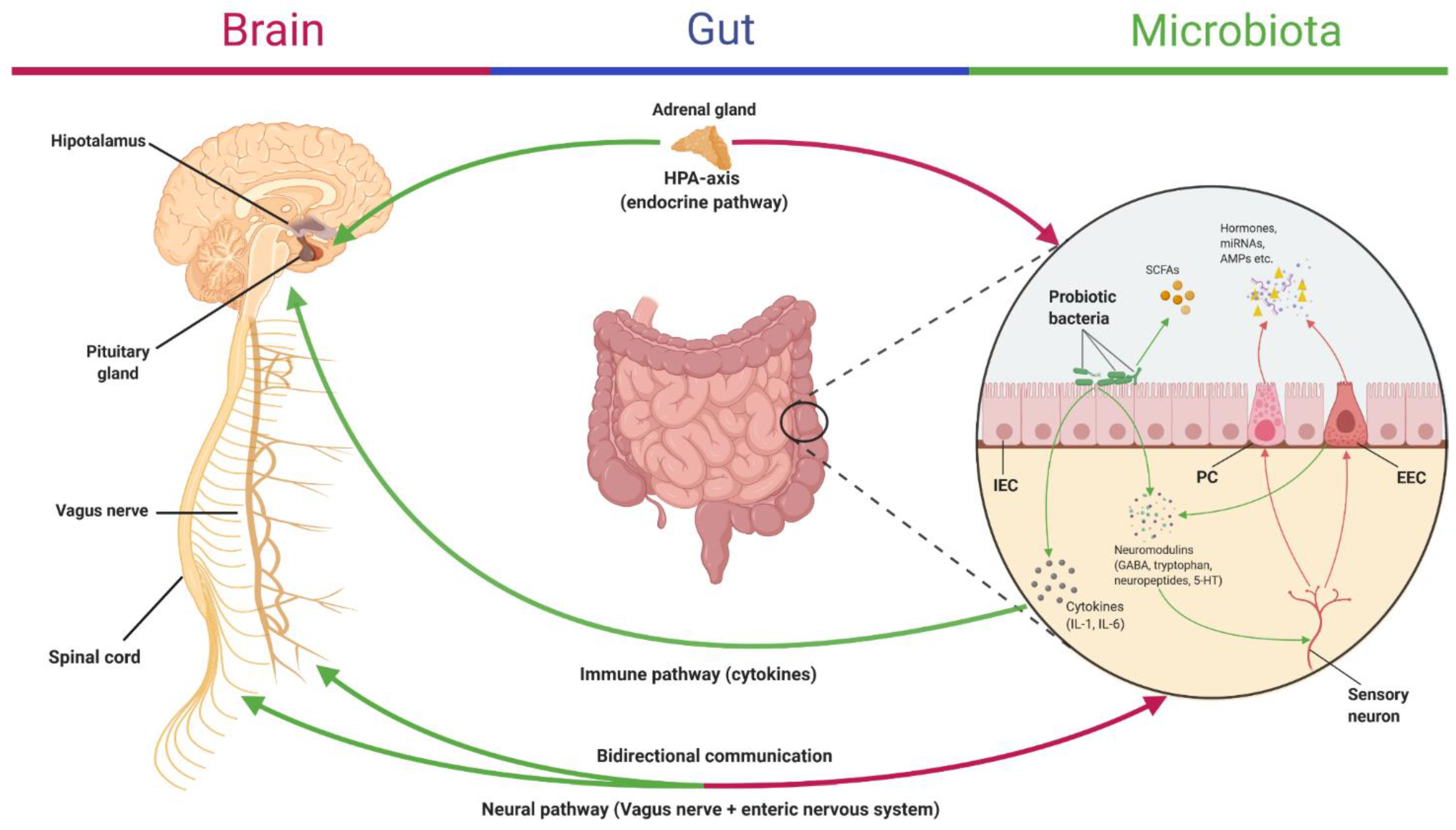
2.5. Microbiota–Gut–Brain Communication in IBS
Depression and Anxiety as Psychologic Comorbidities Associated with IBS
3. Implications of Prebiotics in IBS
4. Synbiotics as Therapy for IBS
Clinical Studies
5. Discussions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayaraman, T.; Wong, R.; Drossman, D.A.; Lee, Y.Y. Communication breakdown between physicians and IBS sufferers: What is the conundrum and how to overcome it? J. R. Coll. Physicians Edinb. 2017, 47, 138–141. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Ballou, S.; Keefer, L. The impact of irritable bowel syndrome on daily functioning: Characterizing and understanding daily consequences of IBS. Neurogastroenterol. Motil. 2016, 29, e12982. [Google Scholar] [CrossRef] [PubMed]
- Buono, J.L.; Carson, R.T.; Flores, N.M. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual. Life Outcomes 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef]
- Agarwal, N.; Spiegel, B.M. The Effect of Irritable Bowel Syndrome on Health-Related Quality of Life and Health Care Expenditures. Gastroenterol. Clin. N. Am. 2011, 40, 11–19. [Google Scholar] [CrossRef]
- Flacco, M.E.; Manzoli, L.; de Giorgio, R.; Gasbarrini, A.; Cicchetti, A.; Bravi, F.; Altini, M.; Caio, G.P.; Ursini, F. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2986–3000. [Google Scholar]
- Lacy, B.E. Review article: An analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 817–830. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Quigley, E.M. The Gut-Brain Axis and the Microbiome: Clues to Pathophysiology and Opportunities for Novel Management Strategies in Irritable Bowel Syndrome (IBS). J. Clin. Med. 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef]
- Labus, J.S.; Hollister, E.B.; Jacobs, J.; Kirbach, K.; Oezguen, N.; Gupta, A.; Acosta, J.; Luna, R.A.; Aagaard, K.; Versalovic, J.; et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N. Current insights into the innate immune system dysfunction in irritable bowel syndrome. Ann. Gastroenterol. 2018, 31, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.-S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Prim. 2016, 2, 1–24. [Google Scholar] [CrossRef]
- Camilleri, M. Management Options for Irritable Bowel Syndrome. Mayo Clin. Proc. 2018, 93, 1858–1872. [Google Scholar] [CrossRef]
- Enck, P.; Junne, F.; Klosterhalfen, S.; Zipfel, S.; Martens, U. Therapy options in irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1402–1411. [Google Scholar] [CrossRef]
- Choi, C.H.; Kwon, J.G.; Kim, S.K.; Myung, S.-J.; Park, K.S.; Sohn, C.-I.; Rhee, P.-L.; Lee, K.J.; Lee, O.Y.; Jung, H.-K.; et al. Efficacy of combination therapy with probiotics and mosapride in patients with IBS without diarrhea: A randomized, double-blind, placebo-controlled, multicenter, phase II trial. Neurogastroenterol. Motil. 2015, 27, 705–716. [Google Scholar] [CrossRef]
- El-Salhy, M.; Mazzawi, T. Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 439–445. [Google Scholar] [CrossRef]
- Quigley, E.M. Probiotics, Prebiotics, Synbiotics, and Other Strategies to Modulate the Gut Microbiota in Irritable Bowel Syndrome (IBS). In Probiotics, Prebiotics, and Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 549–556. [Google Scholar]
- Yu, T.; Zheng, Y.-P.; Tan, J.-C.; Xiong, W.-J.; Wang, Y.; Lin, L. Effects of Prebiotics and Synbiotics on Functional Constipation. Am. J. Med. Sci. 2017, 353, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Butel, M.-J. Probiotics, gut microbiota and health. Méd. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Azpiroz, F. Probiotics in irritable bowel syndrome: Where are we? Neurogastroenterol. Motil. 2018, 30, e13513. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Amerian Córdoba Park Hotel: Córdoba, Argentina, 2001. [Google Scholar]
- Collado, M.C.; Gueimonde, M.; Sanz, Y.; Salminen, S. Adhesion Properties and Competitive Pathogen Exclusion Ability of Bifidobacteria with Acquired Acid Resistance. J. Food Prot. 2006, 69, 1675–1679. [Google Scholar] [CrossRef]
- Vieira, A.T.; Teixeira, M.M.; Martins, F.D.S. The Role of Probiotics and Prebiotics in Inducing Gut Immunity. Front. Immunol. 2013, 4, 445. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Gorbach, S.L. Probiotics: Their role in the treatment and prevention of disease. Expert Rev. Anti-Infect. Ther. 2006, 4, 261–275. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Yu, W.-K.; Heo, T.-R. Identification and screening for antimicrobial activity against Clostridium difficile of Bifidobacterium and Lactobacillus species isolated from healthy infant faeces. Int. J. Antimicrob. Agents 2003, 21, 340–346. [Google Scholar] [CrossRef]
- Collado, M.C.; Grześkowiak, Ł.; Salminen, S. Probiotic Strains and Their Combination Inhibit In Vitro Adhesion of Pathogens to Pig Intestinal Mucosa. Curr. Microbiol. 2007, 55, 260–265. [Google Scholar] [CrossRef]
- Gueimonde, M.; Noriega, L.; Margolles, A.; Reyes-Gavilán, C.G.D.L.; Salminen, S. Ability of Bifidobacterium strains with acquired resistance to bile to adhere to human intestinal mucus. Int. J. Food Microbiol. 2005, 101, 341–346. [Google Scholar] [CrossRef]
- Fujiwara, S.; Hashiba, H.; Hirota, T.; Forstner, J.F. Inhibition of the binding of enterotoxigenic Escherichia coli Pb176 to human intestinal epithelial cell line HCT-8 by an extracellular protein fraction containing BIF of Bifidobacterium longum SBT2928: Suggestive evidence of blocking of the binding receptor gangliotetraosylceramide on the cell surface. Int. J. Food Microbiol. 2001, 67, 97–106. [Google Scholar] [CrossRef]
- Gueimonde, M.; Jalonen, L.; He, F.; Hiramatsu, M.; Salminen, S. Adhesion and competitive inhibition and displacement of human enteropathogens by selected lactobacilli. Food Res. Int. 2006, 39, 467–471. [Google Scholar] [CrossRef]
- Brito, M.B.; Diaz, J.P.; Muñoz-Quezada, S.; Llorente, C.G.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- González-Rodríguez, I.; Sánchez, B.; Ruiz, L.; Turroni, F.; Ventura, M.; Ruas-Madiedo, P.; Gueimonde, M.; Margolles, A. Role of Extracellular Transaldolase from Bifidobacterium bifidum in Mucin Adhesion and Aggregation. Appl. Environ. Microbiol. 2012, 78, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.-L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- De Keersmaecker, S.C.; Verhoeven, T.L.; Desair, J.; Marchal, K.; Vanderleyden, J.; Nagy, I. Strong antimicrobial activity ofLactobacillus rhamnosusGG againstSalmonella typhimuriumis due to accumulation of lactic acid. FEMS Microbiol. Lett. 2006, 259, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; De Vuyst, L. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef]
- Sjögren, J.; Magnusson, J.; Broberg, A.; Schnürer, J.; Kenne, L. Antifungal 3-Hydroxy Fatty Acids from Lactobacillus plantarum MiLAB. Appl. Environ. Microbiol. 2003, 69, 7554–7557. [Google Scholar] [CrossRef] [PubMed]
- Umu, Ö.C.O.; Rudi, K.; Diep, D.B. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb. Ecol. Health Dis. 2017, 28, 1348886. [Google Scholar] [CrossRef]
- Hassan, M.; Kjos, M.; Nes, I.; Diep, D.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef]
- Yildirim, Z.; Winters, D.K.; Johnson, M.G. Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB. J. Appl. Microbiol. 1999, 86, 45–54. [Google Scholar] [CrossRef]
- Nielsen, D.S.; Cho, G.-S.; Hanak, A.; Huch, M.; Franz, C.M.; Arneborg, N. The effect of bacteriocin-producing Lactobacillus plantarum strains on the intracellular pH of sessile and planktonic Listeria monocytogenes single cells. Int. J. Food Microbiol. 2010, 141, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dhaka, P.; Vijay, D.; Vergis, J.; Mohan, V.; Kumar, A.; Kurkure, N.V.; Barbuddhe, S.B.; Malik, S.; Rawool, D.B. Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int. J. Antimicrob. Agents 2016, 48, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Langen, M.L.-V.; Madsen, K.L. Secreted bioactive factors fromBifidobacterium infantisenhance epithelial cell barrier function. Am. J. Physiol. Liver Physiol. 2008, 295, G1025–G1034. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Donato, K.A.; Shen-Tu, G.; Gordanpour, M.; Sherman, P.M. Lactobacillus rhamnosus Strain GG Prevents Enterohemorrhagic Escherichia coli O157:H7-Induced Changes in Epithelial Barrier Function. Infect. Immun. 2008, 76, 1340–1348. [Google Scholar] [CrossRef]
- Masselot, C.R.; Herrmann, A.; Carlstedt, I.; Michalski, J.-C.; Capon, C. Glycosylation of the two O-glycosylated domains of human MUC2 mucin in patients transposed with artificial urinary bladders constructed from proximal colonic tissue. Glycoconj. J. 2008, 25, 213–224. [Google Scholar] [CrossRef]
- Moal, V.L.-L.; Amsellem, R.; Servin, A.L.; Coconnier, M.-H. Lactobacillus acidophilus (strain LB) from the resident adult human gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut 2002, 50, 803–811. [Google Scholar] [CrossRef]
- Phillipson, M.; Johansson, M.E.V.; Henriksnäs, J.; Petersson, J.; Gendler, S.J.; Sandler, S.; Persson, A.E.G.; Hansson, G.C.; Holm, L. The gastric mucus layers: Constituents and regulation of accumulation. Am. J. Physiol. Liver Physiol. 2008, 295, G806–G812. [Google Scholar] [CrossRef] [PubMed]
- Windle, H.J.; Fox, Á.; NíEidhin, D.; Kelleher, D. The Thioredoxin System of Helicobacter pylori. J. Biol. Chem. 2000, 275, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Aristoteli, L.P.; Willcox, M.D.P. Mucin Degradation Mechanisms by Distinct Pseudomonas aeruginosa Isolates In Vitro. Infect. Immun. 2003, 71, 5565–5575. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Woodmansey, E.J.; Macfarlane, G.T. Colonization of Mucin by Human Intestinal Bacteria and Establishment of Biofilm Communities in a Two-Stage Continuous Culture System. Appl. Environ. Microbiol. 2005, 71, 7483–7492. [Google Scholar] [CrossRef]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; A Hollingsworth, M. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef]
- Mattar, A.F.; Teitelbaum, D.H.; Drongowski, R.A.; Yongyi, F.; Harmon, C.M.; Coran, A.G. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.-H.; Whang, K.-Y.; Kim, Y.-J.; Oh, S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008, 18, 1278–1285. [Google Scholar]
- Otte, J.-M.; Podolsky, D.K. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Liver Physiol. 2004, 286, G613–G626. [Google Scholar] [CrossRef]
- Kelsall, B.L. Innate and adaptive mechanisms to control of pathological intestinal inflammation. J. Pathol. 2008, 214, 242–259. [Google Scholar] [CrossRef]
- Möndel, M.; Schroeder, B.; Zimmermann, K.; Huber, H.; Nuding, S.; Beisner, J.; Fellermann, K.; Stange, E.F.; Wehkamp, J. Probiotic E. coli treatment mediates antimicrobial human β-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2008, 2, 166–172. [Google Scholar] [CrossRef]
- Schlee, M.; E Harder, J.; Köten, B.; Stange, E.F.; Wehkamp, J.; Fellermann, K. Probiotic lactobacilli and VSL#3 induce enterocyte β-defensin. Clin. Exp. Immunol. 2008, 151, 528–535. [Google Scholar] [CrossRef]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Meissner, B.W.-V.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-κB- and AP-1-Mediated Induction of Human Beta Defensin-2 in Intestinal Epithelial Cells by Escherichia coli Nissle 1917: A Novel Effect of a Probiotic Bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef]
- Menendez, A.; Finlay, B.B. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 2007, 19, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional Complexes in Various Epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Sherman, P.M.; Johnson-Henry, K.C.; Yeung, H.P.; Ngo, P.S.C.; Goulet, J.; Tompkins, T.A. Probiotics Reduce Enterohemorrhagic Escherichia coli O157:H7- and Enteropathogenic E. coli O127:H6-Induced Changes in Polarized T84 Epithelial Cell Monolayers by Reducing Bacterial Adhesion and Cytoskeletal Rearrangements. Infect. Immun. 2005, 73, 5183–5188. [Google Scholar] [CrossRef]
- Putaala, H.; Salusjärvi, T.; Nordström, M.; Saarinen, M.; Ouwehand, A.C.; Hansen, E.B.; Rautonen, N. Effect of four probiotic strains and Escherichia coli O157:H7 on tight junction integrity and cyclo-oxygenase expression. Res. Microbiol. 2008, 159, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, N.S.; Hyde, L.; Adawi, D.; Kulik, D.; Ahrné, S.; Molin, G.; Jeppsson, B.; MacKenzie, A.; Mack, D.R. Pulse Probiotic Administration Induces Repeated Small Intestinal Muc3 Expression in Rats. Pediatr. Res. 2011, 69, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.D.; Pedersen, M.H.; Cencic, A.; Budde, B.B. Application of Measurements of Transepithelial Electrical Resistance of Intestinal Epithelial Cell Monolayers To Evaluate Probiotic Activity. Appl. Environ. Microbiol. 2005, 71, 7528–7530. [Google Scholar] [CrossRef]
- Dahan, S.; Dalmasso, G.; Imbert, V.; Peyron, J.-F.; Rampal, P.; Czerucka, D. Saccharomyces boulardii Interferes with Enterohemorrhagic Escherichia coli-Induced Signaling Pathways in T84 Cells. Infect. Immun. 2003, 71, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Resta–Lenert, S.; Barrett, K.E. Probiotics and Commensals Reverse TNF-α– and IFN-γ–Induced Dysfunction in Human Intestinal Epithelial Cells. Gastroenterology 2006, 130, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Resta-Lenert, S. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [CrossRef]
- Llorente, C.G.; Muñoz, S.; Gil, A. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. In Proceedings of the Nutrition Society; Cambridge University Press (CUP): Cambridge, UK, 2010; Volume 69, pp. 381–389. [Google Scholar]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Fukumoto, S.; Toshimitsu, T.; Matsuoka, S.; Maruyama, A.; Oh-Oka, K.; Takamura, T.; Nakamura, Y.; Ishimaru, K.; Fujii-Kuriyama, Y.; Ikegami, S.; et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 2014, 92, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Sonowal, R.; Swimm, A.; Sahoo, A.; Luo, L.; Matsunaga, Y.; Wu, Z.; Bhingarde, J.; Ejzak, E.A.; Ranawade, A.; Qadota, H.; et al. Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA 2017, 114, E7506–E7515. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef]
- Alcántara, C.; Coll-Marqués, J.M.; Jadán-Piedra, C.; Vélez, D.; Devesa, V.; Zúñiga, M.; Monedero, V. Polyphosphate in Lactobacillus and Its Link to Stress Tolerance and Probiotic Properties. Front. Microbiol. 2018, 9, 1944. [Google Scholar] [CrossRef]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. BioEssays 2011, 33, 574–581. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Scully, P.; O’Mahony, D.; Murphy, S.; O’Brien, F.; Lyons, A.; Sherlock, G.; Mac Sharry, J.; Kiely, B.; Shanahan, F.; et al. Commensal-Induced Regulatory T Cells Mediate Protection against Pathogen-Stimulated NF-κB Activation. PLoS Pathog. 2008, 4, e1000112. [Google Scholar] [CrossRef]
- Thomas, L.; Suzuki, K.; Zhao, J. Probiotics: A proactive approach to health. A symposium report. Br. J. Nutr. 2015, 114, S1–S15. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotic Bacterium Prevents Cytokine-induced Apoptosis in Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Prokaryotic Regulation of Epithelial Responses by Inhibition of Ikappa B-alpha Ubiquitination. Science 2000, 289, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; A Kamm, M.; Knight, S.C.; Stagg, A.J. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004, 53, 1602–1609. [Google Scholar] [CrossRef]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of action of probiotics: Recent advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef]
- Drakes, M.; Blanchard, T.; Czinn, S. Bacterial Probiotic Modulation of Dendritic Cells. Infect. Immun. 2004, 72, 3299–3309. [Google Scholar] [CrossRef]
- Karlsson, H.; Larsson, P.; Wold, A.; Rudin, A. Pattern of Cytokine Responses to Gram-Positive and Gram-Negative Commensal Bacteria Is Profoundly Changed when Monocytes Differentiate into Dendritic Cells. Infect. Immun. 2004, 72, 2671–2678. [Google Scholar] [CrossRef]
- Braat, H.; Brande, J.V.D.; Van Tol, E.; Hommes, D.; Peppelenbosch, M.; Van Deventer, S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am. J. Clin. Nutr. 2004, 80, 1618–1625. [Google Scholar] [CrossRef]
- Pathmakanthan, S.; Li, C.K.F.; Cowie, J.; Hawkey, C.J. Lactobacillus plantarum 299: Beneficial in vitro immunomodulation in cells extracted from inflamed human colon. J. Gastroenterol. Hepatol. 2004, 19, 166–173. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Guerrero, S.S.M.; Pacheco, A.R.; Garibay, M.G.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Shida, K.; Kiyoshima-Shibata, J.; Kaji, R.; Nagaoka, M.; Nanno, M. Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced byLactobacillus caseithrough Toll-like receptor 2-dependent and independent mechanisms. Immunology 2009, 128, e858–e869. [Google Scholar] [CrossRef]
- He, F.; Morita, H.; Ouwehand, A.C.; Hosoda, M.; Hiramatsu, M.; Kurisaki, J.-I.; Isolauri, E.; Benno, Y.; Salminen, S. Stimulation of the Secretion of Pro-Inflammatory Cytokines byBifidobacteriumStrains. Microbiol. Immunol. 2002, 46, 781–785. [Google Scholar] [CrossRef]
- Van Hemert, S.; Meijerink, M.; Molenaar, D.; A Bron, P.; De Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef]
- Meijerink, M.; van Hemert, S.; Taverne, N.; Wels, M.; De Vos, P.; Bron, P.A.; Savelkoul, H.F.; van Bilsen, J.; Kleerebezem, M.; Wells, J.M. Identification of Genetic Loci in Lactobacillus plantarum That Modulate the Immune Response of Dendritic Cells Using Comparative Genome Hybridization. PLoS ONE 2010, 5, e10632. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigón, G. The Probiotic Bacterium Lactobacillus casei Induces Activation of the Gut Mucosal Immune System through Innate Immunity. Clin. Vaccine Immunol. 2006, 13, 219–226. [Google Scholar] [CrossRef]
- Link-Amster, H.; Rochat, F.; Saudan, K.; Mignot, O.; Aeschlimann, J. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 1994, 10, 55–63. [Google Scholar] [CrossRef]
- Isolauri, E.; Joensuu, J.; Suomalainen, H.; Luomala, M.; Vesikari, T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 1995, 13, 310–312. [Google Scholar] [CrossRef]
- Kaila, M.; Isolauri, E.; Saxelin, M.; Arvilommi, H.; Vesikari, T. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch. Dis. Child. 1995, 72, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Majamaa, H.; Isolauri, E.; Saxelin, M.; Vesikari, T. Lactic Acid Bacteria in the Treatment of Acute Rotavirus Gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 333–338. [Google Scholar] [CrossRef]
- Park, J.-H.; Um, J.-I.; Lee, B.-J.; Goh, J.-S.; Park, S.-Y.; Kim, W.-S.; Kim, P.-H. Encapsulated Bifidobacterium bifidum potentiates intestinal IgA production. Cell. Immunol. 2002, 219, 22–27. [Google Scholar] [CrossRef]
- Vinderola, G.; Matar, C.; Perdigon, G. Role of Intestinal Epithelial Cells in Immune Effects Mediated by Gram-Positive Probiotic Bacteria: Involvement of Toll-Like Receptors. Clin. Vaccine Immunol. 2005, 12, 1075–1084. [Google Scholar] [CrossRef]
- Hoarau, C.; Lagaraine, C.; Martin, L.; Velge-Roussel, F.; Lebranchu, Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 2006, 117, 696–702. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.; Frøkiaer, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Perdigon, G.; Leblanc, A.D.M.D. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Bouladoux, N.; A Hall, J.; Grainger, J.R.; Dos Santos, L.M.; Kann, M.G.; Nagarajan, V.; Verthelyi, D.; Belkaid, Y. Regulatory role of suppressive motifs from commensal DNA. Mucosal Immunol. 2012, 5, 623–634. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar] [CrossRef]
- Weiss, D.S.; Raupach, B.; Takeda, K.; Akira, S.; Zychlinsky, A. Toll-Like Receptors Are Temporally Involved in Host Defense. J. Immunol. 2004, 172, 4463–4469. [Google Scholar] [CrossRef]
- Gourbeyre, P.; Denery, S.; Bodinier, M. Probiotics, prebiotics, and synbiotics: Impact on the gut immune system and allergic reactions. J. Leukoc. Biol. 2011, 89, 685–695. [Google Scholar] [CrossRef]
- Shida, K.; Takahashi, R.; Iwadate, E.; Takamizawa, K.; Yasui, H.; Sato, T.; Habu, S.; Hachimura, S.; Kaminogawa, S. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin. Exp. Allergy 2002, 32, 563–570. [Google Scholar] [CrossRef]
- Shihab, U.S.; Mahfuza, R.; Mollika, P.; Masud, R.; Ranjan Kumar, B.; Mir Imam, I.W.; Rafiqul, I.K. Ameliorative Effect of Probiotic Strains, Lactobacillus acidophilus and Lactobacillus bulgaricus against Acetic Acid-Induced Inflammation in the Mouse Colon. Am. J. Life Sci. 2020, 8, 183–188. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Moore, K.W.; Malefyt, R.D.W.; Coffman, R.L.; O’Garra, A. Interleukin-10 and Theinterleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Pessi, T.; Sütas, Y.; Hurme, M.; Isolauri, E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin. Exp. Allergy 2000, 30, 1804–1808. [Google Scholar] [CrossRef]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef]
- Tsuchiya, J.; Barreto, R.; Okura, R.; Kawakita, S.; Fesce, E.; Marotta, F. Single-blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin. J. Dig. Dis. 2004, 5, 169–174. [Google Scholar] [CrossRef]
- Colecchia, A.; Vestito, A.; La Rocca, A.; Pasqui, F.; Nikiforaki, A.; Festi, D. Effect of a symbiotic preparation on the clinical manifestations of irritable bowel syndrome, constipation-variant. Results of an open, uncontrolled multicenter study. Minerva Gastroenterol. Dietol. 2006, 52, 349–358. [Google Scholar]
- Dughera, L.; Elia, C.; Navino, M.; Cisarò, F. Effects of symbiotic preparations on constipated irritable bowel syndrome symptoms. Acta Bio-Med. Atenei Parm. 2007, 78, 111–116. [Google Scholar]
- Andriulli, A.; Neri, M.; Loguercio, C.; Terreni, N.; Merla, A.; Cardarella, M.P.; Federico, A.; Chilovi, F.; Milandri, G.L.; De Bona, M.; et al. Clinical Trial on the Efficacy of a New Symbiotic Formulation, Flortec, in Patients With Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2008, 42, S218–S223. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Park, S.U.; Jang, Y.S.; Kim, Y.-H.; Rhee, P.-L.; Ko, S.H.; Joo, N.; Kim, S.I.; Kim, C.-H.; Chang, D.K. Effect of composite yogurt enriched with acacia fiber and Bifidobacterium lactis. World J. Gastroenterol. 2012, 18, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Rogha, M.; Esfahani, M.Z.; Zargarzadeh, A.H. The efficacy of a synbiotic containing Bacillus Coagulans in treatment of irritable bowel syndrome: A randomized placebo-controlled trial. Gastroenterol. Hepatol. Bed Bench 2014, 7, 156–163. [Google Scholar] [PubMed]
- Šmid, A.; Strniša, L.; Bajc, K.; Vujić-Podlipec, D.; Matijašić, B.B.; Rogelj, I. Randomized clinical trial: The effect of fermented milk with the probiotic cultures Lactobacillus acidophilus La-5® and Bifidobacterium BB-12® and Beneo dietary fibres on health-related quality of life and the symptoms of irritable bowel syndrome in adults. J. Funct. Foods 2016, 24, 549–557. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cho, D.-Y.; Lee, S.-H.; Han, K.-S.; Yang, S.-W.; Kim, J.-H.; Lee, S.-H.; Kim, S.-M.; Kim, K.-N. A Randomized Clinical Trial of Synbiotics in Irritable Bowel Syndrome: Dose-Dependent Effects on Gastrointestinal Symptoms and Fatigue. Korean J. Fam. Med. 2019, 40, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.C.; Croffut, R.M.; Stranahan, M.C. Prescript-assist™ probiotic-prebiotic treatment for irritable bowel syndrome: A methodologically oriented, 2-week, randomized, placebo-controlled, double-blind clinical study. Clin. Ther. 2005, 27, 755–761. [Google Scholar] [CrossRef]
- Moser, A.M.; Spindelboeck, W.; Halwachs, B.; Strohmaier, H.; Kump, P.; Gorkiewicz, G.; Högenauer, C. Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur. J. Nutr. 2018, 58, 2767–2778. [Google Scholar] [CrossRef]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. eLife 2017, 6. [Google Scholar] [CrossRef]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; de Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The Immune System in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2011, 17, 349–359. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abdollahi, M.; Rahimi, R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J. Neurogastroenterol. Motil. 2016, 22, 558–574. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.; Clarke, G. Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, B.; Verne, N.G. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Quigley, E.M.M.; Brint, E. Microbiota-host interactions in irritable bowel syndrome: Epithelial barrier, immune regulation and brain-gut interactions. World J. Gastroenterol. 2014, 20, 8859–8866. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, Y.-Q.; Zuo, X.-L.; Zhen, Y.-B.; Yang, J.; Liu, C.-H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.F.; Bercík, P.; Bergonzelli, G.E.; Huang, X.-X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthésy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 2004, 127, 826–837. [Google Scholar] [CrossRef]
- Wang, B.; Mao, Y.; Diorio, C.; Wang, L.; Huizinga, J.D.; Bienenstock, J.; Kunze, W. Lactobacillus reuteriingestion and IKCachannel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol. Motil. 2009, 22, 98-e33. [Google Scholar] [CrossRef]
- Verdu, E.F.; Bercik, P.; Verma-Gandhu, M.; Huang, X.-X.; Blennerhassett, P.; Jackson, W.; Mao, Y.; Wang, L.; Rochat, F.; Collins, S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006, 55, 182–190. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay–Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut Microbial Products Regulate Murine Gastrointestinal Motility via Toll-Like Receptor 4 Signaling. Gastroenterology 2012, 143, 1006–1016.e4. [Google Scholar] [CrossRef]
- Brint, E.K.; Mac Sharry, J.; Fanning, A.; Shanahan, F.; Quigley, E.M.M. Differential Expression of Toll-Like Receptors in Patients With Irritable Bowel Syndrome. Am. J. Gastroenterol. 2011, 106, 329–336. [Google Scholar] [CrossRef]
- Harper, A.; Naghibi, M.M.; Garcha, D. The Role of Bacteria, Probiotics and Diet in Irritable Bowel Syndrome. Foods 2018, 7, 13. [Google Scholar] [CrossRef]
- Lee, B.J.; Bak, Y.-T. Irritable Bowel Syndrome, Gut Microbiota and Probiotics. J. Neurogastroenterol. Motil. 2011, 17, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mao, Y.-K.; Wang, B.; Huizinga, J.D.; Bienenstock, J.; Kunze, W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am. J. Physiol. Liver Physiol. 2009, 296, G868–G875. [Google Scholar] [CrossRef] [PubMed]
- McKernan, D.P.; Fitzgerald, P.; Dinan, T.G.; Cryan, J.F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol. Motil. 2010, 22, 1029-e268. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.L.; Altomare, A.; Stasi, E.; Marignani, M.; Severi, C.; Alloni, R.; Dicuonzo, G.; Morelli, L.; Coppola, R.; Cicala, M. Effect of Acute Mucosal Exposure to Lactobacillus rhamnosus GG on Human Colonic Smooth Muscle Cells. J. Clin. Gastroenterol. 2008, 42, S185–S190. [Google Scholar] [CrossRef]
- Bär, F.; Von Koschitzky, H.; Roblick, U.; Bruch, H.P.; Schulze, L.; Sonnenborn, U.; Böttner, M.; Wedel, T.; Bottner, M. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: Evidence from anin vitroorgan bath study. Neurogastroenterol. Motil. 2009, 21, 559-e17. [Google Scholar] [CrossRef]
- Dong, Y.; Han, Y.; Wang, Z.; Qin, Z.; Yang, C.; Cao, J.; Chen, Y. Role of serotonin on the intestinal mucosal immune response to stress-induced diarrhea in weaning mice. BMC Gastroenterol. 2017, 17, 82. [Google Scholar] [CrossRef]
- Casado-Bedmar, M.; Keita, Å.V. Potential neuro-immune therapeutic targets in irritable bowel syndrome. Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Santos, J.; Vanner, S.J.; Vergnolle, N.; Zoetendal, E.G.; Quigley, E.M. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1305–1318.e8. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain–Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflamm. 2021, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef]
- Jašarević, E.; Howerton, C.L.; Howard, C.D.; Bale, T.L. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology 2015, 156, 3265–3276. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Crampton, S.; Desbonnet, L.; Edge, D.; O’Sullivan, O.; Lomasney, K.W.; Zhdanov, A.; Crispie, F.; Moloney, R.D.; Borre, Y.E.; et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015, 60, 58–74. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016, 63, 217–227. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.; Fatimah, A.; Anas, O.M.; Shuhaimi, M.; Yazid, A.; Loong, Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients Versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.A.E.; Vanhoutvin, S.A.L.W.; Troost, F.J.; Rijkers, G.; De Bruïne, A.; Bast, A.; Venema, K.; Brummer, R.-J.M. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; Hamaker, B.R. New View on Dietary Fiber Selection for Predictable Shifts in Gut Microbiota. mBio 2020, 11, 02179-19. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Quigley, E.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Kearney, S.M.; Gibbons, S.M. Designing synbiotics for improved human health. Microb. Biotechnol. 2017, 11, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.O.; Tuffnell, Q.; Lee, A.J. Controlled Trial of Oligofructose in the Management of Irritable Bowel Syndrome. J. Nutr. 1999, 129, 1451S–1453S. [Google Scholar] [CrossRef] [PubMed]
- Olesen, M.; Gudmand-Høyer, E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am. J. Clin. Nutr. 2000, 72, 1570–1575. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.R.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2012, 62, 159–176. [Google Scholar] [CrossRef]
- Ooi, S.L.; Correa, D.; Pak, S.C. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome—What is the current evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef]
- Mego, M.; Accarino, A.; Tzortzis, G.; Vulevic, J.; Gibson, G.; Guarner, F.; Azpiroz, F. Colonic gas homeostasis: Mechanisms of adaptation following HOST-G904 galactooligosaccharide use in humans. Neurogastroenterol. Motil. 2017, 92, e13080. [Google Scholar] [CrossRef]
- Huaman, J.-W.; Mego, M.; Manichanh, C.; Cañellas, N.; Cañueto, D.; Segurola, H.; Jansana, M.; Malagelada, C.; Accarino, A.; Vulevic, J.; et al. Effects of Prebiotics vs a Diet Low in FODMAPs in Patients With Functional Gut Disorders. Gastroenterology 2018, 155, 1004–1007. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Topping, D.L.; Fukushima, M.; Bird, A.R. Resistant starch as a prebiotic and synbiotic: State of the art. In Proceedings of the Nutrition Society; CABI Publishing: Wallingford, UK, 2003; Volume 62, pp. 171–176. [Google Scholar]
- Manning, A.P.; Thompson, W.G.; Heaton, K.W.; Morris, A.F. Towards positive diagnosis of the irritable bowel. BMJ 1978, 2, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A.; Locke, R.G.; Talley, N.J.; Zinsmeister, A.R.; Fett, S.L.; Melton, J.L. A Comparison of The Rome and Manning Criteria for Case Identification in Epidemiological Investigations of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2000, 95, 2816–2824. [Google Scholar] [CrossRef]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Guo, L.-M.; Yang, S.-Y.; Wu, Q.; Meng, F.-J. Effectiveness of probiotics in irritable bowel syndrome: Methodological quality of meta-analyses and systematic reviews. Front. Nurs. 2019, 6, 115–121. [Google Scholar] [CrossRef]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, E.S.; DeLoache, W.C.; Pruss, K.M.; Whitaker, W.R.; Sonnenburg, J.L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 2018, 557, 434–438. [Google Scholar] [CrossRef]
- Ford, A.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1–18. [Google Scholar] [CrossRef]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef]
| Reference | Study Type | Intervention | Number of Subjects (n) | Subjects Metrics | Inclusion Criteria | Trial Duration | Beneficial Effects |
|---|---|---|---|---|---|---|---|
| Tsuchiya et al., 2004 [122] | Single-blind, preliminary, controlled trial | 10 mL of SCM-III synbiotic consisting of 1.25 × 105 cfu/mL L. acidophilus, 1.3 × 108 cfu/mL L. helveticus, 4.95 × 108 cfu/mL Bifidobacterium and vitamin- and phytoextracts-enriched medium; Complementary/synergistic synbiotic (NS *) | 68 | 20 males, 48 females; mean age, 46 years, range: 36–65 years | Adults with IBS according to Rome II criteria, free from lactose malabsorption, abdominal surgery, overt psychiatric disorders and ongoing psychotropic drug therapy or ethanol abuse | 12 weeks | Decrease in intensity of bowel habits and abdominal bloating compared to control group; p < 0.01 vs. baseline values and control; <5% reported “not effective” as the final evaluation compared with >40% of patients in the control group. |
| Colecchia et al., 2006 [123] | Open, uncontrolled, multicenter study | 3 g/day synbiotic consisting of B. longum W11 and short chain oligosaccharide Fos-Actilight; Complementary/synergistic synbiotic (NS *) | 636 | 250 men, 386 women with age >18 years | Adults diagnosed with constipation-type IBS according to the Rome II criteria | 36 days | Increase of stool frequency in patients with IBS-C variant and reduction of abdominal bloating and pain in patients presenting moderate/severe symptoms; ‘‘no symptom’’ class raised from 3% to 26.7% for bloating and from 8.4% to 44.1% for abdominal pain (p < 0.0001); for more severe symptoms classes (moderate-severe), symptom frequency decreased from 62.9% to 9.6% and from 38.8% to 4.1% for bloating and abdominal pain, respectively. |
| Dughera et al., 2007 [124] | Open, uncontrolled, multicenter study | 3 g/ day of synbiotic consisting of 5 × 109 cfu/mL B. longum W11 Short chain oligosaccharide Fos-Actilight; Complementary/synergistic synbiotic (NS *) | 129 | NS * | Adults with IBS meeting the Rome II criteria, free from lactose malabsorption, abdominal surgery, overt psychiatric disorders and ongoing psychotropic drug therapy or ethanol abuse | 3 months | Increase in stool frequency and lowering of abdominal bloating and pain in patients with moderate and severe symptoms; mean stool frequency before treatment was 12.8 ± 7.1; a significant (p < 0.001) increase of movements per month (14.7 ± 8.7 during the first month, 15.8 ± 7.8 during the second month and 16.96 ± 7.8 at the end of treatment) was recorded |
| Andriulli et al., 2008 [125] | Randomized, double-blind, controlled trial | 7 g twice a day of synbiotic Flortec consisting of 5 × 109 cfu/mL L. paracasei B21060, 500 mg lutamine, 700 mg xylo-oligosaccharides, and 1243 mg arabinogalactone; Synergistic synbiotic | 267 | Males and females with age between 18 and 75 years | Adults with IBS meeting the Rome II criteria patients complained about abdominal pain or discomfort as dominant symptom | 12 weeks | Decrease of number of bowel movements in comparison with the control group; Number of patients with absent/mild pain increased in the Flortec group (p = 0.019) |
| Min et al., 2012 [126] | Randomized, double-blind, controlled trial | 150 mL twice a day of synbiotic product containing B. animalis subsp. lactis Bb-12 1011 cfu/bottle S. thermophile 3 × 109 cfu/bottle L. acidophilus 109 cfu/bottle, and acacia dietary fibre; Synergistic synbiotic | 130 | Males and females with age between 18 and 70 years | Adults with IBS who met the Rome III criteria | 8 weeks | Improvement of bowel habit satisfaction in IBS-D predominant patients and overall symptoms in IBS-C predominant patients compared to baseline. Bowel habit satisfaction improved more in the test group compared with the control group (change from baseline of 27.16 vs. 15.51, p = 0.010); the improvement in general IBS symptoms was higher in the test group towards the control group (64.2 ± 17.0 vs. 50.4 ± 20.5, p < 0.001) |
| Rogha, Esfahani, and Zargarzadeh, 2014 [127] | Randomized, double-blind, controlled trial | 1 tablet three times a day of synbiotic Lactol containing 15 × 107 spores B. Coagulans and 100 g fructo-oligosaccharides; Complementary/synergistic synbiotic (NS *) | 85 | Male and females, mean age: 40 years | Adults with IBS who met the Rome III criteria, predominant symptoms—abdominal pain, diarrhea, constipation | 12 weeks | Decrease in abdominal pain frequency (score reduction 4.2 ± 1.8 vs. 1.9 ± 1.5, p < 0.001); diarrhea frequency decreased in the synbiotic group but not in the placebo one (score reduction 1.9 ± 1.2 vs. 0.0 ± 0.5, p < 0.001); constipation frequency decrease within the two groups (score reduction 0.9 ± 1.2 vs. 0.8 ± 1.1, p = 0.561) |
| Šmid et al., 2016 [128] | Randomized, double-blind, controlled trial | 180 g twice a day of LCA synbiotic product consisting of 1.8 × 107 cfu/g L. acidophilus La-5 2.5 × 107 cfu/g B. BB-12 S. thermophilus 2% Beneo dietary fibres; Complementary/synergistic synbiotic (NS *) | 76 | Males and females with age between 18 and 65 years; test-33 patients, control-43patients | Adults who met the Rome III criteria for a diagnosis of constipation –predominant IBS with symptoms being present for >6 months, and had had symptoms such as abdominal pain, bloating and general digestive discomfort at least twice a week in the last 3 months prior to the clinical trial | 12 weeks | No beneficial effects in comparison with placebo group; It was observed an overall improvement of 18% in the total quality of life score from the baseline to the end of the product/placebo consumption period in all the included patients (both control and test groups) |
| Lee et al., 2019 [129] | Randomized, double-blind, controlled trial | 1 capsule of synbiotic (Ultra-Probiotics-500) / day consisting of 109 cfu of each strain (L. rhamnosus, L. acidophilus, L. casei, L. bulgaricus, L. plantarum, L. salivarius, B. bifidum, B. longum), 175 mg offructo-oligosaccharides, 150 mg of slippery elm bark powder, 10 mg of herb bennet powder, and 100 g of inulin; Complementary/synergistic synbiotic (NS *) | 30 | Males and females with age ≥19 years | Adults meeting Rome III criteria for IBS free of IBD, celiac disease, antibiotic treatments, abdominal surgery, pregnancy or breastfeeding, or psychiatric diseases | 8 weeks | High doses improved the bowel symptoms and fatigue in comparison with the placebo group. Abdominal discomfort, abdominal bloating, frequency of formed stool, and fatigue were significantly improved in the high-dose group compared with those in the placebo group (p = 0.002, 0.003, 0.002, and 0.013, respectively) |
| Bittner, Croffut, and Stranahan, 2005 [130] | Randomized, double-blind, controlled trial | Once a day one 500 mg capsule of Prescript-AssistTM Safer Medical, Inc., Fort Benton, Montana (Probiotic-Prebiotic complex based on 29 soil microorganisms combined with several substances with leonardite being the predominant component); Complementary/synergistic synbiotic (NS *) | 25 | 23 women, 2 men; age between 20–70 years | Adults with IBS who met Rome II criteria | 2 weeks | Reduction in general ill feelings/nausea (reduced by 0.345 standard score units (F (1,46) = 4.26; p = 0.042), indigestion/flatulence (reduced by 0.544 standard score units (F (1,46) = 7.83; p = 0.008), and colitis (reduced by 0.826 standard score units (F (1,46) = 10.20; p = 0.003) compared with placebo group |
| Moser et al., 2019 [131] | Pilot study | Twice a day of synbiotic mixture (OMNi-BiOTiC Stress Repair) containing 7.5 × 109 cfu of each strain (L. casei W56, L. acidophilus W22, L. paracasei W20, L. salivarius W24, L. plantarum W62, L. lactis W19, B. lactis W51, W52, B. bifidum W23), Corn starch, Maltodextrin, Inulin, Fructo-oligosaccharides, Potassium chloride, Magnesium sulphate, Manganese sulphate; Complementary/synergistic synbiotic (NS *) | 10 | Males and females, age between 18–65 years | Adults with IBS symptoms–free of: Chronic inflammatory diseases, immune- or neoplastic diseases, recent application of immune-modifying medication, pregnancy, and alcohol or drug abuse | 4 weeks | Increase of SCFA levels, microbial abundance and reduction of symptom severity and fecal zonulin in comparison to baseline (p = 0.002). Increased microbial diversity in gastric (p = 0.008) and duodenal (p = 0.025) mucosal specimens |
| Diagnosis Criteria | Manning Criteria (1978) | Rome Criteria (1992) | Rome II Criteria (1999) | Rome III Criteria (2006) | Rome IV Criteria (2016) |
|---|---|---|---|---|---|
| Main diagnosis symptoms | Abdominal pain that is relieved with a bowel movement | Continuous or recurrent symptoms of: abdominal pain, relieved with defecation, and/or disturbed defecation, usually with bloating or feeling of abdominal distension | At least 12 weeks, which need not be consecutive, in the preceding 12 months of abdominal discomfort or pain | * Recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months | * Recurrent abdominal pain on average at least 1 day/week in the last 3 months |
| Pain and/or defecation associated features | Looser and more frequent stools Sensation of incomplete evacuation Passage of mucus Abdominal distention | Two or more of: Altered stool frequency Altered stool form (hard or loose/watery) Altered stool passage (straining or urgency, feeling of incomplete evacuation) Passage of mucus | At least two of three following features: Relieved with defecation Change in frequency of stool Change in form (appearance) of stool | Two or more of the following: Improvement with defecation Change in the frequency of stool Change in the form (appearance) of stool | Two or more of the following: Related to defecation Change in the frequency of stool Change in the form (appearance) of stool |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. https://doi.org/10.3390/nu13062112
Simon E, Călinoiu LF, Mitrea L, Vodnar DC. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients. 2021; 13(6):2112. https://doi.org/10.3390/nu13062112
Chicago/Turabian StyleSimon, Elemer, Lavinia Florina Călinoiu, Laura Mitrea, and Dan Cristian Vodnar. 2021. "Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome" Nutrients 13, no. 6: 2112. https://doi.org/10.3390/nu13062112
APA StyleSimon, E., Călinoiu, L. F., Mitrea, L., & Vodnar, D. C. (2021). Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients, 13(6), 2112. https://doi.org/10.3390/nu13062112








