Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
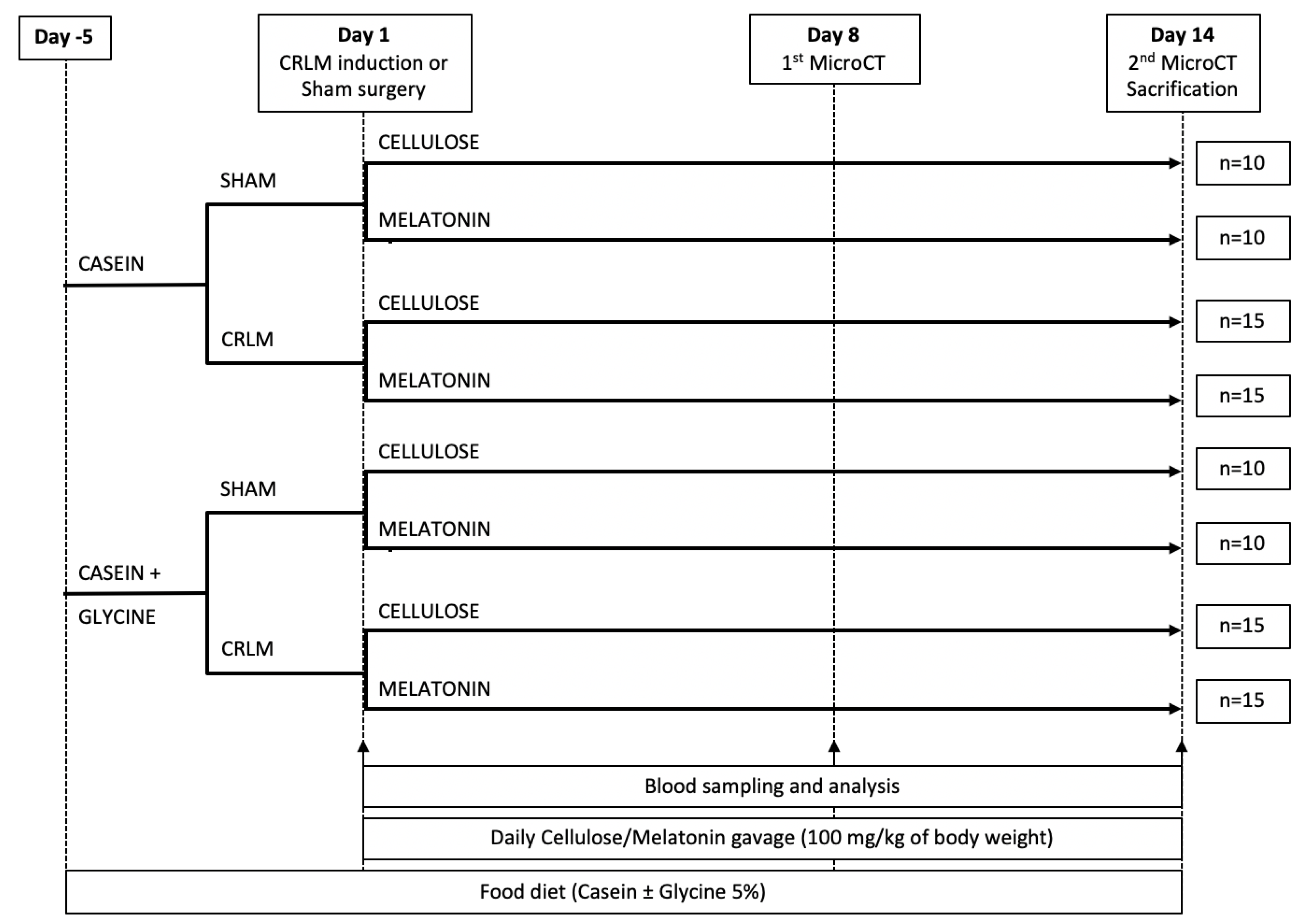
2.2. Animal Groups and Experimental Design
2.3. CC-531 Cell Line Maintenance
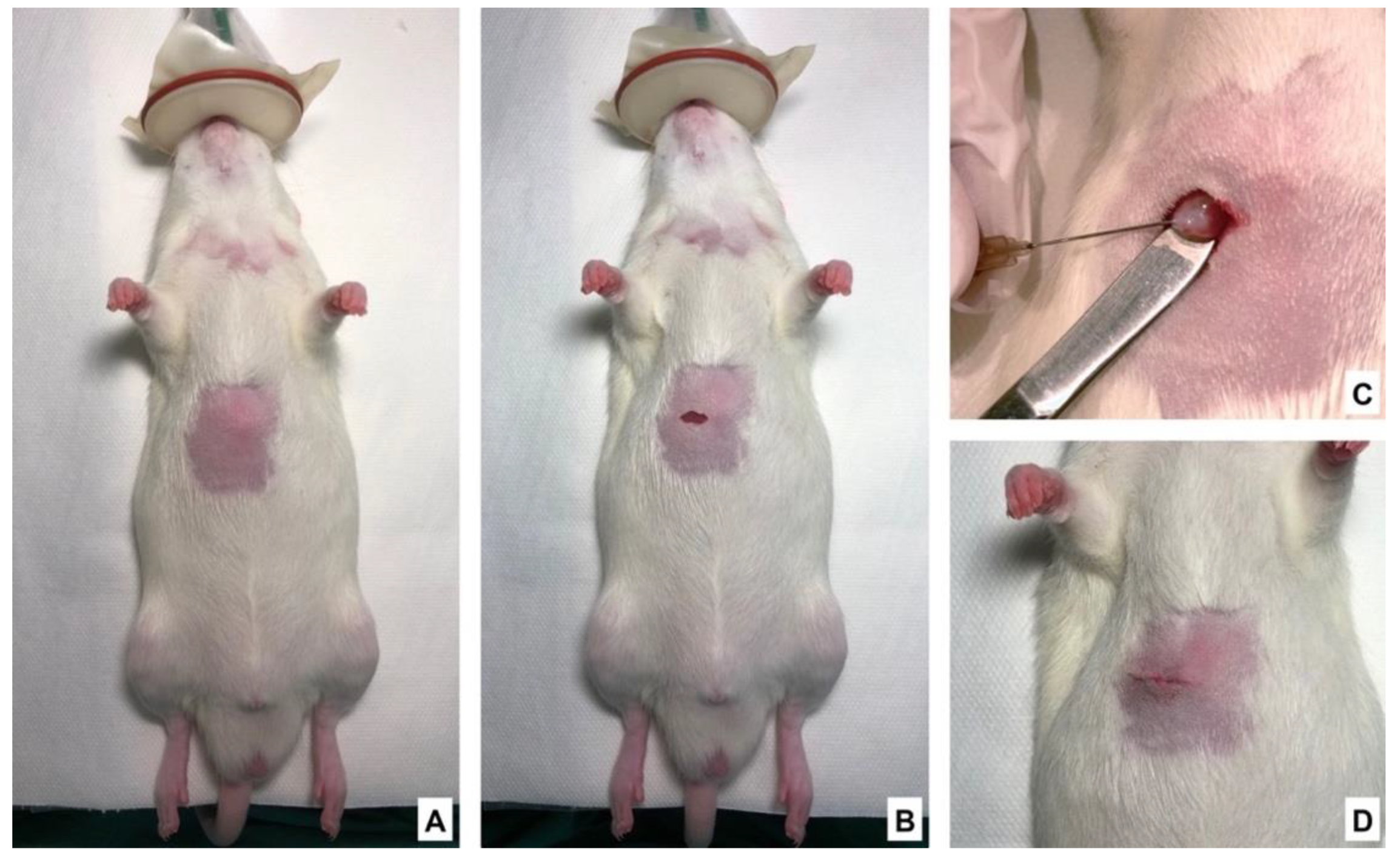
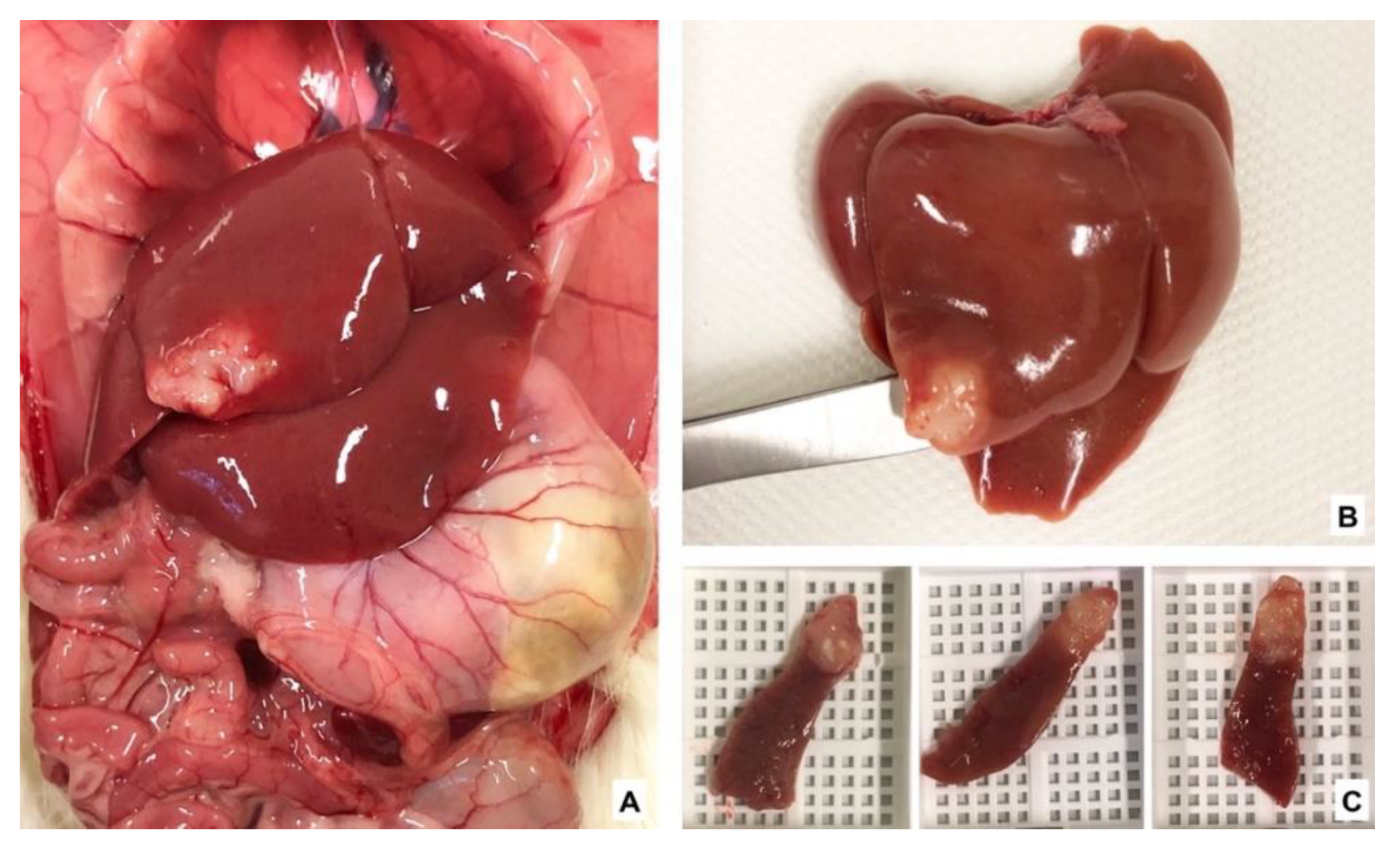
2.4. Tumor Implantation and Sham Surgery
2.5. MicroCT Scanning Procedure and Analysis
2.6. Blood Sample Analysis
2.7. Immunohistochemical Staining
2.8. Statistical Analyses
3. Results
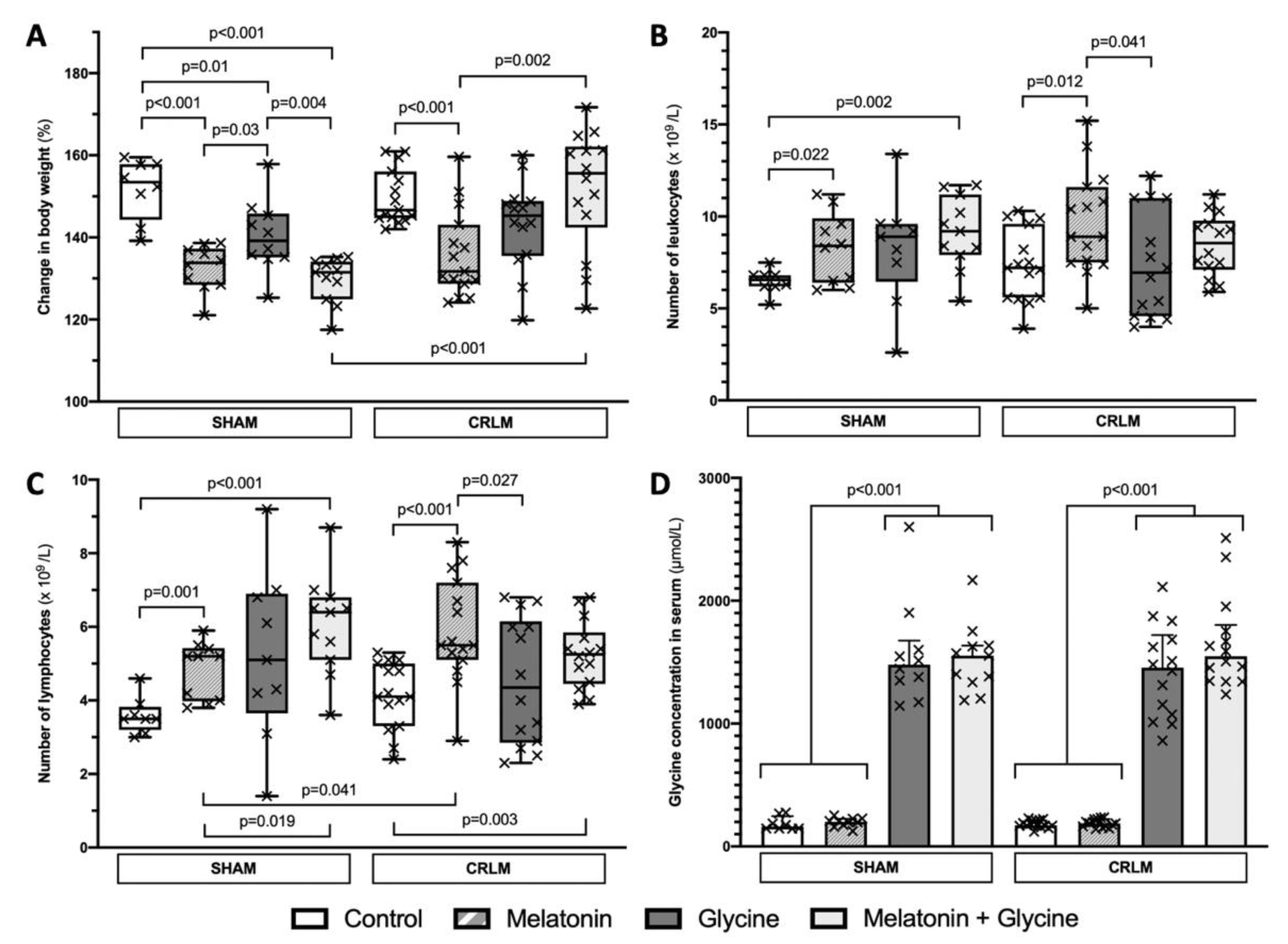
3.1. General Data
3.2. Blood Test Results and Glycine Concentration
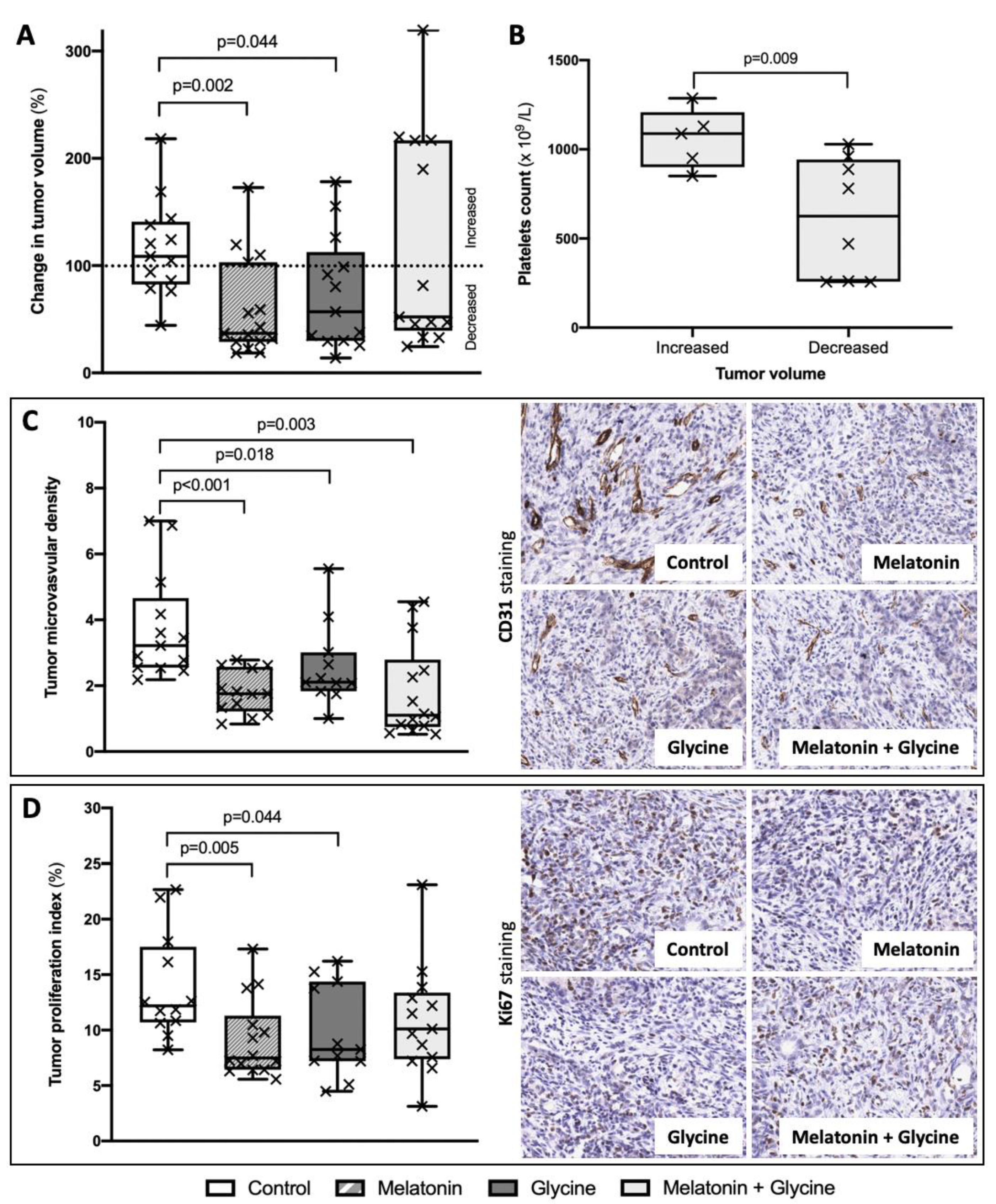
3.3. Change in Tumor Volume
3.4. Tumor Microvascular Density (MVD) and Proliferation Index
3.5. Subgroup Analysis: Combined Treatment with Melatonin and Glycine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Del Cornò, M.; Gessani, S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit. Rev. Oncol. 2020, 145, 102834. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Sveen, A.; Kopetz, S.; Lothe, R.A. Biomarker-guided therapy for colorectal cancer: Strength in complexity. Nat. Rev. Clin. Oncol. 2020, 17, 11–32. [Google Scholar] [CrossRef]
- Xue, L.; Williamson, A.; Gaines, S.; Andolfi, C.; Paul-Olson, T.; Neerukonda, A.; Steinhagen, E.; Smith, R.; Cannon, L.; Polite, B.; et al. An Update on Colorectal Cancer. Curr. Probl. Surg. 2018, 55, 76–116. [Google Scholar] [CrossRef] [PubMed]
- Kvietkauskas, M.; Zitkute, V.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. The role of melatonin in colorectal cancer treatment: A comprehensive review. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Primrose, J.N. Surgery for colorectal liver metastases. Br. J. Cancer 2010, 102, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Smedman, T.M.; Line, P.; Hagness, M.; Syversveen, T.; Grut, H.; Dueland, S. Liver transplantation for unresectable colorectal liver metastases in patients and donors with extended criteria (SECA-II arm D study). BJS Open 2020, 4, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Çetinkaya, E.; Ersöz, Ş.; Tez, M. Role of surgery in colorectal cancer liver metastases. World J. Gastroenterol. 2014, 20, 6113. [Google Scholar] [CrossRef] [PubMed]
- Sivesgaard, K.; Larsen, L.P.; Sørensen, M.; Kramer, S.; Schlander, S.; Amanavicius, N.; Bharadwaz, A.; Nielsen, D.T.; Mortensen, F.V.; Pedersen, E.M. Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur. Radiol. 2018, 28, 4735–4747. [Google Scholar] [CrossRef]
- van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. Faculty Opinions recommendation of ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2018, 27, 1386–1422. [Google Scholar] [CrossRef]
- López, R.I.; Castro, J.L.; Cedeño, H.; Cisneros, D.; Corrales, L.; González-Herrera, I.; Lima-Pérez, M.; Prestol, R.; Salinas, R.; Soriano-García, J.L.; et al. Consensus on management of metastatic colorectal cancer in Central America and the Caribbean: San José, Costa Rica, August 2016. ESMO Open 2018, 3, e000315. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Aprile, G.; Arnoldi, E.; Aschele, C.; Carnaghi, C.; Cosimelli, M.; Maiello, E.; Normanno, N.; Sciallero, S.; Valvo, F.; et al. Management of metastatic colorectal cancer patients: Guidelines of the Italian Medical Oncology Association (AIOM). ESMO Open 2017, 2, e000147. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.-J.; Xu, D.-P.; Li, H.-B. Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef]
- Kong, X.; Gao, R.; Wang, Z.; Wang, X.; Fang, Y.; Gao, J.; Reiter, R.J.; Wang, J. Melatonin: A Potential Therapeutic Option for Breast Cancer. Trends Endocrinol. Metab. 2020, 31, 859–871. [Google Scholar] [CrossRef]
- Hong, Y.; Won, J.; Lee, Y.; Lee, S.; Park, K.; Chang, K.-T.; Hong, Y. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J. Pineal Res. 2014, 56, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Cao, Y.; Li, Z.; Zhou, X.; Ding, R.; Chen, K.; Liu, Y.; Qiu, Y.; Wu, Z.; Fang, M. Glycine metabolomic changes induced by anticancer agents in A549 cells. Amino Acids 2020, 52, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Petrulionis, M.; Schultze, D.; Al Saeedi, M.; Lin, S.; Yamanaka, K.; Ambrazevičius, M.; Strupas, K.; Schemmer, P. Glycine inhibits angiogenic signaling in human hepatocellular carcinoma cells. Amino Acids 2014, 46, 969–976. [Google Scholar] [CrossRef]
- Maneikyte, J.; Bausys, A.; Leber, B.; Feldbacher, N.; Hoefler, G.; Kolb-Lenz, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Glycine Prevents FOLFOX Chemotherapy-Induced Heart Injury: A Colorectal Cancer Liver Metastasis Treatment Model in Rats. Nutrients 2020, 12, 2634. [Google Scholar] [CrossRef]
- Maneikyte, J.; Bausys, A.; Leber, B.; Horvath, A.; Feldbacher, N.; Hoefler, G.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary glycine decreases both tumor volume and vascularization in a combined colorectal liver metastasis and chemotherapy model. Int. J. Biol. Sci. 2019, 15, 1582–1590. [Google Scholar] [CrossRef]
- Bruns, H.; Kazanavicius, D.; Schultze, D.; Al Saeedi, M.; Yamanaka, K.; Strupas, K.; Schemmer, P. Glycine inhibits angiogenesis in colorectal cancer: Role of endothelial cells. Amino Acids 2016, 48, 2549–2558. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zitkute, V.; Kvietkauskas, M.; Maskoliunaite, V.; Leber, B.; Ramasauskaite, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Custodiol-N Is Superior to Custodiol((R)) Solution in Experimental Rat Uterus Preservation. Int. J. Mol. Sci. 2020, 21, 8015. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.-A.; Akhondzadeh, S.; Mohammadi, M.R.; Keshtkar, A.-A.; Hosseini, S.; Eshraghian, M.R.; Motlagh, T.A.; Alipour, R.; Keshavarz, S.A. Role of Melatonin in Body Weight: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2017, 23, 3445–3452. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in the regulation of brown adipose tissue metab-olism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Vale, M.I.; Peres, S.B.; Vernochet, C.; Farmer, S.R.; Lima, F.B. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBPbeta tran-scriptional activity. J. Pineal. Res. 2009, 47, 221–227. [Google Scholar] [CrossRef]
- Graubert, T.; Ley, T.J. How do lymphocytes kill tumor cells? Clin. Cancer Res. 1996, 2, 785–789. [Google Scholar] [PubMed]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Goradel, N.H.; Asghari, M.H.; Moloudizargari, M.; Negahdari, B.; Haghi-Aminjan, H.; Abdollahi, M. Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence. Toxicol. Appl. Pharmacol. 2017, 335, 56–63. [Google Scholar] [CrossRef]
- González-González, A.; Rueda, N.; Alonso-González, C.; Menéndez, J.M.; Martínez-Campa, C.; Mitola, S.; Cos, S. Usefulness of melatonin as complementary to chemotherapeutic agents at different stages of the angiogenic process. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, H.; Gu, C.; Liu, Y.; Shao, J.; Zhu, R.; He, Y.; Zhu, X.; Li, M. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int. J. Mol. Med. 2018, 43, 945–955. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Yin, Y.; Zhang, D.; Xia, P.; Zhu, G. Therapeutic Opportunities in Colorectal Cancer: Focus on Melatonin Antioncogenic Action. BioMed. Res. Int. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Casado, J.; Ruiz, S.M.J.; Zurita, M.S.; González-Puga, C.; Rejón, J.D.; Gila, A.; De Rueda, P.M.; Pavón, E.J.; Reiter, R.J.; et al. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-κβ. J. Pineal Res. 2014, 56, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.L.; Cattley, R.C.; Dunn, C.; Wong, V.; Li, X.; Thurman, R.G. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 1999, 20, 2075–2081. [Google Scholar] [CrossRef]
- Rose, M.L.; Madren, J.; Bunzendahl, H.; Thurman, R.G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 1999, 20, 793–798. [Google Scholar] [CrossRef]
- Rose, M.L.; Germolec, D.; Arteel, G.E.; Schoonhoven, A.R.; Thurman, R.G. Dietary Glycine Prevents Increases in Hepatocyte Proliferation Caused by the Peroxisome Proliferator WY-14,643. Chem. Res. Toxicol. 1997, 10, 1198–1204. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.-H. Melatonin attenuates TNF-alpha and IL-1beta expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal. Res. 2019, 66, e12560. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.D.; Zhang, H.; Xu, Z.S.; Cheng, H.; Shen, W.; Wang, X.P. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: A meta-analysis. Medicine 2018, 97, e10831. [Google Scholar] [CrossRef] [PubMed]
- Sierko, E.; Wojtukiewicz, M. Platelets and Angiogenesis in Malignancy. Semin. Thromb. Hemost. 2004, 30, 95–108. [Google Scholar] [CrossRef] [PubMed]
| SHAM | CRLM | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Melatonin | Glycine | Melatonin + Glycine | Control | Melatonin | Glycine | Melatonin + Glycine | |
| Leukocytes (× 109/L) | 6.5 (6.2; 6.8) | 8.4 (6.4; 9.9) | 8.9 (6.5; 9.6) | 9.2 (7.9; 11.2) | 7.2 (5.6; 9.6) | 8.9 (7.5; 11.6) | 7 (4.6; 11) | 8.6 (7.1; 9.8) |
| Lymphocytes (× 109/L) | 3.5 (3.2; 3.8) | 5.2 (4; 5.4) | 5.1 (3.7; 6.9) | 6.4 (5.1; 6.8) | 4.1 (3.3; 5) | 5.5 (5.1; 7.2) | 3.4 (2.9; 6.2) | 5.3 (4.5; 5.9) |
| Glycine (µmol/L) | 161.1 (146; 247.2) | 201 (171.8; 225.1) | 1480.4 (1307.1; 1675.6) | 1553.9 (1336.8; 1634.9) | 171.1 (167; 210.7) | 188.8 (162.6; 221.7) | 1455.5 (1058.2; 1721.1) | 1548.3 (1345.6; 1803.5) |
| Control | Melatonin | Glycine | Melatonin + Glycine | |
|---|---|---|---|---|
| Change in tumor volume (%) | 108.7 (82.5; 140.9) | 36.8 (28.9; 103.1) | 56.9 (29.9; 112.6) | 52.3 (39.4; 216.9) |
| Tumor MVD (number of vessels/area (mm2) × 10−4) | 3.2 (2.5; 4.7) | 1.8 (1.2; 2.6) | 2.1 (1.8; 3) | 1.1 (0.7; 2.8) |
| Tumor proliferation index (%) | 12.2 (10.7; 17.5) | 7.5 (6.4; 11.3) | 8.3 (7.2; 14.4) | 10.1 (7.4; 13.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvietkauskas, M.; Zitkute, V.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis. Nutrients 2021, 13, 2035. https://doi.org/10.3390/nu13062035
Kvietkauskas M, Zitkute V, Leber B, Strupas K, Stiegler P, Schemmer P. Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis. Nutrients. 2021; 13(6):2035. https://doi.org/10.3390/nu13062035
Chicago/Turabian StyleKvietkauskas, Mindaugas, Viktorija Zitkute, Bettina Leber, Kestutis Strupas, Philipp Stiegler, and Peter Schemmer. 2021. "Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis" Nutrients 13, no. 6: 2035. https://doi.org/10.3390/nu13062035
APA StyleKvietkauskas, M., Zitkute, V., Leber, B., Strupas, K., Stiegler, P., & Schemmer, P. (2021). Dietary Melatonin and Glycine Decrease Tumor Growth through Antiangiogenic Activity in Experimental Colorectal Liver Metastasis. Nutrients, 13(6), 2035. https://doi.org/10.3390/nu13062035









