A Systematic Review and Meta-Analysis of Human Milk Feeding and Short-Term Growth in Preterm and Very Low Birth Weight Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Registration and Reporting
2.2. Eligibility Criteria
2.2.1. Types of Studies
2.2.2. Types of Participants
2.2.3. Types of Intervention
2.2.4. Type of Outcome Measure
2.3. Information Sources and Study Selection
2.4. Data Extraction, Risk of Bias in Individual Studies, and Data Synthesis
3. Results
3.1. Risk of Bias and General Characteristics
3.2. Weight Gain and Human Milk Feeding
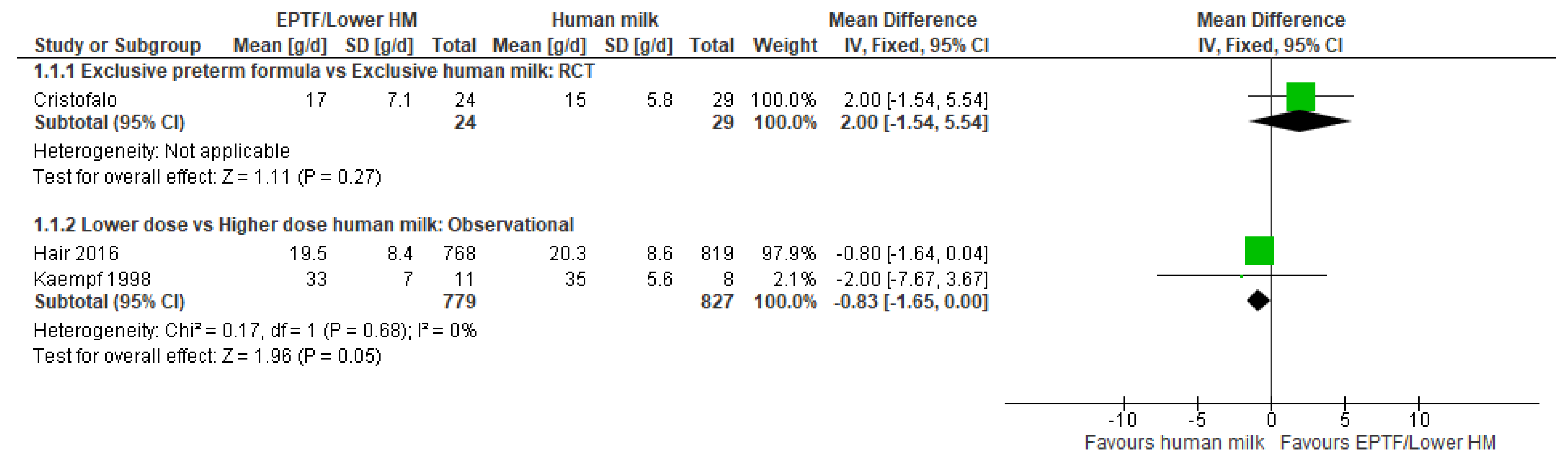
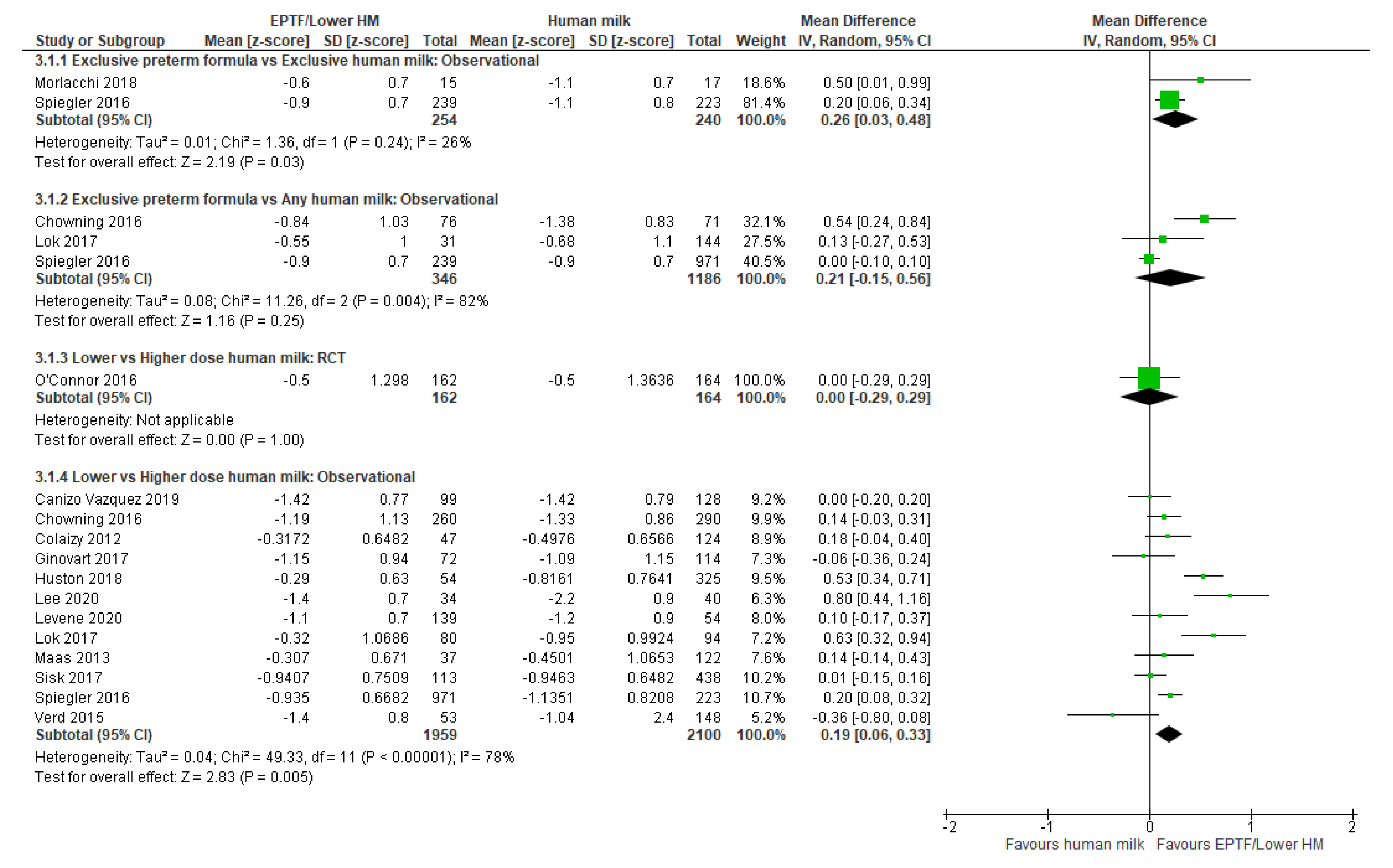
3.2.1. Comparison 1: Exclusive Preterm Formula vs. Exclusive Human Milk
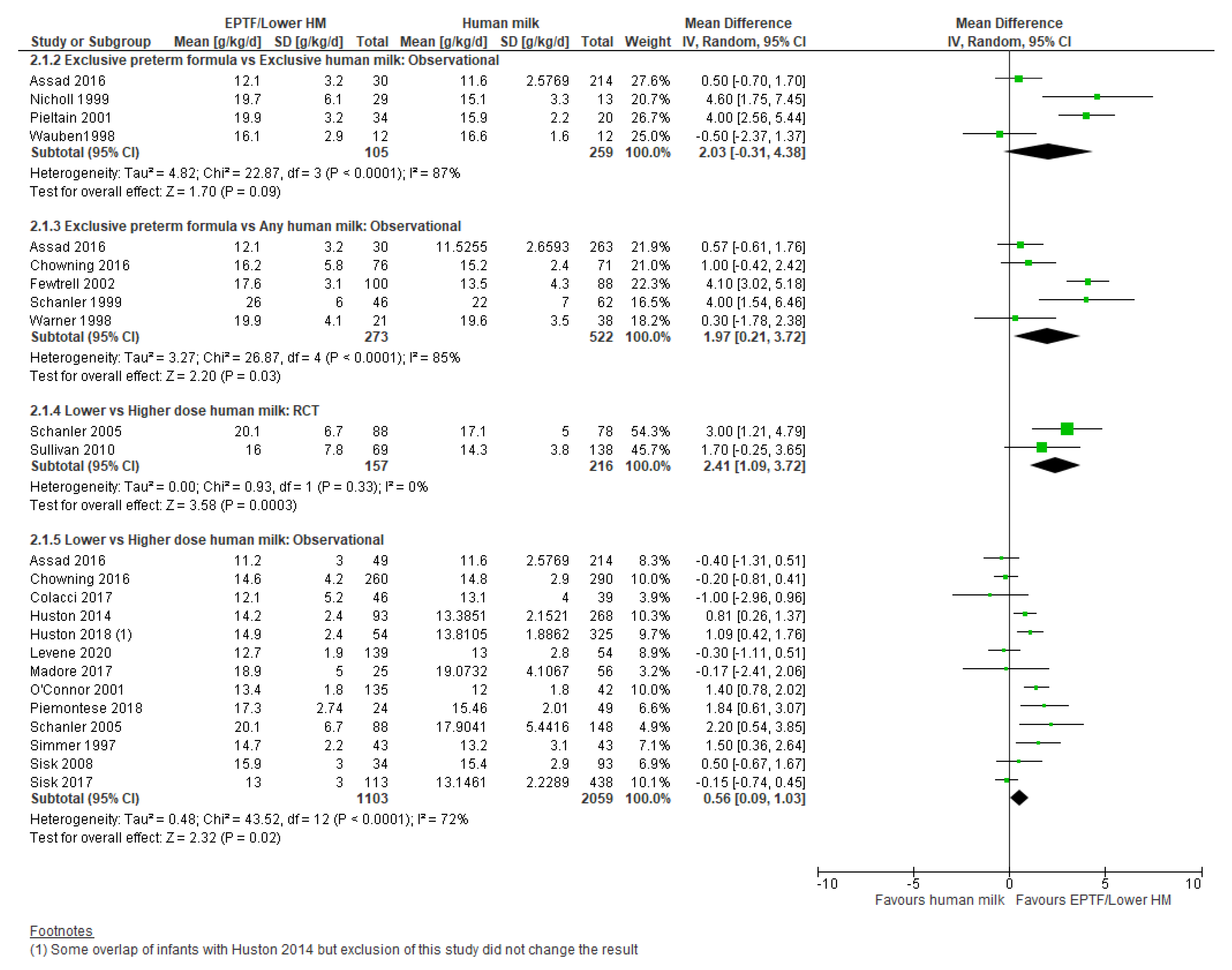
3.2.2. Comparison 2: Exclusive Preterm Formula vs. Any Human Milk
3.2.3. Comparison 3: Lower- vs. Higher-Dose Human Milk
3.3. Head Circumference Gain and Human Milk Feeding
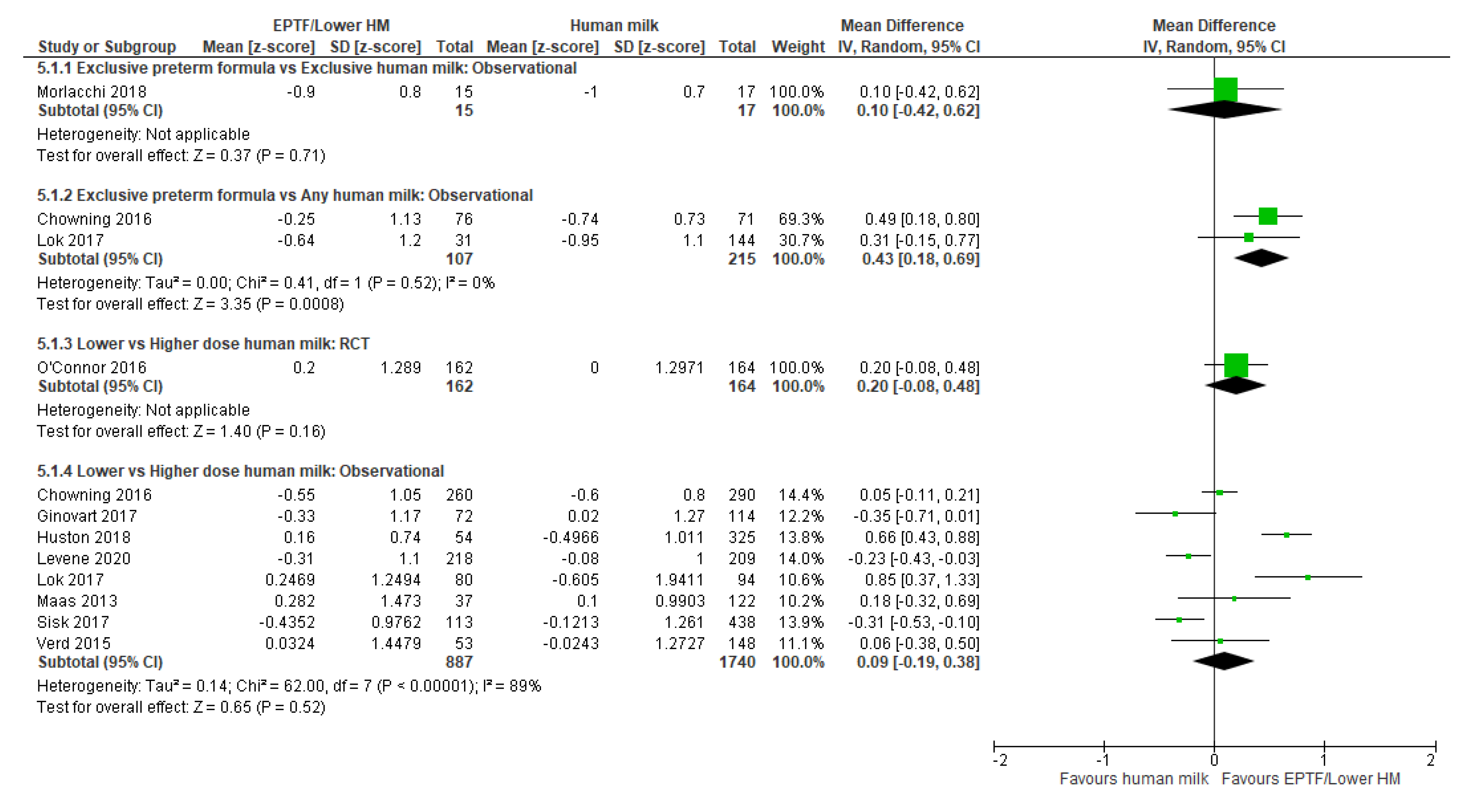
3.3.1. Comparison 1: Exclusive Preterm Formula vs. Exclusive Human Milk
3.3.2. Comparison 2: Exclusive Preterm Formula vs. Any Human Milk
3.3.3. Comparison 3: Lower- vs. Higher-Dose Human Milk
3.4. Length Gain and Human Milk Feeding
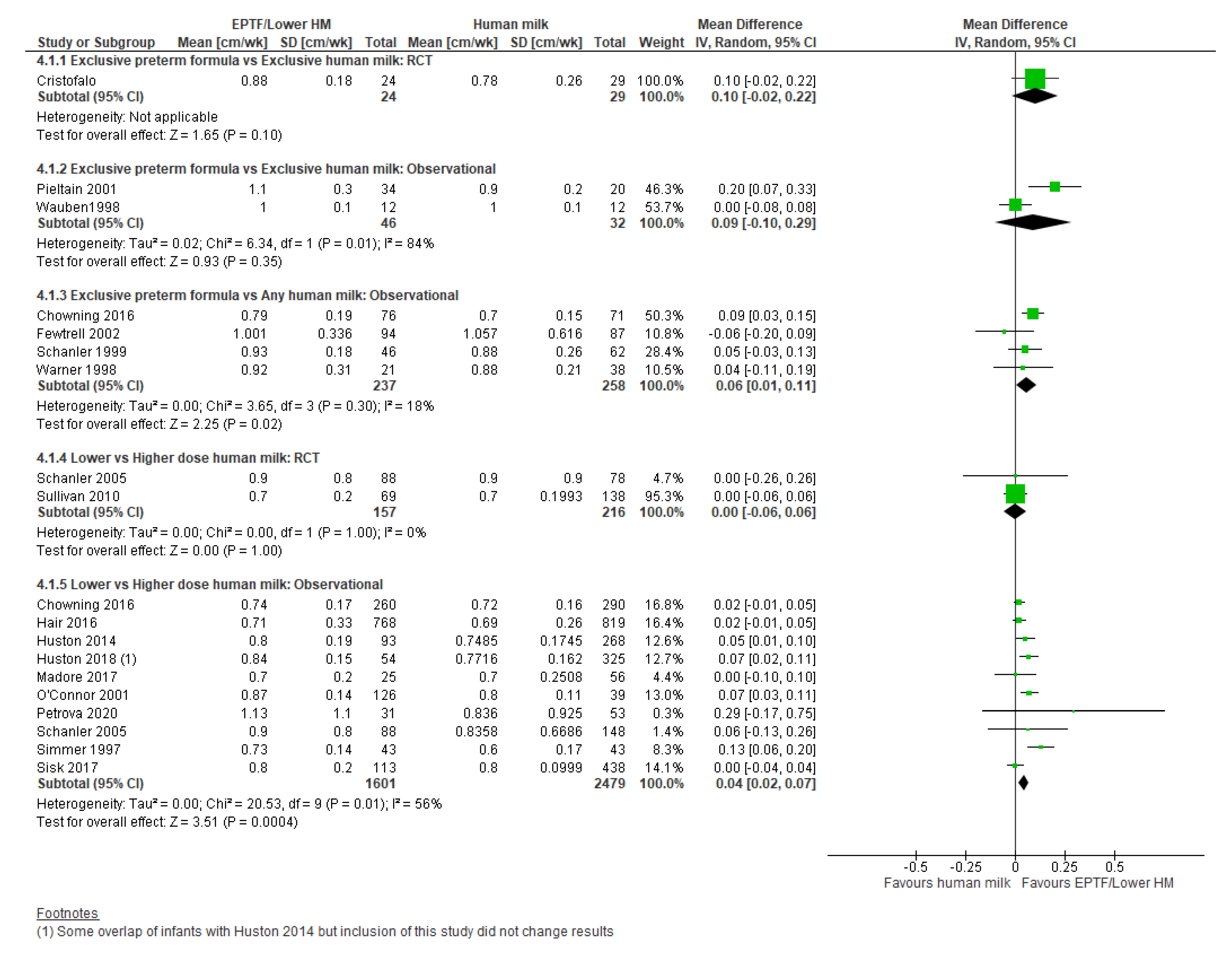
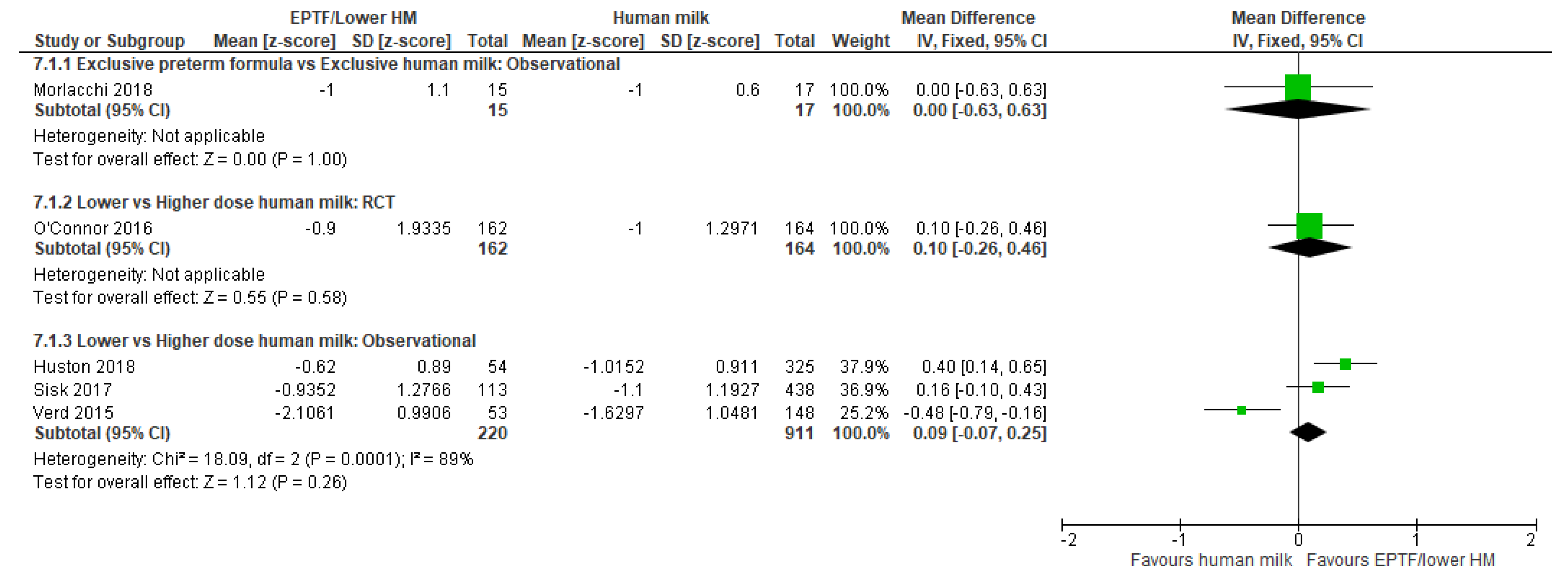
3.4.1. Comparison 1: Exclusive Preterm Formula vs. Exclusive Human Milk
3.4.2. Comparison 2: Exclusive Preterm Formula vs. Any Human Milk
3.4.3. Comparison 3: Lower- vs. Higher-Dose Human Milk
3.5. Body Composition and Human Milk Feeding
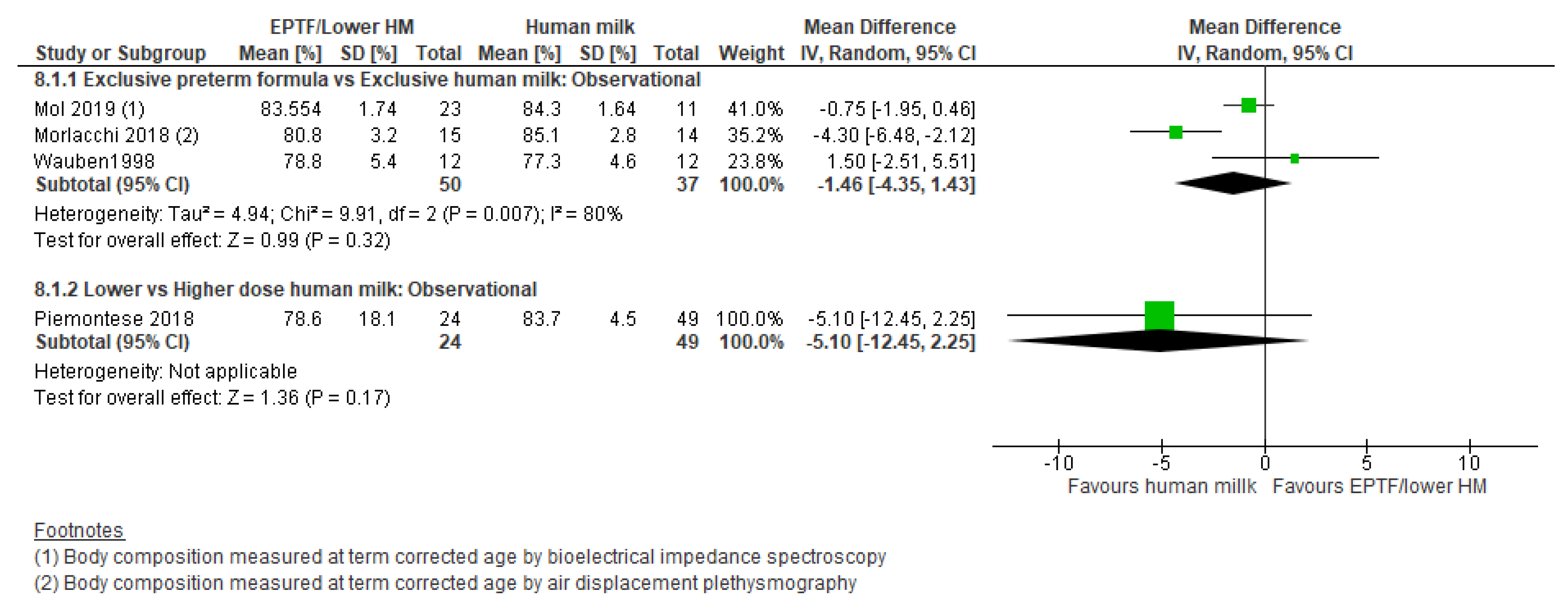
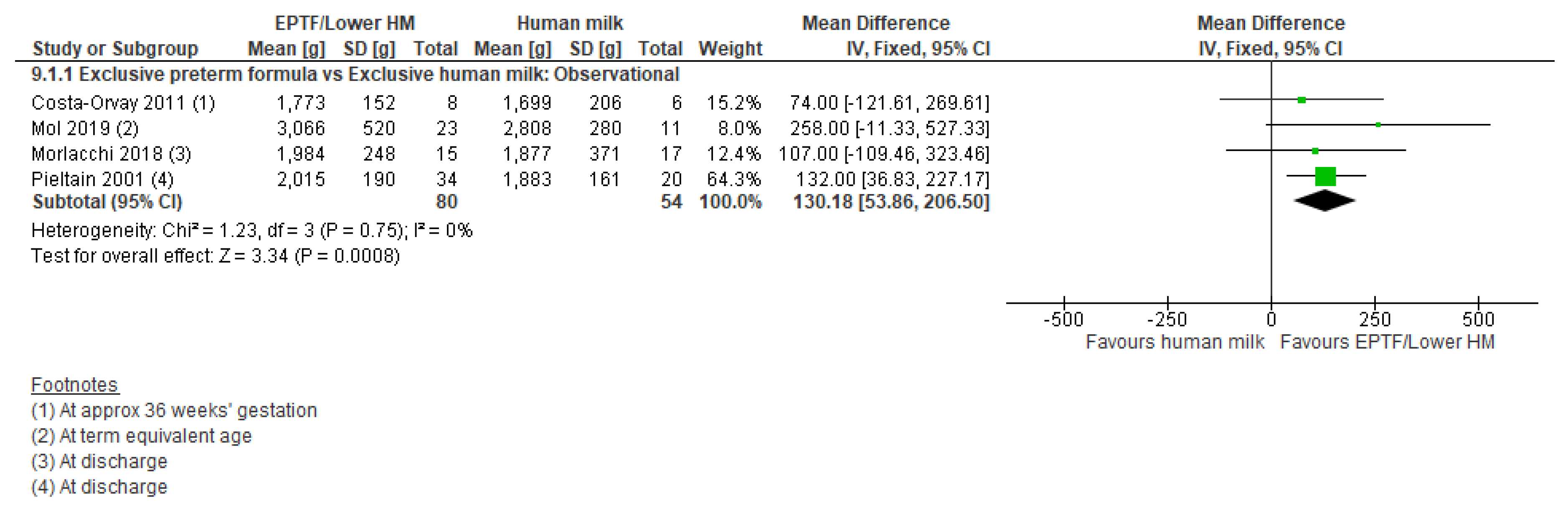
3.5.1. Comparison 1: Exclusive Preterm Formula vs. Exclusive Human Milk
3.5.2. Comparison 2: Any Human Milk vs. Exclusive Preterm Formula
3.5.3. Comparison 3: Lower- vs. Higher-Dose Human Milk Intake
4. Discussion
4.1. Summary of Main Results
4.2. Findings from Other Reviews
4.3. Implications for Clinical Practice and Research
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edmond, K.; Bahl, R. Optimal Feeding of Low-Birth-Weight Infants; Technical Review; World Health Organisation: Geneva, Switzerland, 2006. [Google Scholar]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; de Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the european society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Tonkin, E.; Damarell, R.; McPhee, A.; Suganuma, M.; Suganuma, H.; Middleton, P.; Makrides, M.; Collins, C. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 2018, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- AAP Committee on Nutrition; AAP Section on Breastfeeding; AAP Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.V.; Lin, L.; Embleton, N.D.; Harding, J.E.; McGuire, W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst. Rev. 2020, 5, CD000343. [Google Scholar] [CrossRef]
- Arslanoglu, S.; Boquien, C.-Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of human milk for preterm infants: Update and recommendations of the european milk bank association (emba) working group on human milk fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Colaizy, T.T.; Carlson, S.; Saftlas, A.F.; Morriss, F.H., Jr. Growth in vlbw infants fed predominantly fortified maternal and donor human milk diets: A retrospective cohort study. BMC Pediatr. 2012, 12, 124. [Google Scholar] [CrossRef]
- Ginovart, G.; Gich, I.; Gutierrez, A.; Verd, S. A fortified donor milk policy is associated with improved in-hospital head growth and weight gain in very low-birth-weight infants. Adv. Neonatal Care 2017, 17, 250–257. [Google Scholar] [CrossRef]
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999, 103, 1150–1157. [Google Scholar] [CrossRef]
- Brown, J.V.E.; Walsh, V.; McGuire, W. Formula versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 8, CD002972. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 7, CD002971. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, J.; Yin, Y.; Jing, W.; Luo, B.; Wang, J. Effects of breast-feeding compared with formula-feeding on preterm infant body composition: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 132–141. [Google Scholar] [CrossRef]
- Leppanen, M.; Lapinleimu, H.; Lind, A.; Matomaki, J.; Lehtonen, L.; Haataja, L.; Rautava, P.; Group, P.S. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics 2014, 133, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Costa-Orvay, J.A.; Figueras-Aloy, J.; Romera, G.; Closa-Monasterolo, R.; Carbonell-Estrany, X. The effects of varying protein and energy intakes on the growth and body composition of very low birth weight infants. Nutr. J. 2011, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Rifas-Shiman, S.L.; Sullivan, T.; Collins, C.T.; McPhee, A.J.; Ryan, P.; Kleinman, K.P.; Gillman, M.W.; Gibson, R.A.; Makrides, M. Infant growth before and after term: Effects on neurodevelopment in preterm infants. Pediatrics 2011, 128, e899–e906. [Google Scholar] [CrossRef]
- Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; Hay, W.W., Jr.; Bloomfield, F.H. Comparing apples with apples: It is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016, 79, 810–820. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia. Available online: www.covidence.org (accessed on 20 June 2020).
- Fewtrell, M.S.; Morley, R.; Abbott, R.A.; Singhal, A.; Isaacs, E.B.; Stephenson, T.; MacFadyen, U.; Lucas, A. Double-blind, randomized trial of long-chain polyunsaturated fatty acid supplementation in formula fed to preterm infants. Pediatrics 2002, 110, 73–82. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Hall, R.T.; Adamkin, D.H.; Auestad, N.; Castillo, M.; Connor, W.E.; Connor, S.L.; Fitzgerald, K.M.; Groh-Wargo, S.; Hartmann, E.E.; et al. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: A prospective, randomized controlled trial. Pediatrics 2001, 108, 359–371. [Google Scholar] [CrossRef]
- Wauben, I.P.; Atkinson, S.A.; Grad, T.L.; Shah, J.K.; Paes, B. Moderate nutrient supplementation of mother’s milk for preterm infants supports adequate bone mass and short-term growth: A randomized, controlled trial. Am. J. Clin. Nutr. 1998, 67, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Lok, K.Y.W.; Chau, P.H.; Fan, H.S.L.; Chan, K.M.; Chan, B.H.; Fung, G.P.C.; Tarrant, M. Increase in weight in low birth weight and very low birth weight infants fed fortified breast milk versus formula milk: A retrospective cohort study. Nutrients 2017, 9, 520. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Hoboken, NI, USA; Available online: www.training.cochrane.org/handbook (accessed on 20 April 2020).
- Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2016. [Google Scholar]
- Review Manager (RevMan) [Computer Program]; Version 5.4; The Cochrane Collaboration: Copenhagen, Denmark; The Nordic Cochrane Centre: Copenhagen, Denmark, 2020.
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2016, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Huston, R.K.; Markell, A.M.; McCulley, E.A.; Gardiner, S.K.; Sweeney, S.L. Improving growth for infants ≤250 grams receiving an exclusive human milk diet. Nutr. Clin. Pract. 2018, 33, 671–678. [Google Scholar] [CrossRef]
- Huston, R.K.; Markell, A.M.; McCulley, E.A.; Pathak, M.; Rogers, S.P.; Sweeney, S.L.; Dolphin, N.G.; Gardiner, S.K. Decreasing necrotizing enterocolitis and gastrointestinal bleeding in the neonatal intensive care unit: The role of donor human milk and exclusive human milk diets in infants ≤1500 g birth weight. Infant Child. Adolesc. Nutr. 2014, 6, 86–93. [Google Scholar] [CrossRef]
- Gradepro Gdt: Gradepro Guideline Development Tool [Software]; McMaster University: Hamilton, ON, Canada, 2015; (developed by Evidence Prime, Inc.); Available online: gradepro.org (accessed on 23 November 2020).
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1591. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E.; et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016, 316, 1897–1905. [Google Scholar] [CrossRef]
- Schanler, R.J.; Lau, C.; Hurst, N.M. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawöger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567.e561. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, R.M.; Gamsu, H.R. Changes in growth and metabolism in very low birthweight infants fed with fortified breast milk. Acta Paediatr. 1999, 88, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Kaempf, D.E.; Pfluger, M.S.; Thiele, A.M.; Hermanussen, M.; Linderkamp, O. Influence of nutrition on growth in premature infants: Assessment by knemometry. Ann. Hum. Biol. 1998, 25, 127–136. [Google Scholar] [CrossRef]
- Jacobi-Polishook, T.; Collins, C.T.; Sullivan, T.R.; Simmer, K.; Gillman, M.W.; Gibson, R.A.; Makrides, M.; Belfort, M.B. Human milk intake in preterm infants and neurodevelopment at 18 months corrected age. Pediatr. Res. 2016, 80, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Modi, N.; Uthaya, S. Impact of breast milk intake on body composition at term in very preterm babies: Secondary analysis of the nutritional evaluation and optimisation in neonates randomised controlled trial. Arch. Dis. Child. Fetal. Neonatal Ed. 2019, 104, F306–F312. [Google Scholar] [CrossRef] [PubMed]
- Assad, M.; Elliott, M.J.; Abraham, J.H. Decreased cost and improved feeding tolerance in vlbw infants fed an exclusive human milk diet. J. Perinatol. 2016, 36, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Colacci, M.; Murthy, K.; DeRegnier, R.O.; Khan, J.Y.; Robinson, D.T. Growth and development in extremely low birth weight infants after the introduction of exclusive human milk feedings. Am. J. Perinatol. 2017, 34, 130–137. [Google Scholar] [CrossRef]
- Hoban, R.; Schoeny, M.E.; Esquerra-Zwiers, A.; Kaenkumchorn, T.K.; Casini, G.; Tobin, G.; Siegel, A.H.; Patra, K.; Hamilton, M.; Wicks, J.; et al. Impact of donor milk on short- and long-term growth of very low birth weight infants. Nutrients 2019, 11, 241. [Google Scholar] [CrossRef]
- Verd, S.; Porta, R.; Botet, F.; Gutierrez, A.; Ginovart, G.; Barbero, A.H.; Ciurana, A.; Plata, II. Hospital outcomes of extremely low birth weight infants after introduction of donor milk to supplement mother’s milk. Breastfeed. Med. 2015, 10, 150–155. [Google Scholar] [CrossRef]
- Brownell, E.A.; Matson, A.P.; Smith, K.C.; Moore, J.E.; Esposito, P.A.; Lussier, M.M.; Lerer, T.J.; Hagadorn, J.I. Dose-response relationship between donor human milk, mother’s own milk, preterm formula, and neonatal growth outcomes. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 90–96. [Google Scholar] [CrossRef]
- Cañizo Vázquez, D.; Salas García, S.; Izquierdo Renau, M.; Iglesias-Platas, I. Availability of donor milk for very preterm infants decreased the risk of necrotizing enterocolitis without adversely impacting growth or rates of breastfeeding. Nutrients 2019, 11, 1895. [Google Scholar] [CrossRef]
- Carlson, S.J.; Ziegler, E.E. Nutrient intakes and growth of very low birth weight infants. J. Perinatol. 1998, 18, 252–258. [Google Scholar]
- Castellano Yáñez, C.; Castillo Barrio, B.; Muñoz Labián, M.D.C.; Ortiz Movilla, R.; García Lara, N.R.; Royuela Vicente, A.; Marín Gabriel, M.A. Providing very preterm infants with donor human milk led to faster breastfeeding rates but worse biometric gains. Acta Paediatr. 2019, 108, 766–767. [Google Scholar] [CrossRef]
- Chowning, R.; Radmacher, P.; Lewis, S.; Serke, L.; Pettit, N.; Adamkin, D.H. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J. Perinatol. 2016, 36, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Hair, A.B.; Peluso, A.M.; Hawthorne, K.M.; Perez, J.; Smith, D.P.; Khan, J.Y.; O’Donnell, A.; Powers, R.J.; Lee, M.L.; Abrams, S.A. Beyond necrotizing enterocolitis prevention: Improving outcomes with an exclusive human milk–based diet. Breastfeed. Med. 2016, 11, 70–74. [Google Scholar] [CrossRef]
- Lee, L.Y.; Lee, J.; Niduvaje, K.; Seah, S.S.; Atmawidjaja, R.W.; Cheah, F.C. Nutritional therapies in the neonatal intensive care unit and post-natal growth outcomes of preterm very low birthweight asian infants. J. Paediatr. Child. Health 2020, 56, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Levene, I.; McCormick, K. Improved growth of extremely and very preterm babies: Evaluation of a quality-of-care initiative. J. Paediatr. Child. Health 2020, 56, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Maas, C.; Wiechers, C.; Bernhard, W.; Poets, C.F.; Franz, A.R. Early feeding of fortified breast milk and in-hospital-growth in very premature infants: A retrospective cohort analysis. BMC Pediatr. 2013, 13, 178. [Google Scholar] [CrossRef]
- Madore, L.S.; Bora, S.; Erdei, C.; Jumani, T.; Dengos, A.R.; Sen, S. Effects of donor breastmilk feeding on growth and early neurodevelopmental outcomes in preterm infants: An observational study. Clin. Ther. 2017, 39, 1210–1220. [Google Scholar] [CrossRef]
- Manea, A.; Boia, M.; Iacob, D.; Dima, M.; Iacob, R.E. Benefits of early enteral nutrition in extremely low birth weight infants. Singapore Med. J. 2016, 57, 616–618. [Google Scholar] [CrossRef]
- Mol, N.; Zasada, M.; Kwinta, P. Does type of feeding affect body composition in very low birth weight infants?—A prospective cohort study. Pediatr. Neonatol. 2019, 60, 135–140. [Google Scholar] [CrossRef]
- Morlacchi, L.; Roggero, P.; Gianni, M.L.; Bracco, B.; Porri, D.; Battiato, E.; Menis, C.; Liotto, N.; Mallardi, D.; Mosca, F. Protein use and weight-gain quality in very-low-birth-weight preterm infants fed human milk or formula. Am. J. Clin. Nutr. 2018, 107, 195–200. [Google Scholar] [CrossRef]
- Petrova, A.; Eccles, S.; Mehta, R. Role of the proportional intake of fortified mother’s own milk in the weight gain pattern of their very-preterm-born infants. Nutrients 2020, 12, 1571. [Google Scholar] [CrossRef]
- Pieltain, C.; de Curtis, M.; Gerard, P.; Rigo, J. Weight gain composition in preterm infants with dual energy x-ray absorptiometry. Pediatr. Res. 2001, 49, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, P.; Liotto, N.; Mallardi, D.; Roggero, P.; Puricelli, V.; Gianni, M.L.; Morniroli, D.; Tabasso, C.; Perrone, M.; Menis, C.; et al. The effect of human milk on modulating the quality of growth in preterm infants. Front. Pediatr. 2018, 6, 291. [Google Scholar] [CrossRef]
- Simmer, K.; Metcalf, R.; Daniels, L. The use of breastmilk in a neonatal unit and its relationship to protein and energy intake and growth. J. Paediatr. Child. Health 1997, 33, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; Lovelady, C.A.; Gruber, K.J.; Dillard, R.G.; O’Shea, T.M. Human milk consumption and full enteral feeding among infants who weigh ≤1250 grams. Pediatrics 2008, 121, e1528–e1533. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; Lambeth, T.M.; Rojas, M.A.; Lightbourne, T.; Barahona, M.; Anthony, E.; Auringer, S.T. Necrotizing enterocolitis and growth in preterm infants fed predominantly maternal milk, pasteurized donor milk, or preterm formula: A retrospective study. Am. J. Perinatol. 2017, 34, 676–683. [Google Scholar]
- Soldateli, B.; Parker, M.; Melvin, P.; Gupta, M.; Belfort, M. Human milk feeding and physical growth in very low-birth-weight infants: A multicenter study. J. Perinatol. 2020, 40, 1246–1252. [Google Scholar] [CrossRef]
- Spiegler, J.; Preuss, M.; Gebauer, C.; Bendiks, M.; Herting, E.; Gopel, W.; German Neonatal Network. Does breastmilk influence the development of bronchopulmonary dysplasia? J. Pediatr. 2016, 169, 76–80.e74. [Google Scholar] [CrossRef]
- Warner, J.T.; Linton, H.R.; Dunstan, F.D.; Cartlidge, P.H. Growth and metabolic responses in preterm infants fed fortified human milk or a preterm formula. Int. J. Clin. Pract. 1998, 52, 236–240. [Google Scholar]
- Wauben, I.P.; Atkinson, S.A.; Shah, J.K.; Paes, B. Growth and body composition of preterm infants: Influence of nutrient fortification of mother’s milk in hospital and breastfeeding post-hospital discharge. Acta Paediatr. 1998, 87, 780–785. [Google Scholar] [CrossRef]
- Hamatschek, C.; Yousuf, E.I.; Mollers, L.S.; So, H.Y.; Morrison, K.M.; Fusch, C.; Rochow, N. Fat and fat-free mass of preterm and term infants from birth to six months: A review of current evidence. Nutrients 2020, 12, 288. [Google Scholar] [CrossRef]
- Franz, A.R.; Pohlandt, F.; Bode, H.; Mihatsch, W.A.; Sander, S.; Kron, M.; Steinmacher, J. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009, 123, e101–e109. [Google Scholar] [CrossRef] [PubMed]
- Roze, J.C.; Darmaun, D.; Boquien, C.Y.; Flamant, C.; Picaud, J.C.; Savagner, C.; Claris, O.; Lapillonne, A.; Mitanchez, D.; Branger, B.; et al. The apparent breastfeeding paradox in very preterm infants: Relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, epipage and lift. BMJ Open 2012, 2, e000834. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Miller, J.; Collins, C.T.; Rumbold, A.R. Comparison of different protein concentrations of human milk fortifier for promoting growth and neurological development in preterm infants. Cochrane Database Syst. Rev. 2020, 11, CD007090. [Google Scholar] [CrossRef]
- Premkumar, M.H.; Pammi, M.; Suresh, G. Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates. Cochrane Database Syst. Rev. 2019, 11, CD013145. [Google Scholar] [CrossRef] [PubMed]
- Grace, E.; Hilditch, C.; Gomersall, J.; Collins, C.T.; Rumbold, A.; Keir, A.K. Safety and efficacy of human milk-based fortifier in enterally fed preterm and/or low birthweight infants: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 137–142. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Sun, R.; Maillart, L.; Schaefer, A.; Spence, E.H.; Perrin, M.T. Macronutrient variability in human milk from donors to a milk bank: Implications for feeding preterm infants. PLoS ONE 2019, 14, e0210610. [Google Scholar] [CrossRef]
- Paulaviciene, I.J.; Liubsys, A.; Eidukaite, A.; Molyte, A.; Tamuliene, L.; Usonis, V. The effect of prolonged freezing and holder pasteurization on the macronutrient and bioactive protein compositions of human milk. Breastfeed. Med. 2020, 15, 583–588. [Google Scholar] [CrossRef]

| Study Country | Design | Participants BW, g; GA, wk; n | Intervention (Proportion of HM % Unless Stated Elsewhere) | Comparisons for This Review C1: EHM vs. EPTF C2: Any HM vs. EPTF C3: High- vs. Low-Dose HM | Study Duration | Outcomes | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Randomised Controlled Trials | |||||||
| Cristofalo (2013) [31] USA and Australia | RCT | 53 infants Gp1: 996 ± 152; 27.7 ± 1.5; 29 Gp2: 983 ± 207; 27.5 ± 2.4; 24 | Gp1: EHM (HMDF) (100%) Gp2: EPTF (BovF) (0%) | C1: Gp1 vs. Gp2 | SS: Initiation of enteral nutrition SE: Earliest of 91 d of age; DC; or 50% oral feedings | Growth (from regained BW to SE): wt gain (g/d), HC gain (cm/wk), Length gain (cm/wk) | Moderate (sequence generation and allocation concealment unclear) |
| O’Connor 2016 [32] Canada | RCT | 363 infants Gp1: 995 ± 273; 27.5 ± 2.4; 181 Gp2: 996 ± 272; 27.8 ± 2.7; 182 | Gp1: EHM (MOM + DHM) (100%; MOM 58.4% (13.6, 96.0)) Gp2: Mixed (MOM + PTF) (MOM 63.3% (9.6, 97.2)) | C3: Gp1 vs. Gp2 | SS: Start enteral feeds SE: 90 d or DC | Growth (during intervention): change in wt, HC and length z-score | Low |
| Schanler 2005 [33] USA | RCT (randomised arms) Cohort (non-randomised arm) | 243 infants Gp1: 947 ± 233; 27 ± 2; 81 Gp2: 957 ± 267; 27 ± 2; 92 Gp3: 999 ± 259; 27 ±2; 70 | Gp1: EHM (MOM + DHM) (100%) Gp2: Mixed (MOM + PTF) (NR) Gp3: EHM (MOM) (100%) (reference, not randomised) | C3 (RCT): Gp1 vs. Gp2 C3 (non-RCT): Gp 1+ Gp3 vs. Gp2 | SS: 4 d after birth SE: 90 d of age or DC | Growth (during study): wt gain (g/kg/d), HC gain (cm/wk), Length gain (cm/wk) | Low (sequence generation unclear) |
| Sullivan 2010 [34] USA and Austria | RCT | 207 infants Gp1: 945 ± 202; 27.2 ± 2.2; 67 Gp2: 909 ± 193; 27.1 ± 2.3; 71 Gp3: 922 ± 197; 27.3 ± 2.0; 69 | Gp1: EHM (MOM + DHM + HMDF) (100%; MOM 73% (16, 82)) (fortified at 100 mL/kg/d) Gp2: EHM (MOM + DHM + HMDF) (100%; MOM 70% (18, 80)) (fortified at 40 mL/kg/d) Gp3: Mixed (MOM + PTF + BovF) (NR; MOM 82% (38, 100)) (fortified at 100 mmL/kg/d) | C3: Gp1 + 2 vs. Gp 3 | SS: Start of enteral nutrition SE: Earliest of 91 d of age, DC, 50% oral feedings | Growth (from SS to SE): mean wt gain (g/kg/day), HC and length gain (cm/wk); HC and length gain reported as median, IQR, and converted to mean, SD. | High (no blinding of caregivers who likely measured growth) |
| Observational Studies | |||||||
| Assad 2016 [39] USA | Interrupted time series | 293 infants BW range: 490 to 1700 GA Gp1: 27.7 ± 2.7; 87 Gp2: 28.3 ± 2.8; 127 Gp3: 27.6 ± 2.8; 49 Gp4: 29.8 ± 2.5; 30 | Gp1: EHM (MOM + DHM + HMDF) (100%) Gp2: EHM (MOM + BovF) (100%) Gp3: Mixed (MOM + BovF + PTF) (NR) Gp4: EPTF (0%) | C1: Gp1 + Gp2 vs. Gp4 C2: Gp1 + Gp2 + Gp3 vs. Gp4 C3: Gp1 + Gp2 vs. G3 | NR | Growth (from birth to DC): wt gain (g/kg/d) | Low |
| Brownell 2018 [43] USA | Cohort | 314 infants Whole cohort: 1233 ± 373; 29.5 ± 2.9; 314 | 10% incremental exposure to MOM, DHM, and PTF | C3: synthesised narratively | SS: enteral feedings start SE: 36 wk PMA or DC | Growth (from birth to 36 wk PMA): wt gain (g/kg/day), HC gain (cm/wk), length gain (cm/wk), change in wt, length, and HC z-scores | Low |
| Canizo Vazquez 2019 [44] Spain | Cohort | 227 infants Gp 1: 1283 ± 393; 29.5 ± 2.3; 99 Gp 2: 1197 ± 370; 29.1 ± 2.3; 128 | Gp1: MOM, PTF Gp2: MOM, DHM | C3: Gp2 vs. Gp1 | Hospital stay | Growth (from birth to discharge): change in wt z-score; length, and HC z-score at SE | Moderate (Proportion of HM not reported) |
| Carlson 1998 [45] USA | Cohort | 51 infants Whole cohort: 899 ± 205; 27.1 ± 1.9; 51 | Gp1: EHM (MOM) (100%) Gp2: Mixed (MOM + PTF) (NR) Gp3: EPTF (0%) | C1, C2, C3: synthesised narratively | NR | Growth (from birth to DC): wt gain (g/kg/day), measured in time periods: 0–14 d, 15–35 d, 36–56 d, 57 d to TCA | Low |
| Castellano Yanez 2019 [46] Spain | Cohort | 130 infants Gp 1: 1430 ± 262; 31.2 (30.1, 31.7); 52 Gp 2: 1343 ± 233; 31.5 (30.0, 32.7); 78 | Gp1: MOM, PTF Gp2: MOM, DHM | C3: synthesised narratively | Hospital stay | Growth (from birth to DC): change in wt, length, and HC z-scores—difference between groups | Moderate (some differences between Gps) |
| Chowning 2016 [47] USA | Cohort | 550 infants Gp1: 1030 ± 290; 28.1 ± 2.7; 260 Gp2: 1080 ± 280; 28.6 ± 2.5; 290 Gp3: 1150 ± 290; 29.3 ± 2.8; 76 Gp4: 1160 ± 240; 29.1 ± 2.4; 71 | Gp1: <50% d received HM Gp2: ≥50% d received HM Separate analysis done for extremes of intake Gp3: 0% d received HM Gp4: ≥90% d received HM | C2: Gp4 vs. Gp3 C3: Gp2 vs. Gp1 | Hospital stay | Growth: wt gain (from regained BW to DC, g/kg/d), HC gain (from birth to DC, cm/wk), Change in wt and HC z-score (from birth to DC) | Low |
| Colacci 2017 [40] USA | Interrupted time series | 85 infants Gp1: 783 ± 143; 26.0 ± 1.9; 39 Gp2: 770 ± 137; 26.0 ± 1.9; 46 | Gp1: EHM (MOM + DHM + HMDF) (100%) Gp2: Mixed (MOM + PTF + BovF) (83% of feeding as formula) | C3: Gp1 vs. Gp2 | SS: birth SE: at least for 4 wk and wt ≥1500 g or 34 wk PMA (whichever occur first) | Growth (from birth to DC): wt gain (g/kg/d) | Low |
| Colaizy 2012 [7] USA | Cohort | 171 infants Gp1: 1083 (778, 1184); 28.4 (25.4, 29.6); 17 Gp2: 861 (736, 1091); 26.89(25.4, 29.0); 30 Gp3: 848 (717, 1011); 26.6(25.7, 28.5); 36 Gp4: 880 (719, 1052); 27(25.6, 28.8); 88 | Gp1: <25% HM (MOM + DHM + PTF) Gp2: 25–50% HM (MOM + DHM + PTF) Gp3: 51–75% HM (MOM + DHM + PTF) Gp4: >75% HM (MOM + DHM + PTF) | C3: Gp1 + Gp2 vs. Gp3 + Gp4 | Hospital stay | Growth (from birth to DC): change in wt z-score (reported as median, IQR and converted to mean, SD) | Low |
| Costa-Orvay 2011 [15] Spain | RCT with non-randomised reference group | 38 infants Gp1: 1138 ± 173; 29.0 ± 1.7; 6 Gp2: 1196 ± 243; 29.6 ± 1.6; 8 Gp3: 1220 ± 221; 30.2 ± 1.4; 12 Gp4: 1313 ± 336; 29.8 ± 1.7; 12 | Gp1: EHM (MOM + BovF) (100%), (reference, not randomised) Gp2: EPTF (0%) Gp3: EPTF (high energy and protein formula) (0%) Gp4: EPTF (high energy and protein formula) (0%) | C1: Gp1 vs. Gp2 | Intervention: 4 wks from regain BW | Body composition (BIA) at SE: FM (g), FFM (g) | Low |
| Fewtrell 2002 [19] United Kingdom | RCT with non-randomised reference group | 283 infants Gp1: 1353 ± 274; 30.3 ± 2.4; 100 Gp2: 1336 ± 284; 30.4 ± 2.3; 95 Gp3: 1395 ± 262; 30.3 ± 2.0; 88 | Gp1: Control PTF (0%) Gp2: LCPUFA-supplemented formula (0%) Gp3: MOM (NR) (reference, not randomised) | C2: Gp3 vs. Gp1 | SS: 10 d of age SE: 18 m CA | Growth (from birth to DC): wt gain (g/kg/d), HC gain (cm/wk) | Low |
| Ginovart 2017 [8] Spain | Interrupted time series | 182 infants Gp1: 1108 ± 273; 29+4 ± 2+6; 72 Gp2: 1078 ± 289; 29+1 ± 2+6; 114 | Gp1: Any PTF (PTF + MOM) (NR) Gp2: EHM (MOM + DHM + BovF) (100%) | C3: Gp2 vs. Gp1 | Hospital stay | Growth (from birth to DC): change in wt and HC z-score | Low |
| Hair 2016 [48] USA | Cohort | 1587 infants Gp1: 823 ± 205; 26.4 ± 2.3; 768 Gp2: 844 ± 210; 26.5 ± 2.5; 819 | Gp1: MOM + BovF + PTF (NR) Gp2: EHM (MOM+ DHM + HMDF) (100%) | C3: Gp2 vs. Gp1 | SS: NR SE: Varied between sites: 34 wk PMA, 60 d of age, at 1500 g or 34 wk PMA, 32 wk PMA | Growth (time frame for measures NR): wt gain (g/d), HC gain (cm/wk) | Moderate (some differences between Gps) |
| Hoban, 2019 [41] USA | Interrupted time series | 321 infants Gp1: Median (IQR) 1050 (750, 1220); 2707 (26.1, 29.4); 160 Gp2: 1000 (800, 1180); 27.7 (25.9, 29.3); 161 | Gp1: Pre-DM era (97% (35, 100) Gp2: DM era (100%) | C3: Gp 2 vs. Gp1 Synthesised narratively | Feeding collected 1st 28 days of life | Growth (birth to DC): change wt, length, and HC z-scores | Low |
| Huston 2014 [29] USA | Cohort | 361 infants Gp1: 1177 ± 222; 29.1 ± 1.8; 93 Gp2: 1104 ± 262; 28.1 ± 2.2; 224 Gp3: 919 ± 269; 26.7 ± 2.4; 44 | Gp1: EPTF or MOM + BovF + PTF for >48 h (NR) Gp2: MOM + DHM + BovF (100%) Gp3: EHM (MOM + DHM + HMDF) (100%) | C3: Gp2 + Gp3 vs. Gp1 | NR | Growth (during hospital stay): wt gain (g/kg/d), HC gain (cm/wk), length gain (cm/wk) | Moderate (some differences between Gps) |
| Huston 2018 [28] USA Some overlap of infants between this report and Huston 2014 | Interrupted time series | 379 infants Gp1: 1025 ± 164; 28.4 ± 1.9; 54 Gp2: 944 ± 199; 27.4 ± 2.0; 87 Gp3: 959 ± 174; 27.1 ± 2.0; 111 Gp4: 855 ± 209; 26.2 ± 2.2; 33 Gp5: 904 ± 200; 26.6 ± 2.4; 94 | Gp1: PTF (MOM + PTF + BovF) (NR) Gp2: HMBF1 (MOM + DHM + BovF) (before implementation of the feeding protocol) (100%) Gp3: HMBF2 (MOM + DHM + BovF) (after implementation of the protocol) Gp4: EHM1 (MOM + HMDF) (before implementation of the feeding protocol) (100%) Gp5: EHM2 (MOM + HMDF) (after implementation of the feeding protocol) | C3: Gp2 + Gp4 vs. Gp1 | Hospital stay | Growth (during hospital stay): change in wt, HC and length z-score | Moderate (some differences between Gps) |
| Jacobi-Polishook 2016 [37] Australia | Secondary analysis of RCT | 611 infants median (range) Gp1: 1580 (720, 2280); 31.0 (25.0, 32.0); 46 Gp2: 1360 (530, 2620); 30.0 (24.0, 32.0); 141 Gp3: 1390 (420, 2400); 30.0 (23.0, 33.0); 141 Gp4: 1290 (500, 2090); 29.0 (23.0, 33.0); 142 Gp5: 1240 (320, 2480); 30.0 (24.0, 33.0); 141 | Gp1: EPTF (0%) Gp2: Q1 (HM median (range) 49 (0.1, 85) mL/kg/d) Gp3: Q2 (HM 103 (85, 114) ml/kg/d) Gp4: Q3 (HM 124 (114, 134) ml/kg/d) Gp5: Q4 (HM 149 (134, 180) ml/kg/d) | C2: Gp2 + Gp3 + Gp4 + Gp5 vs. G1 C3: Gp4 + Gp5 vs. Gp2 + Gp3 BMI gain synthesised narratively | Hospital stay | Growth (from birth to DC): length gain (cm/wk), BMI gain | Moderate (some differences between Gps) |
| Kaempf 1998 [36] Germany | Non-randomised trial | 19 infants Gp1: 1220 ± 310; 29 ± 1.1; 11 Gp2: 1220 ± 270; 30 ± 1.6; 8 | Gp1: PTF (>80% PTF) Gp2: HM (fortified MOM) (>80% fortified MOM) | C3: Gp1 vs. Gp2 for wt gain (g/d); lower leg length synthesised narratively | SS: Gp1; age 8 ± 5 d, Gp2; age 10 ± 5 d Study duration: Gp1; 51 ± 12 d, Gp2; 48 ± 18 d | Growth (time frame for measures NR): wt gain (g/d), lower leg length (mm/d) | Moderate (some differences between Gps) |
| Lee 2020 [49] Singapore, Malaysia | Cohort | 236 infants Gp 1: 855 ± 123; 27.1 ± 1.9; 40 Gp 2: 849 ±120; 27.6 ± 2.4; 34 | Gp1 (Singapore): MOM 97% Gp2 (Malaysia): MOM 26%, Mix MOM/PTF 62%, EPTF 12% | C3: Gp1 vs. Gp2 | Birth to 36 wk PMA | Growth (birth to 36 wk PMA): change in wt z-score | Low |
| Levene 2020 [50] United Kingdom | Cohort | 193 infants Infants in ‘after’ cohort: 1117 ± 335; 28.1 ± 2.2; 209 | Gp1: Exclusive HM (DHM until 34 wks PMA) (+package of key nutritional changes) Gp2: Any PTF (+package of key nutritional changes) | C3: Gp1 vs. Gp2 | Hospital stay | Growth (birth to DC): wt gain (g/kg/d); change in wt z-score | Low |
| Li 2019 [38] United Kingdom | Secondary analysis of RCT | 133 infants Gp1: 997 (780, 1178); 28.1 (26.5, 29.5); 56 Gp2: 1140 (885, 1398); 28.6 (26.8, 30.1); 39 Gp3: 1132 (905, 1334); 28.2 (26.9, 30.0); 38 | (RCT: four PN intervention groups) Gp1: EHM; MOM (100%) Gp2: Predominantly HM; MOM + DHM + PTF (NR) Gp3: Predominantly PTF; MOM + DHM + PTF (NR) | C3: % FFM Gp1 + Gp2 vs. Gp 3; remainder of outcomes synthesised narratively | Nutritional intake: from birth until 34 wks PMA | Growth (birth to TCA): wt, length, and HC change in z-scores Body composition (MRI) at TCA: FM (g and %), FFM (g), FFM % (reported as median IQR and converted to mean, SD) | Low |
| Lok 2017 [22] Hong Kong | Cohort | 175 VLBW infants Gp1: 1269.3 ± 180.6; NR; 31 Gp2: 1139.2 ± 205.9; NR; 144 Gp3: 1213 ± 204.3; NR; 55 Gp4: 1202 ± 189.5; NR; 25 Gp5: 1135 ± 221.5; NR; 47 Gp6: 1106 ± 191.4; NR; 47 | Gp1: EPTF (0%) Gp2: Any HM (NR) Group by proportion of breast milk intake Gp3: <25% HM Gp4: 25–50% HM Gp5: 50–75% HM Gp6: >75% HM | C2: Gp2 vs. Gp1 C3: Gp5 + Gp6 vs. Gp3 + Gp4 | The first 30 d of hospitalization | Growth (from birth to DC): change in wt and HC z-score | Low |
| Maas 2013 [51] Germany | Cohort | 206 infants Gp1: 846 (705, 1160); 28.6 (25.5, 30.5); 37 Gp2: 925 (665, 1175); 27.6 (25.5, 29.7); 122 | Gp1: <25% HM Gp2: >75% HM | C3: Gp 2 vs. Gp1 | Hospital stay | Growth (from birth to day 28): wt and HC changes in z-score (reported as median, IQR and converted to mean, SD) | Moderate (some loss to FU) |
| Madore 2017 [52] USA | Cohort | 81 infants Gp1: 936.6 ± 211.0; 27.0 ± 1.5; 29 Gp2: 913.8 ± 222.6; 27.3 ± 2.1; 25 Gp3: 890.5 ± 175.8; 27.1 ± 1.9; 27 | Gp1: EHM; MOM (100%) Gp2: Predominantly PTF (>50% PTF) Gp3: DHM (>50% DHM) | C3: Gp1 + Gp3 vs. Gp2 | The first month of life | Growth (from birth to day 30 and 60): wt gain (g/kg/d), HC gain (cm/wk), length gain (cm/wk) | Low |
| Manea 2016 [53] Romania | Cohort | 34 infants Birth wt range; 850–1000 g Birth GA; 25–33 weeks Gp1: n = 16 Gp2: n = 18 | Gp1: EPTF (0%) Gp2: EHM; MOM + BovF (after reached 100 mL/kg/day) (100%) | C1: synthesised narratively | Hospital stay | Growth (from birth to 5 wk of age): wt gain (g/d) | High (Gp characteristics and participant flow not described. SD and p values NR) |
| Mol 2019 [54] Poland | Cohort | 53 infants BW; mean ± SD, GA; median (IQR) Gp1: 1240 ± 180; 29 (28–31.8); 23 Gp2: 1210 ± 161; 29 (28–32); 11 Gp3: 3320 ± 399; 39 (37–40); 19 | Gp1: EPTF (0%) Gp2: Fortified HM (MOM + BovF) (100%) Gp3: full-term infants | C1: Gp2 vs. Gp1 | Hospital stay | Body composition (BIS) at TCA: FM (g and %), FFM (g and %) | Moderate (milk amount not described) |
| Morlacchi 2018 [55] Italy | Cohort | 32 infants Gp1: 1214.8 ± 246; 29.2 ± 1.6; 17 Gp2: 1293.0 ± 138; 30.3 ± 1; 15 | Gp1: Fortified HM (MOM + BovF) (100%) Gp2: EPTF (0%) | C1: Gp1 vs. Gp2 Change in z-scores synthesised narratively | SS: at DC SE: at TCA Intervention start from birth to DC | Growth (from birth to DC): wt, length, and HC change in z-scores Body composition (ADP (PeaPod) at DC): FM (g and %), FFM (g and %) | Low |
| Nicholl 1999 [35] United Kingdom | RCT with non-randomised arm | 52 infants Gp1: 1074 ± 216; 29 ± 2.1; 10 Gp2: 1002 ± 286; 28.1 ±2.4; 13 Gp3: 1087 ± 252; 28.7 ± 2.5; 29 | Gp1: EHM (MOM + DHM) (100%) Gp2: EHM (MOM + DHM + BovF) (100%) Gp3: EPTF (0%) (not randomised) | C1: Gp2 vs. Gp3 Lower leg length synthesised narratively | SS: enteral feed ≥ 150 mL/kg/day SE: nasogastric feeds ceased | Growth (from SS to SE): wt gain (g/kg/d), lower leg length gain | Low |
| O’Connor 2001 [20] USA and United Kingdom | RCT with non-randomised reference group | 470 infants Gp1: 1287 ± 272; 29.6 ± 1.9; 142 Gp2: 1305 ± 293; 29.8 ± 2.1; 138 Gp3: 1309 ± 286; 29.7 ± 2.0; 140 Gp4: 1275 ± 312; 29.7 ± 2.1; 43 | Gp1: HM + PTF (NR) Gp2: HM + PTF + AA + DHA from fish/fungal oil Gp3: HM + PTF + AA + DHA from egg-derived triglyceride/fish oil G4: HM (>80% at term corrected age) (reference, not randomised) | C3: Gp4 vs. Gp1 | SS: first enteral feed SE: 12 m CA | Growth (from SS to TCA): wt gain(g/kg/d), HC gain (cm/wk), length gain (cm/wk) | Low |
| Petrova 2020 [56] USA | Cohort | 84 infants Gp 1: 1027 (95% CI 924, 1321); 27.7 (95% CI 26.8, 28.6); 37 Gp 2: 1285 (95% CI 1130, 1439); 29.8 (95% CI 28.6, 31.0); 16 Gp 3: 1272 (95% CI 1102, 1442); 29.1 (95% CI 28.2, 30.3); 31 | Gp1: Predominantly HM (≥97% HM) Gp2: Partial HM (55–70% HM) Gp3: Predominantly PTF (≤9% HM) | C3: Gp1 + Gp2 vs. Gp3 | SS: full enteral feeding had been achieved SE: 2 wks post full enteral feeds | Growth (from SS to SE): wt gain (g/kg/d) reported as median, IQR in figure format only; HC gain (cm/wk) reported as mean, 95% CI and converted to mean, SD | Moderate (some differences between Gps) |
| Pieltain 2001 [57] Belgium | Cohort | 54 infants Gp1: 1298 ± 317; 31 ± 2; 20 Gp2: 1269 ± 261; 30 ± 2; 34 | Gp1: Fortified HM; MOM + DHM + BovF (100%) Gp2: EPTF (0%) | C1: Gp1 vs. Gp2 | SS: full enteral feeding had been achieved SE: at DC | Growth (from SS to SE): wt gain (g/kg/d), HC gain (cm/wk), length gain (cm/wk) Body composition (DEXA at SS and around DC): FFM (g), FM (g and %) | Low |
| Piemontese, 2018 [58] Italy | Cohort | 73 infants Gp1: 1207 ± 208; 30 ± 2.4; 24 Gp2: 1269 ± 193; 30.3 ± 1.8; 49 | Gp1: Fortified HM <50% intake; MOM + DHM + BovF + PTF (34.9% ± 12.5) Gp2: Fortified HM ≥50% intake; MOM + DHM + BovF + PTF (80.9% ± 15.5) | C3: Gp2 vs. Gp1 | Hospital stay Targeted fortification commenced when intake ≥80 mL/kg | Growth (from birth to TCA): wt z-score SE, wt gain g/kg/day Body composition (ADP at TCA); FM (%), FFM (%) | Low |
| Schanler 1999 [9] USA | Secondary analysis of RCT | 108 infants Gp1: 1069 ± 169; 27.9 ± 1.2; 62 Gp2: 1044 ± 185; 27.9 ± 1.1; 46 | Gp1: Fortified HM; MOM + BovF (84 ± 20%, median 93%) Gp2: EPTF (0%) | C2: Gp1 vs. Gp2 Knee-heel length synthesised narratively | Hospital stay | Growth (from minimum wt to DC): wt gain (g/kg/d), HC gain (cm/wk), length gain (cm/wk) | Low |
| Simmer 1997 [59] Australia | Cohort | 86 infants Gp1: 1486 ± 450; 30.8 ± 2.6, 43 Gp2: 1379 ± 347; 29.8 ± 2.5; 43 | Gp1: Predominantly MOM; >50% (84 ± 15%) Gp2: Predominantly PTF; >50% PTF (16 ± 17%) | C3: Gp1 vs. Gp2 | Hospital stay | Growth: wt gain (from week 2 to DC, g/kg/day), HC gain (during admission in the neonatal unit, cm/wk), length gain (during admission in the neonatal unit, cm/wk) | Low |
| Sisk 2008 [60] USA | Cohort | 127 infants Gp1: 978 ± 149; 27.8 ± 2.1; 34 Gp2: 1000 ± 149; 27.4 ± 1.6; 93 | Gp1: Lower HM (<50% of HM) Gp2: Higher HM (≥50% of HM) | C3: Gp 1 vs. Gp 2 | Hospital stay | Growth (from regained BW to DC): wt gain (g/kg/day) (reported as median, IQR, and converted to mean, SD) | Low |
| Sisk 2017 [61] USA | Cohort | 551 infants Gp1: 1017 ±291; 27.8 ± 2.4; 299 Gp2: 1026 ± 270; 28.0 ± 2.4; 139 Gp3: 1036 ± 21; 28.1 ± 2.6; 113 | Gp1: MOM; ≥50% MOM (MOM 97%) Gp2: PDHM; ≥50% PDHM (PDHM 86%) Gp3: PTF; ≥50% PTF (PTF 91%) | C3: Gp1 + Gp2 vs. Gp3 | SS: birth SE: NEC diagnosis or 34 wk PMA | Growth (from birth to DC): wt gain (g/kg/day), HC gain (cm/wk), length gain (cm/wk), change in z-scores (reported as median, IQR and converted to mean, SD) | Low |
| Soldateli 2020 [62] USA | Cohort (secondary analysis of data collected for QI initiative) | 1429 infants Whole cohort: 1080 (861, 1285); 28 (27, 30) | Gp1: 0–25% HM Gp2: 26–50% HM Gp3: 51–75% HM Gp4: 76–99% Gp5: 100% HM Diet recorded on days of life 7, 14, 21, 28, 42, 56, 70, 84, and at discharge or transfer | C3: synthesised narratively | Birth to DC or transfer | Growth (from birth to DC or transfer): wt gain (g/kg/day), change in wt and length z-scores (only mean reported in text) | Moderate (unable to determine if Gps comparable) |
| Spielger 2016 [63] Germany | Cohort | 1433 infants Gp1: 1080 (830, 1330); 28.7 (26.6, 30.1); 239 Gp2: 1100 (865, 1340); 29.0 (26.9, 30.0); 223 Gp3: 1050 (805, 1295); 28.4 (26.6, 30.0); 971 | Gp1: EPTF; PTF (0%) Gp2: EHM; MOM + DHM (100%) Gp3: Mixed; MOM + DHM + PTF (NR) | C1: Gp1 vs. Gp2 C2: Gp2 + Gp3 vs. Gp1 C3: Gp2 vs. Gp1 + Gp2 | Hospital stay | Growth (from birth to DC): change in wt z-score, (reported as median, IQR, and converted to mean, SD) | Low |
| Verd 2015 [42] Spain | Interrupted time series | 201 infants Gp1: 800 (410, 995); GA (d) 185 (161, 236); 148 Gp2: 830 (440, 998); 190 (166, 239); 53 | Gp1: EHM; MOM + DHM (100%) Gp2: Any PTF; MOM + DHM + PTF (NR) | C3: Gp1 vs. Gp2 | Hospital stay | Growth (from birth to DC): change in wt, length, and HC z-scores reported as median, IQR, and converted to mean, SD | Low |
| Warner 1998 [64] United Kingdom | Cohort | 59 infants, median(range) Gp1: 1178 (685, 1510); 29.6 (25, 33); 38 Gp2: 1120 (840, 1580); 29.1 (25, 34); 21 | Gp1: HM; MOM + BovF + (term infant formula if MOM was insufficient) (NR) Gp2: EPTF (0%) | C2: Gp1 vs. Gp2 | SS: at birth SE: wt of 1800 g was reached | Growth: wt gain (from start of full feeds to 1800 g, g/kg/d), HC gain (from SS to SE, cm/wk), length gain (from SS to SE, cm/wk) | Low |
| Wauben 1998 [21,65] Canada | RCT with non-randomised reference group | 37 infants Gp1: 1400 ± 200; 29.9 ± 1.9; 12 Gp2: 1300 ± 200; 30.1 ± 1.5; 13 Gp3: 1200 ± 200; 29.7 ± 1.7, 12 | Gp1: MOM + multinutrient fortifier (100%) Gp2: MOM + calcium and phosphorus alone (100%) Gp3: PTF (0%) (comparison group, not randomised) | C1: Gp1 vs. Gp3 Body composition C3: Gp1 vs. Gp3 (Gp2 not fortified therefore excluded) | SS: full oral feeds ≥5 d SE: Discharge home or >38 wk PMA (whichever came first) | Growth (from SS to DC): wt gain (g/kg/d), HC gain (cm/wk), length gain (cm/wk) [21] Body composition (DXA) at TCA: FM (%), FFM (%) [65] | Low |
| Outcome | EPTF vs. EHM Anticipated Absolute Effects MD (95% CI); N Participants; N Studies GRADE Certainty of Evidence Interpretation | EPTF vs. Any HM Anticipated Absolute Effects MD (95% CI); N Participants; N Studies GRADE Certainty of Evidence Interpretation | Low- vs. High-Dose HM Anticipated Absolute Effects MD (95% CI); N Participants; N Studies GRADE Certainty of Evidence Interpretation |
|---|---|---|---|
| WEIGHT GAIN, G/DAY | Randomised controlled trial MD 2 (−1.54 to 5.54); n = 53; 1 study Certainty: not graded; ROB moderate Interpretation: Inconclusive | No studies identified | Observational studies MD −0.83 (−1.65 to 0); n = 1606; 2 studies Certainty: low Interpretation: Possible effect of a small decrease in weight gain (g/day) with lower-dose HM versus higher-dose HM |
| WEIGHT GAIN, G/KG/DAY | Observational studies MD 2.03 (−0.31 to 4.38); n = 364; 4 studies Certainty: very low Interpretation: Inconclusive | Observational studies MD 1.97 (0.21 to 3.72); n = 795; 5 studies Certainty: very low Interpretation: Inconclusive | Randomised controlled trial MD 2.41 (1.09 to 3.72); n = 373; 2 studies Certainty: low Observational studies MD 0.56 (0.09 to 1.03); n = 3162; 13 studies; Certainty: very low Interpretation: Possible effect of a small increase in weight gain (g/kg/day) with lower-dose HM versus high-dose HM |
| CHANGE IN WEIGHT Z-SCORE | Observational studies MD 0.26 (0.03 to 0.48); n = 49; 2 studies Certainty: low Interpretation: Possible effect of a small increase in weight z-score with EPTF vs. EHM | Observational studies MD 0.21 (−0.15 to 0.56); n = 1532; 3 studies Certainty: very low Interpretation: Inconclusive | Randomised controlled trial MD 0 (−0.29 to 0.29); n = 326; 1 study Certainty: not graded; ROB low Observational studies MD 0.19 (0.6 to 0.33); n = 4059; 12 studies Certainty: very low Interpretation: Inconclusive |
| HC GAIN, CM/WK | Randomised controlled trial MD 0.1 (−0.02 to 0.22); n = 53; 1 study Certainty: not graded; ROB moderate Observational studies MD 0.09 (−0.10 to 0.29); n = 78; 2 studies Certainty: very low Interpretation: Inconclusive | Observational studies MD 0.06 (0.1 to 0.11); n = 495; 4 studies Certainty: low Interpretation: Possible effect of small increase in HC gain in infants fed EPTF versus any human milk | Randomised controlled trial MD 0 (−0.06 to 0.06); n = 373; 2 studies Certainty: moderate Observational Studies MD 0.04 (0.02 to 0.07); n = 4080; 10 studies Certainty: very low Interpretation: Possibly no effect of HM dose on HC gain (cm/wk) |
| CHANGE IN HC Z-SCORE | Observational study MD 0.1 (−0.42 to 0.62); n = 32, 1 study Certainty: not graded; ROB low Interpretation: Inconclusive | Observational studies MD 0.43 (0.18 to 0.69); n = 322; 2 studies Certainty: low Interpretation: Possible effect of small increase in HC z-score with EPTF versus any HM. | Randomised controlled trial MD 0.2 (−0.08 to 0.48); n = 326; 1 study Certainty: not graded; ROB low Observational studies MD 0.09 (−0.19 to 0.38); n = 2627; 8 studies Certainty: very low Interpretation: Inconclusive |
| LENGTH GAIN, CM/WK | Randomised controlled trial MD 0.28 (0.14 to 0.42); n = 53; 1 study Certainty: not graded; ROB moderate Observational studies MD 0.06 (−0.07 to 0.19); n = 78; 2 studies Certainty: very low Interpretation: Inconclusive | Observational studies MD 0.09 (−0.05 to 0.22); n = 778; 3 studies Certainty: very low Interpretation: Inconclusive | Randomised controlled trial MD −0.04 (−0.28 to 0.21); n = 373; 2 studies Certainty: low Observational studies MD 0.05 (0.02 to 0.08); n = 2423; 8 studies Certainty: low Interpretation: Possibly no effect of dose of human milk on length gain (cm/wk) |
| CHANGE IN LENGTH Z-SCORE | Observational study MD 0.0 (−0.63 to 0.63); n = 32, 1 study Certainty: not graded; ROB low Interpretation: Inconclusive | No studies detected | Randomised controlled trial MD 0.1 (−0.26 to 0.46); n = 326; 1 study Certainty: not graded; ROB low Observational study MD 0.09 (−0.07 to 0.25); n = 1131, 3 studies Certainty: very low Interpretation: Inconclusive |
| FAT FREE MASS % | Observational studies MD −1.46 (−4.35 to 1.43); n = 87; 3 studies Certainty: very low Interpretation: Inconclusive | No studies identified | Observational studies MD −5.1 (−12.45 to 2.25); n = 73; 1 study Certainty: not graded; ROB low Interpretation: Inconclusive |
| FAT FREE MASS G | Observational studies MD 130.18 (53.86 to 206.5); n = 134; 4 studies Certainty: very low Interpretation: Inconclusive | No studies identified | No studies identified |
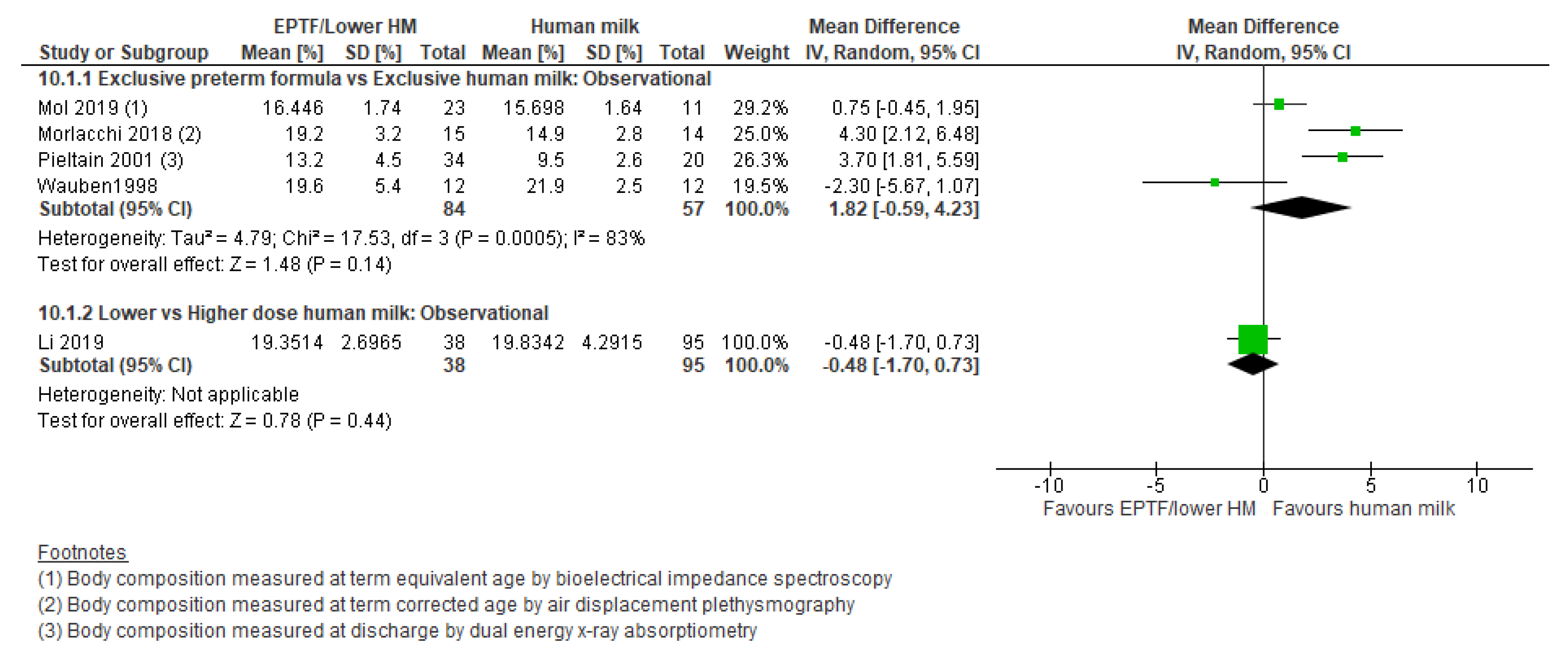
| FAT MASS % | Observational studies MD 1.82 (−0.59 to 4.23); n = 141; 4 studies Certainty: very low Interpretation: Inconclusive | No studies identified | Observational studies MD −0.48 (−1.7 to 0.73); n = 133; 1 study Certainty: not graded; ROB low Interpretation: Inconclusive |
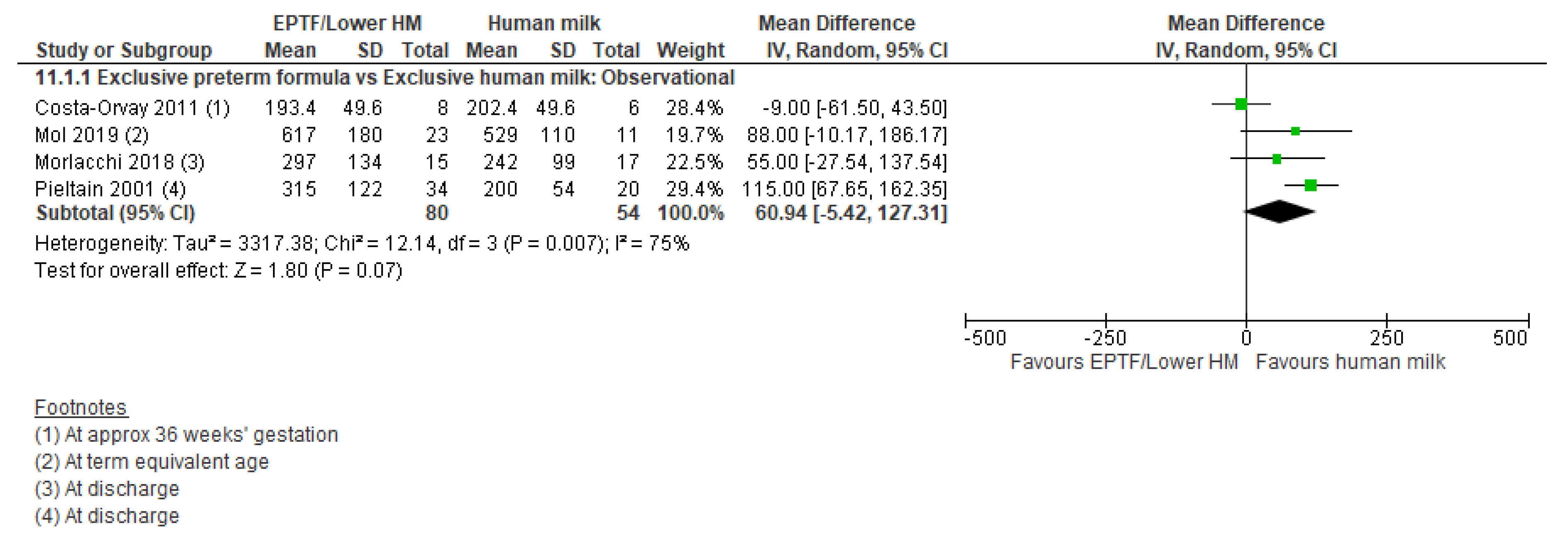
| FAT MASS G | Observational studies MD 60.94 (−5.42 to 127.31); n = 134; 4 studies Certainty: very low Interpretation: Inconclusive | No studies identified | No studied identified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suganuma, M.; Rumbold, A.R.; Miller, J.; Chong, Y.F.; Collins, C.T. A Systematic Review and Meta-Analysis of Human Milk Feeding and Short-Term Growth in Preterm and Very Low Birth Weight Infants. Nutrients 2021, 13, 2089. https://doi.org/10.3390/nu13062089
Suganuma M, Rumbold AR, Miller J, Chong YF, Collins CT. A Systematic Review and Meta-Analysis of Human Milk Feeding and Short-Term Growth in Preterm and Very Low Birth Weight Infants. Nutrients. 2021; 13(6):2089. https://doi.org/10.3390/nu13062089
Chicago/Turabian StyleSuganuma, Machiko, Alice R. Rumbold, Jacqueline Miller, Yan Fong Chong, and Carmel T. Collins. 2021. "A Systematic Review and Meta-Analysis of Human Milk Feeding and Short-Term Growth in Preterm and Very Low Birth Weight Infants" Nutrients 13, no. 6: 2089. https://doi.org/10.3390/nu13062089
APA StyleSuganuma, M., Rumbold, A. R., Miller, J., Chong, Y. F., & Collins, C. T. (2021). A Systematic Review and Meta-Analysis of Human Milk Feeding and Short-Term Growth in Preterm and Very Low Birth Weight Infants. Nutrients, 13(6), 2089. https://doi.org/10.3390/nu13062089







