Free and Total Amino Acids in Human Milk in Relation to Maternal and Infant Characteristics and Infant Health Outcomes: The Ulm SPATZ Health Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Human Milk Sample Collection
2.3. Amino Acid Analyses
2.4. Infant and Maternal Characteristics and Anthropometric Measurements
2.5. Infant Immune Outcome
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Participating Mothers and Their Infants
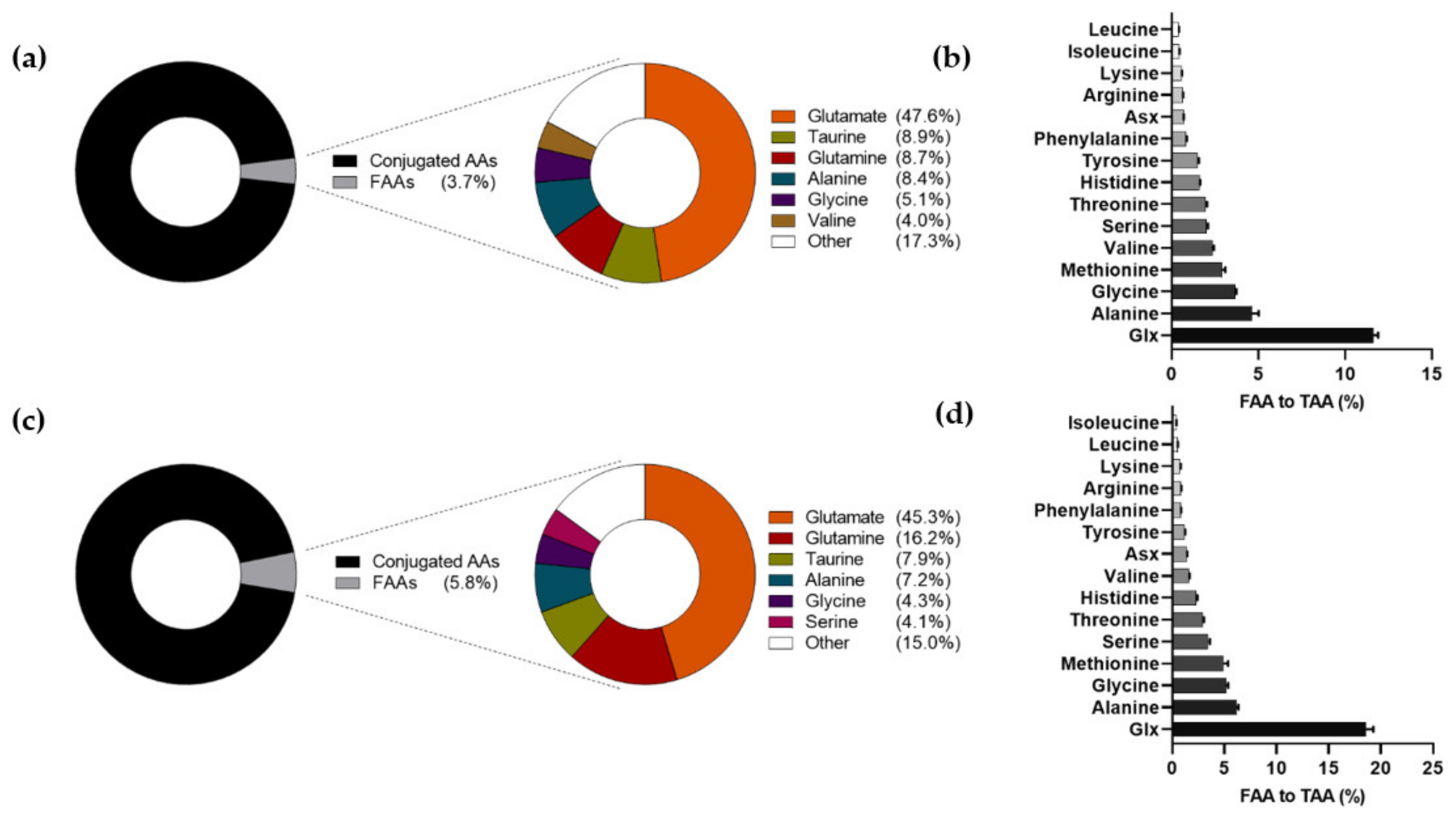
3.2. Amino Acid Levels and Composition in Human Milk at 6 Weeks and 6 Months of Lactation
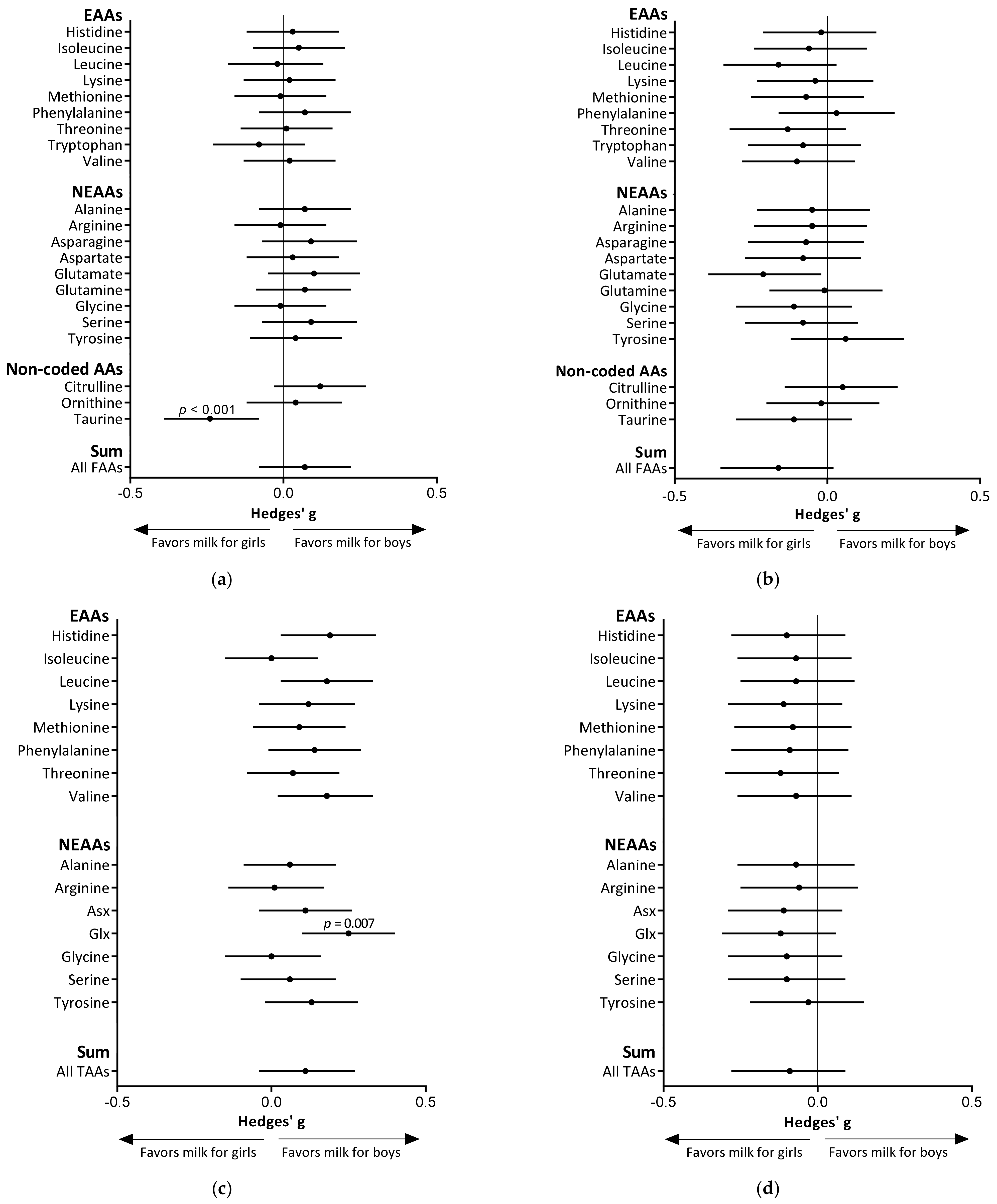
3.3. Infant Sex Differences in Amino Acid Levels in Human Milk
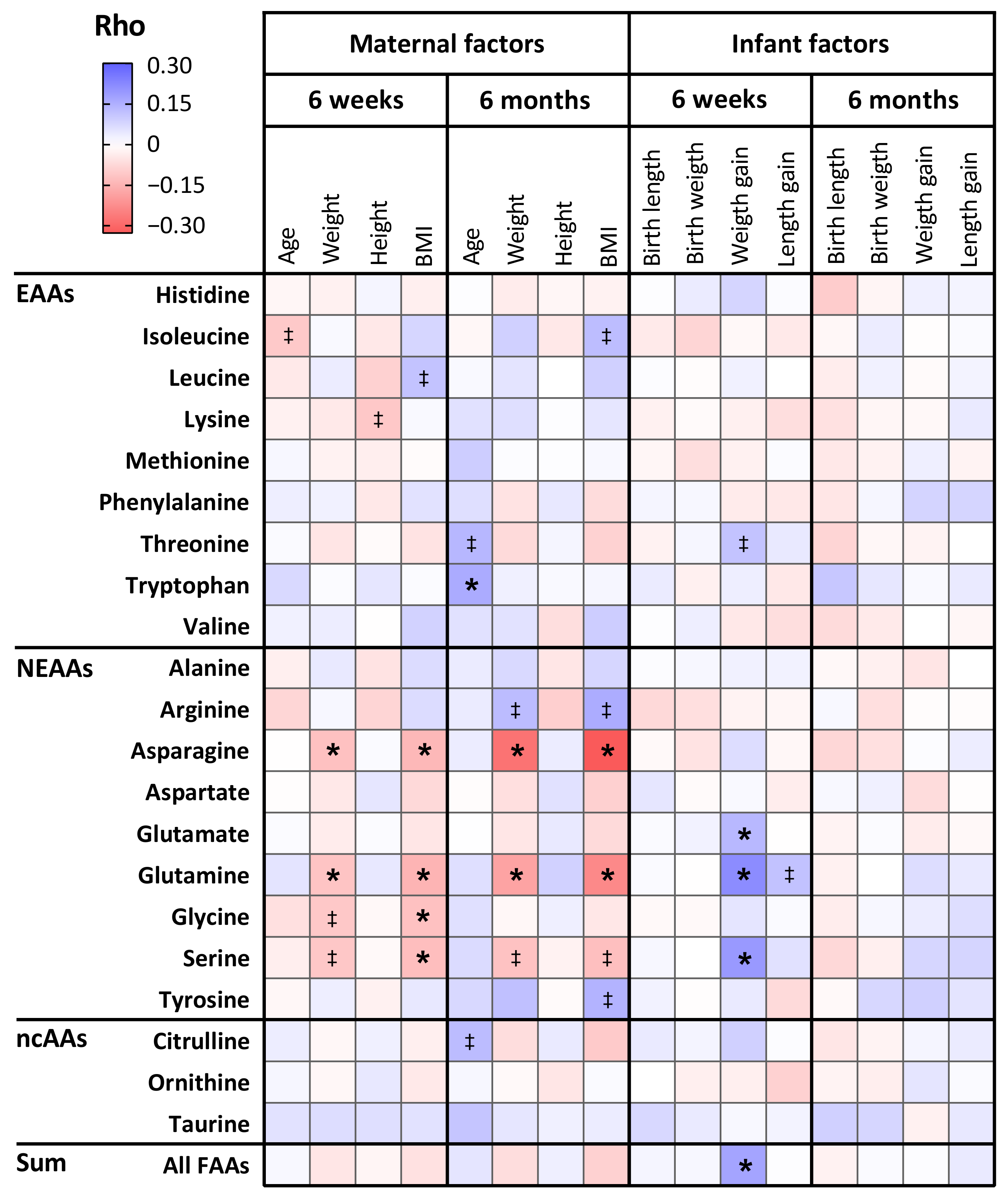
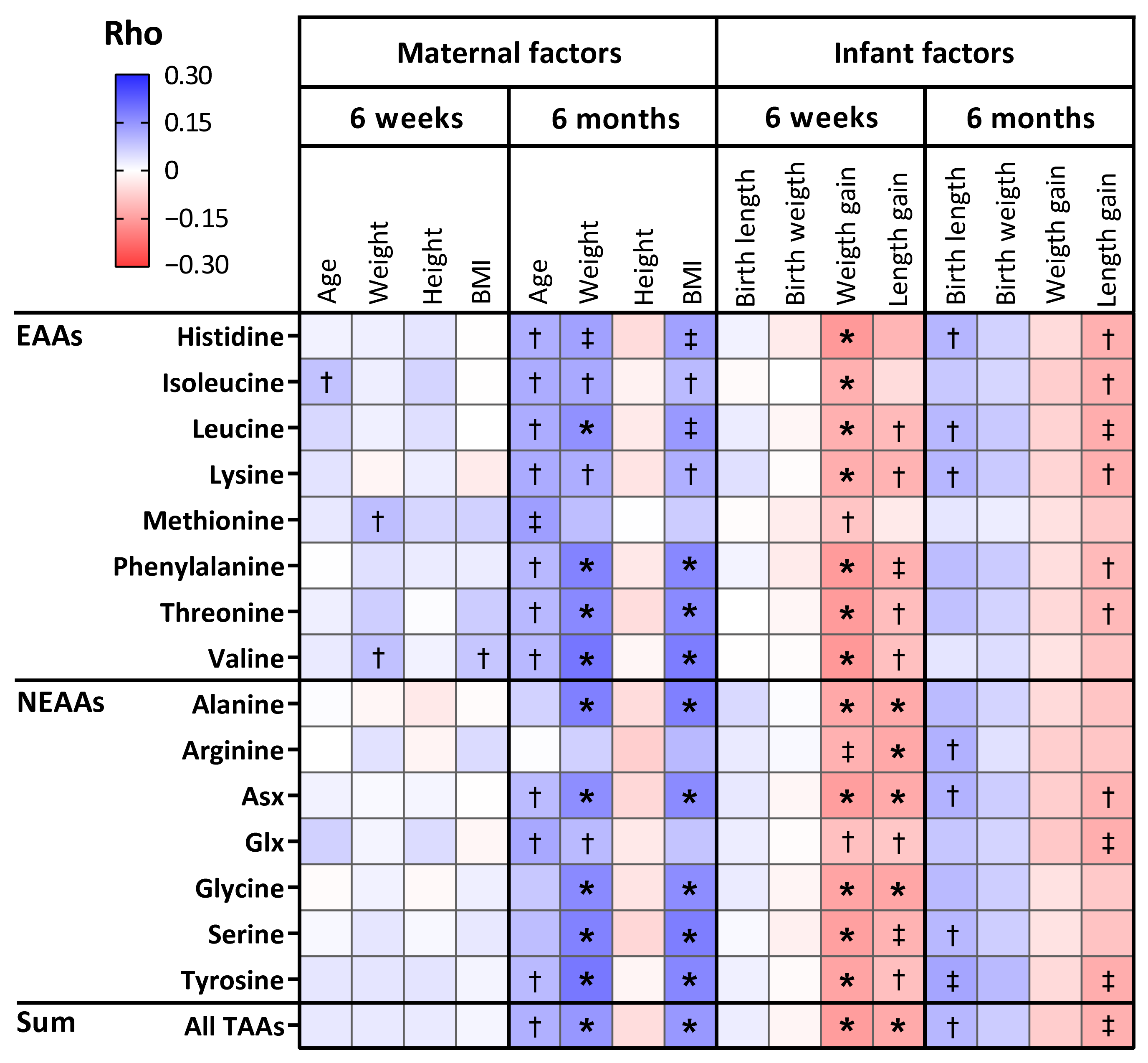
3.4. The Relation Between Maternal Characteristics and Amino Acid Levels in Human Milk
3.5. The Relation Between Amino Acid Levels in Human Milk and Infant Anthropometrics
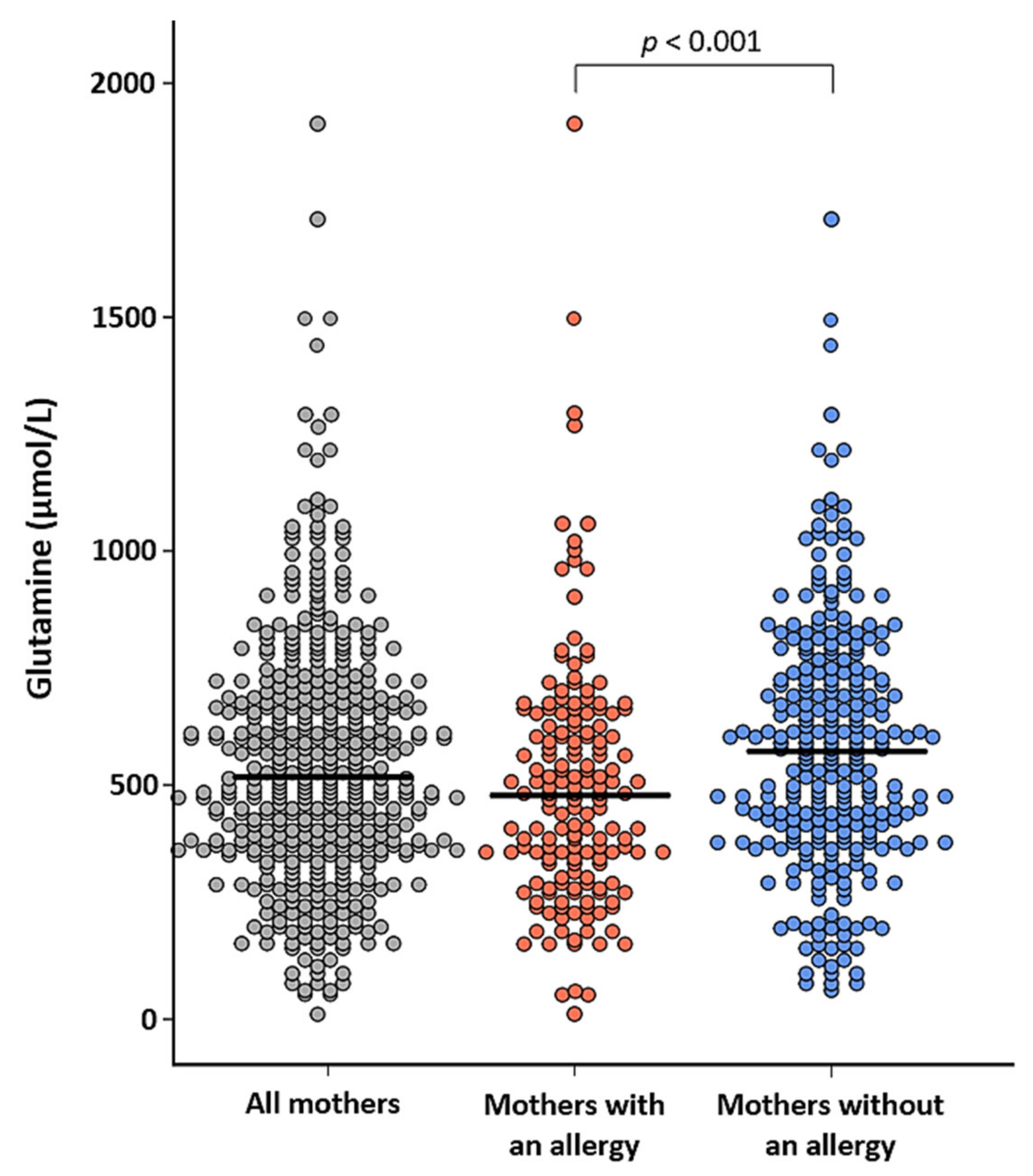
3.6. Associations of Free Amino Acids in Human Milk and Infant Health Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duijts, L.; Jaddoe, V.W.; Hofman, A.; Moll, H.A. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Yao, X.D.; Nasser, L.; Roozrogousheh, A.; Rosenthal, K.L. Breastfeeding behaviors and the innate immune system of human milk: Working together to protect infants against inflammation, hiv-1, and other infections. Front. Immunol. 2017, 8, 1631. [Google Scholar] [CrossRef] [PubMed]
- Tromp, I.; Kiefte-de Jong, J.; Raat, H.; Jaddoe, V.; Franco, O.; Hofman, A.; de Jongste, J.; Moll, H. Breastfeeding and the risk of respiratory tract infections after infancy: The generation r study. PLoS ONE 2017, 12, e0172763. [Google Scholar] [CrossRef]
- Bjorksten, B.; Sepp, E.; Julge, K.; Voor, T.; Mikelsaar, M. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 2001, 108, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory effects of breast milk on food allergy. Ann. Allergy Asthma Immunol. 2019, 123, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, U.M.; Kajosaari, M. Breastfeeding as prophylaxis against atopic disease: Prospective follow-up study until 17 years old. Lancet 1995, 346, 1065–1069. [Google Scholar] [CrossRef]
- Zhu, J.; Dingess, K.A. The functional power of the human milk proteome. Nutrients 2019, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lonnerdal, B. Amino acid profiles in term and preterm human milk through lactation: A systematic review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef] [PubMed]
- Van Sadelhoff, J.H.J.; van de Heijning, B.J.M.; Stahl, B.; Amodio, S.; Rings, E.; Mearin, M.L.; Garrsen, J.; Hartog, A. Longitudinal variation of amino acid levels in human milk and their associations with infant gender. Nutrients 2018, 10, 1233. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodenas, C.L.; Affolter, M.; Vinyes-Pares, G.; De Castro, C.A.; Karagounis, L.G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Amino acid composition of breast milk from urban chinese mothers. Nutrients 2016, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Ruth, M.R.; Field, C.J. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van Sadelhoff, J.H.J.; Perez Pardo, P.; Wu, J.; Garssen, J.; van Bergenhenegouwen, J.; Hogenkamp, A.; Hartog, A.; Kraneveld, A.D. The gut-immune-brain axis in autism spectrum disorders; a focus on amino acids. Front. Endocrinol. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Van Sadelhoff, J.; Wiertsema, S.; Garssen, J.; Hogenkamp, A. Free amino acids in human milk: A potential role for glutamine and glutamate in the protection against neonatal allergies and infections. Front. Immunol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Van Sadelhoff, J.H.J.; Hogenkamp, A.; Wiertsema, S.P.; Harthoorn, L.F.; Loonstra, R.; Hartog, A.; Garssen, J. A free amino acid-based diet partially prevents symptoms of cow’s milk allergy in mice after oral sensitization with whey. Immun. Inflamm. Dis. 2020, 8, 93–105. [Google Scholar] [CrossRef]
- Baldeon, M.E.; Zertuche, F.; Flores, N.; Fornasini, M. Free amino acid content in human milk is associated with infant gender and weight gain during the first four months of lactation. Nutrients 2019, 11, 2239. [Google Scholar] [CrossRef]
- Larnkjær, A.; Bruun, S.; Pedersen, D.; Zachariassen, G.; Barkholt, V.; Agostoni, C.; Mlgaard, C.; Husby, S.; Michaelsen, K.F. Free amino acids in human milk and associations with maternal anthropometry and infant growth. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.; Zittel, T.; Striebel, S.; Reister, F.; Brenner, H.; Rothenbacher, D.; Genuneit, J. Changing societal and lifestyle factors and breastfeeding patterns over time. Pediatrics 2016, 137, e20154473. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using Spss, 2nd ed.; Sage Publications Ltd.: London, UK, 2005. [Google Scholar]
- MacCallum, R.; Widaman, K.; Zhang, S.; Hong, S. Sample size in factor analysis. Psychol. Methods 1999, 4, 84–99. [Google Scholar] [CrossRef]
- Naimi, A.I.; Whitcomb, B.W. Estimating risk ratios and risk differences using regression. Am. J. Epidemiol. 2020, 189, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814S–821S. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M. Citrulline: Is it ready for primetime. Its uses and limitations in neonatal medicine. J. Neonatal. Biol. 2014, 3, 147. [Google Scholar] [CrossRef]
- Vlaardingerbroek, H.; van den Akker, C.; Groof, F.; Hogewind-Schoonenboom, J.E.; Huang, L.; Riedijk, M.A.; van der Schoor, S.R.D.; Huang, Y.; van Goudoever, J.B. Amino acids for the neonate: Search for the ideal dietary composition. NeoReviews 2011, 12, e506–e516. [Google Scholar] [CrossRef]
- Cotter, D.G.; d’Avignon, D.A.; Wentz, A.E.; Weber, M.L.; Crawford, P.A. Obligate role for ketone body oxidation in neonatal metabolic homeostasis. J. Biol. Chem. 2011, 286, 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar] [PubMed]
- Wharton, B.A.; Morley, R.; Isaacs, E.B.; Cole, T.J.; Lucas, A. Low plasma taurine and later neurodevelopment. Arch. Dis. Child. Fetal Neonatal. Ed. 2004, 89, F497–F498. [Google Scholar] [CrossRef][Green Version]
- Watkins, J.B.; Järvenpää, A.L.; Szczepanik-Van Leeuwen, P.; Klein, P.D.; Rassin, D.K.; Gaull, G.; Räihä, N.C. Feeding the low-birth weight infant: V. Effects of taurine, cholesterol, and human milk on bile acid kinetics. Gastroenterology 1983, 85, 793–800. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Taneja, V. Chapter 39—Microbiome: Impact of gender on function & characteristics of gut microbiome. In Principles of Gender-specific Medicine, 3rd ed.; Legato, M.J., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 569–583. [Google Scholar]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Olędzka, G.; Szostak-Węgierek, D.; Weker, H.; Wesołowska, A. Maternal nutrition and body composition during breastfeeding: Association with human milk composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Jung, J.A.; Kim, H.; Jo, A.; Kang, S.; Lee, S.W.; Hyunju, Y.; Kim, J.; Yim, J.G.; Jung, B.M. Macronutrient composition of human milk from korean mothers of full term infants born at 37-42 gestational weeks. Nutr. Res. Pract. 2015, 9, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jochum, F.; Colling, S.; Meinardus, P.; Alteheld, B.; Stehle, P.; Fusch, C. Total glutamine content in human milk is not influenced by gestational age. Acta Paediatr. 2006, 95, 985–990. [Google Scholar] [CrossRef]
- Miller, A.L. Therapeutic considerations of l-glutamine: A review of the literature. Altern. Med. Rev. 1999, 4, 239–248. [Google Scholar] [PubMed]
- Newsholme, P.; Lima, M.M.R.; Procopio, J.; Pithon-Curi, T.C.; Doi, S.Q.; Bazotte, R.B.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef]
- Soeters, P.B.; Grecu, I. Have we enough glutamine and how does it work? A clinician’s view. Ann. Nutr. Metab. 2012, 60, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.; van Zwol, A.; Moll, H.A.; Fetter, W.P.F.; van Elburg, R.M. Glutamine-enriched enteral nutrition in very low-birth-weight infants: Effect on the incidence of allergic and infectious diseases in the first year of life. Arch. Pediatr. Adolesc. Med. 2007, 161, 1095–1101. [Google Scholar] [CrossRef][Green Version]
- Van den Berg, A.; van Elburg, R.M.; Westerbeek, E.A.M.; Twisk, J.W.R.; Fetter, W.P.F. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: A randomized controlled trial. Am. J. Clin. Nutr. 2005, 81, 1397–1404. [Google Scholar] [CrossRef]
- Fujimura, T.; Lum, S.Z.C.; Nagata, Y.; Kawamoto, S.; Oyoshi, M.K. Influences of maternal factors over offspring allergies and the application for food allergy. Front. Immunol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wu, X.; Wan, D.; Yin, Y. Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol. Biochem. 2018, 48, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zheng, G.; Fang, X.; Jiang, J. Effects of glutamine on growth performance of weanling piglets. Czech J. Anim. Sci. 2006, 51, 444–448. [Google Scholar]
- Teixeira, A.D.O.; Nogueira, E.T.; Kutschenko, M.; Rostagno, H.S.; Lopes, D.C. Inclusion of glutamine associated with glutamic acid in the diet of piglets weaned at 21 days of age. Revista Brasileira de Saúde e Produção Animal 2014, 15, 881–896. [Google Scholar] [CrossRef]
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The darling study. Am. J. Clin. Nutr. 1993, 58, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.O.; Ellis, K.J. Infant feeding mode affects early growth and body composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.; Ong, K.K.; Schoemaker, M.H.; van Tol, E.A.F.; Vervoort, J.; Hughes, I.A.; Acerini, C.L.; Dunger, D.B. Breast milk nutrient content and infancy growth. Acta Paediatr. 2016, 105, 641–647. [Google Scholar] [CrossRef]
- Lee, Y.C.; Su, Y.T.; Liu, T.Y.; Tsai, C.M.; Chang, C.H.; Yu, H.R. L-arginine and l-citrulline supplementation have different programming effect on regulatory t-cells function of infantile rats. Front. Immunol. 2018, 9, 2911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Pi, D.; Leng, W.; Wang, X.; Hu, C.A.A.; Hou, Y.; Xiong, J.; Wang, C.; Qin, Q.; Lio, Y. Asparagine preserves intestinal barrier function from lps-induced injury and regulates crf/crfr signaling pathway. Innate Immun. 2017, 23, 546–556. [Google Scholar] [CrossRef]
- Torres, A.; Luke, J.D.; Kullas, A.L.; Kapilashrami, K.; Botbol, Y.; Koller, A.; Tonge, P.J.; Chen, E.I.; Macian, F.; van der Velden, A.W.M. Asparagine deprivation mediated by salmonella asparaginase causes suppression of activation-induced t cell metabolic reprogramming. J. Leukoc. Biol. 2016, 99, 387–398. [Google Scholar] [CrossRef]
- Van Aalderen, W.M. Childhood asthma: Diagnosis and treatment. Scientifica 2012, 2012, 674204. [Google Scholar] [CrossRef]
- Sopi, R.B.; Zaidi, S.I.A.; Mladenov, M.; Sahiti, H.; Istrefi, Z.; Gjorgoski, I.; Lajçi, A.; Jakupaj, M. L-citrulline supplementation reverses the impaired airway relaxation in neonatal rats exposed to hyperoxia. Respir. Res. 2012, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Winnica, D.; Que, L.G.; Baffi, C.; Grasemann, H.; Fiedler, K.; Yang, Z.; Etling, E.; Wasil, K.; Wenzel, S.; Freeman, B.; et al. L-citrulline prevents asymmetric dimethylarginine-mediated reductions in nitric oxide and nitrosative stress in primary human airway epithelial cells. Clin. Exp. Allergy 2017, 47, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Grasemann, H.; Sharma, S.; Winnica, D.; Wasil, K.; Smith, V.; Cruse, M.H.; Perez, N.; Colemon, E.; Scialla, T.; et al. L-citrulline increases nitric oxide and improves control in obese asthmatics. JCI Insight 2019, 4, e131733. [Google Scholar] [CrossRef]
- Van Sadelhoff, J.H.J.; Mastorakou, D.; Weenen, H.; Stahl, B.; Garssen, J.; Hartog, A. Short communication: Differences in levels of free amino acids and total protein in human foremilk and hindmilk. Nutrients 2018, 10, 1828. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Cubero, J.; Sánchez Alarcón, J.; Franco, L.; Rodríguez, A.; Rivero, M.; Barriga, C. Evolution of the circadian profile of human milk amino acids during breastfeeding. J. Appl. Biomed. 2013, 11, 59–70. [Google Scholar] [CrossRef]
- Calkins, K.L.; Sanchez, L.A.; Tseng, C.H.; Faull, K.F.; Yoon, A.J.; Ryan, C.M.; Le, T.; Shew, S.B. Effect of high-dose cysteine supplementation on erythrocyte glutathione: A double-blinded, randomized placebo-controlled pilot study in critically ill neonates. JPEN J. Parenter. Enteral Nutr. 2016, 40, 226–234. [Google Scholar] [CrossRef]
- Herrera, O.; Storm, M.; Tillman, E.; Helms, R. Cysteine supplementation to parenteral nutrition improves red blood cell glutathione concentrations of critically ill preterm neonates. Food Sci. Nutr. 2018, 9, 619–631. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef]
- Tonon, M.K.; de Morais, B.M.; Abrão, F.V.A.C.; Miranda, A.; Morais, T.B. Maternal and infant factors associated with human milk oligosaccharides concentrations according to secretor and lewis phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Much, D.; Brunner, S.; Vollhardt, C.; Schmid, D.; Sedlmeier, E.M.; Brüderl, M.; Heimberg, E.; Bartke, N.; Boehm, G.; Bader, B.L.; et al. Breast milk fatty acid profile in relation to infant growth and body composition: Results from the infat study. Pediatr. Res. 2013, 74, 230–237. [Google Scholar] [CrossRef] [PubMed]
| All 6-Week Samples (n = 671) | All 6-Month Samples (n = 441) | |||
|---|---|---|---|---|
| n | % or Mean | n | % or Mean | |
| Maternal Characteristics | ||||
| Age (Years) | 671 | 33.1 | 441 | 33.5 |
| Pre-Pregnancy BMI | ||||
| Underweight (BMI <18.50) | 15 | 2.3% | 8 | 1.9% |
| Normal (BMI 18.50–24.99) | 411 | 63.3% | 274 | 64.6% |
| Overweight (BMI 25.00–29.99) | 135 | 20.8% | 88 | 20.8% |
| Obese (BMI ≥ 30.00) | 88 | 13.6% | 54 | 12.7% |
| Weight (kg) | 617 | 70.5 | 417 | 69.9 |
| Height (cm) | 653 | 167.6 | 430 | 167.7 |
| Parity (n Births of Foetus ≥ 24 weeks) | ||||
| 0 births | 347 | 51.8% | 224 | 50.8% |
| ≥ 1 birth | 323 | 48.2% | 217 | 49.2% |
| Maternal School Education | ||||
| Low | 40 | 6.1% | 14 | 3.2% |
| Intermediate | 182 | 27.5% | 104 | 24.0% |
| High | 439 | 66.4% | 316 | 72.8% |
| History of Smoking (Ever in Life) | ||||
| Yes | 288 | 43.2% | 169 | 38.5% |
| No | 378 | 56.8% | 270 | 61.5% |
| Maternal Allergy (Either Active or Non-Active) | ||||
| Yes | 225 | 33.7% | 166 | 37.7% |
| No | 443 | 66.3% | 274 | 62.3% |
| Exclusive Breastfeeding at the Time of Sample Collection | ||||
| Yes | 502 | 74.8% | 366 | 83.0% |
| No | 169 | 25.2% | 75 | 17.0% |
| Infant Characteristics | ||||
| Sex | ||||
| Boys | 354 | 52.8% | 232 | 52.6% |
| Girls | 317 | 47.2% | 209 | 47.4% |
| Gestational Age at Birth | ||||
| Early (37–38 weeks) | 239 | 35.6% | 148 | 33.6% |
| Full (39–40 weeks) | 352 | 52.5% | 240 | 54.4% |
| Late (41–42 weeks) | 80 | 11.9% | 53 | 12.0% |
| Method of Birth | ||||
| Vaginal spontaneous | 455 | 67.9% | 312 | 70.7% |
| Elective caesarean | 67 | 10.0% | 36 | 8.2% |
| Emergency caesarean | 86 | 12.8% | 52 | 11.8% |
| Vaginal assisted | 62 | 9.3% | 41 | 9.3% |
| Weight | ||||
| Weight at birth (g) | 670 | 3212.5 | 441 | 3250.1 |
| Weight at 4–5 weeks (g) | 594 | 4365.5 | 416 | 4372.6 |
| Weight at 6–7 months (g) | 554 | 7852.0 | 385 | 7849.1 |
| Length | ||||
| Length at birth (cm) | 669 | 51.1 | 440 | 51.1 |
| Length at 6 weeks (cm) | 592 | 62.9 | 416 | 62.9 |
| Length at 6 months (cm) | 553 | 68.8 | 384 | 68.9 |
| Atopic Dermatitis (AD) | ||||
| Parent-reported diagnosis | 44 | 7.9% | 27 | 7.0% |
| Paediatrician-reported diagnosis | 64 | 12.6% | 37 | 10.5% |
| Parent- and paediatrician-reported diagnosis | 35 | 6.2% | 20 | 5.1% |
| Wheeze Phenotypes | ||||
| Transient wheeze | 47 | 12.1% | 30 | 11.2% |
| Persistent wheeze | 31 | 8.0% | 18 | 6.7% |
| Intermediate wheeze | 31 | 8.0% | 27 | 10.0% |
| Lower respiratory tract infections | 151 | 30.0% | 108 | 31.0% |
| Food allergy | 13 | 2.6% | 7 | 2.0% |
| AA | FAAs | TAAs | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 Weeks (n = 671) | 6 Weeks Restricted (n = 411) | 6 Months (n = 441) | p-Value 1 | 6 Weeks (n = 671) | 6 Weeks Restricted (n = 411) | 6 Months (n = 441) | p-Value 2 | |
| EAAs | ||||||||
| Histidine | 28.2 (9.1) | 28.0 (9.1) | 29.7 (13.9) | 0.944 | 1795.2 (264.3) | 1803.3 (255.6) | 1326.2 (201.4) | <0.001 b |
| Isoleucine | 16.5 (10.3) | 15.9 (9.8) | 12.7 (5.9) | <0.001 b | 4034.8 (599.5) | 4094.8 (609.8) | 3381.8 (461.0) | <0.001 b |
| Leucine | 33.9 (17.8) | 32.8 (15.7) | 34.9 (11.4) | <0.001 a | 8690.4 (1232.6) | 8728.5 (1188.1) | 6791.8 (922.6) | <0.001 b |
| Lysine | 34.9 (24.1) | 32.3 (20.4) | 30.2 (15.0) | 0.333 | 6359.2 (949.1) | 6371.2 (895.8) | 3963.3 (626.2) | <0.001 b |
| Methionine | 27.8 (24.2) | 29.5 (22.7) | 34.6 (22.7) | <0.001 a | 1010.6 (200.7) | 1019.2 (208.4) | 750.4 (220.2) | <0.001 b |
| Phenylalanine | 21.4 (10.1) | 21.3 (10.1) | 16.5 (5.6) | <0.001 b | 2610.9 (422.4) | 2594.4 (382.6) | 2057.7 (305.0) | <0.001 b |
| Threonine | 71.0 (29.3) | 70.8 (30.6) | 97.1 (37.2) | <0.001 a | 3707.5 (578.2) | 3688.9 (552.9) | 3367.3 (507.9) | <0.001 b |
| Tryptophan | 14.6 (7.5) | 14.5 (7.4) | 29.0 (11.8) | <0.001 a | - | - | - | - |
| Valine | 109.7 (58.2) | 110.5 (54.9) | 66.7 (27.4) | <0.001 b | 4669.9 (755.3) | 4678.7 (740.3) | 4198.7 (628.1) | <0.001 b |
| NEAAs/conditionally EAAs | ||||||||
| Alanine | 231.7 (64.8) | 228.2 (62.6) | 242.7 (72.2) | <0.001 a | 5354.5 (988.8) | 5294.8 (864.6) | 3974.9 (639.3) | <0.001 b |
| Arginine | 17.0 (11.3) | 15.9 (9.5) | 17.0 (6.4) | 0.029 | 2924.9 (1048.9) | 2925.1 (1006.3) | 2141.1 (589.8) | <0.001 b |
| Asparagine | 24.9 (14.9) | 25.7 (14.8) | 17.7 (8.6) | <0.001 b | 8558.3 (1324.2) 3 | 8527.1 (1221.1) 3 | 6240.4 (932.3) 3 | <0.001 b |
| Aspartate | 30.9 (18.2) | 30.0 (17.4) | 65.3 (39.1) | <0.001 a | ||||
| Glutamate | 1307.0 (410.3) | 1270.8 (395.3) | 1530.1 (328.5) | <0.001 a | 13,477.7 (1630.0) 4 | 13,533.9 (1589.8) 4 | 11,478.3 (1550.2) 4 | <0.001 b |
| Glutamine | 239.0 (161.0) | 249.9 (162.9) | 548.5 (268.6) | <0.001 a | ||||
| Glycine | 139.1 (40.4) | 138.7 (37.1) | 146.3 (48.5) | 0.056 | 3902.8 (709.3) | 3837.5 (583.5) | 2868.4 (516.4) | <0.001 b |
| Serine | 100.2 (34.6) | 100.0 (33.1) | 137.8 (61.7) | <0.001 a | 5120.4 (817.2) | 5068.5 (719.1) | 4094.4 (626.5) | <0.001 b |
| Tyrosine | 27.5 (20.1) | 28.0 (21.0) | 17.5 (7.8) | <0.001 b | 1897.6 (296.9) | 1896.1 (281.8) | 1506.3 (224.8) | <0.001 b |
| Non-coded AAs | ||||||||
| Citrulline | 10.7 (4.7) | 10.7 (5.0) | 20.8 (6.1) | <0.001 a | - | - | - | - |
| Ornithine | 14.8 (16.7) | 14.1 (15.1) | 15.7 (27.0) | <0.001 a | - | - | - | - |
| Taurine | 243.6 (93.1) | 240.9 (94.3) | 266.2 (106.1) | <0.001 a | - | - | - | - |
| Sum | ||||||||
| All AAs | 2744.5 (645.1) | 2708.5 (627.4) | 3377.1 (676.4) | <0.001 a | 74,114.8 (11,817.4) | 74,062.2 (11,099.8) | 58,140.9 (8951.5) | <0.001 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Sadelhoff, J.H.J.; Siziba, L.P.; Buchenauer, L.; Mank, M.; Wiertsema, S.P.; Hogenkamp, A.; Stahl, B.; Garssen, J.; Rothenbacher, D.; Genuneit, J. Free and Total Amino Acids in Human Milk in Relation to Maternal and Infant Characteristics and Infant Health Outcomes: The Ulm SPATZ Health Study. Nutrients 2021, 13, 2009. https://doi.org/10.3390/nu13062009
van Sadelhoff JHJ, Siziba LP, Buchenauer L, Mank M, Wiertsema SP, Hogenkamp A, Stahl B, Garssen J, Rothenbacher D, Genuneit J. Free and Total Amino Acids in Human Milk in Relation to Maternal and Infant Characteristics and Infant Health Outcomes: The Ulm SPATZ Health Study. Nutrients. 2021; 13(6):2009. https://doi.org/10.3390/nu13062009
Chicago/Turabian Stylevan Sadelhoff, Joris H. J., Linda P. Siziba, Lisa Buchenauer, Marko Mank, Selma P. Wiertsema, Astrid Hogenkamp, Bernd Stahl, Johan Garssen, Dietrich Rothenbacher, and Jon Genuneit. 2021. "Free and Total Amino Acids in Human Milk in Relation to Maternal and Infant Characteristics and Infant Health Outcomes: The Ulm SPATZ Health Study" Nutrients 13, no. 6: 2009. https://doi.org/10.3390/nu13062009
APA Stylevan Sadelhoff, J. H. J., Siziba, L. P., Buchenauer, L., Mank, M., Wiertsema, S. P., Hogenkamp, A., Stahl, B., Garssen, J., Rothenbacher, D., & Genuneit, J. (2021). Free and Total Amino Acids in Human Milk in Relation to Maternal and Infant Characteristics and Infant Health Outcomes: The Ulm SPATZ Health Study. Nutrients, 13(6), 2009. https://doi.org/10.3390/nu13062009






