Abstract
Excessive body fat at birth is a risk factor for the development of childhood obesity. The aim of the present systematic review with meta-analysis was to evaluate the effect of lifestyle interventions in pregnant women with overweight or obesity on neonatal adiposity. The PubMed, Embase, Web of Science, Scopus, and LILACS databases were used as information sources. Original articles from randomized clinical trials of lifestyle intervention studies on pregnant women with excessive body weight and the effect on neonatal adiposity were considered eligible. The risk of bias was assessed using Cochrane criteria. The meta-analysis was calculated using the inverse variance for continuous data expressed as mean difference (MD), using the random effect model with a 95% confidence interval (CI). The outcomes were submitted to the GRADE evaluation. Of 2877 studies, four were included in the qualitative and quantitative synthesis (n = 1494). All studies were conducted in developed countries, with three including pregnant women with overweight or obesity, and one only pregnant women with obesity. The interventions had no effect on neonatal adiposity [Heterogeneity = 56%, MD = −0.21, CI = (−0.92, 0.50)] with low confidence in the evidence, according to GRADE. Studies are needed in low- and medium-developed countries with different ethnic-racial populations. PROSPERO (CRD42020152489).
1. Introduction
The prevalence of childhood obesity is characterized as a pandemic and constitutes one of the greatest public health challenges of the 21st century [1]. In the period from 2000 to 2018, the number of children with overweight or obesity up to five years of age worldwide increased from 30.1 to 40.1 million, an increase of 33.2% in the period [2,3]. According to the World Health Organization (WHO), approximately 340 million children and adolescents aged 5–19 years were overweight in 2016 [4].
Increasing trends in child overweight are taking place in most world regions, not only in high-income countries, where prevalence is the highest (15% in 2011). The epidemic has been growing most rapidly in low- and middle-income countries, particularly in Northern and Southern Africa, the Middle East, and the Pacific Islands. In Africa, the estimated prevalence of overweight in children younger than 5 years increased from 4% in 1990 to 7% in 2011 and is expected to reach 11% in 2025. The prevalence of overweight is lower in Asia (5% in 2011), but the number of affected children is higher compared with Africa (17 and 12 million, respectively). A systematic review showed that in Latin America, the prevalence of overweight in children aged 0–19 years is about 25% [5,6,7].
Excess body weight can have immediate consequences for the physical and mental health of children and adolescents. It is considered a risk factor for developing chronic non-communicable diseases, such as obesity, diabetes, and cardiovascular diseases, culminating in premature death in adulthood. In addition, childhood obesity can negatively influence children’s learning and socialization in the school environment due to the stigma associated with being overweight [8,9].
Body composition at birth is an intrauterine growth marker [10]. The excessive accumulation of body fat at birth is considered a risk factor for the development of childhood obesity [11]. Data from observational studies suggest a positive association between the maternal preconception Body Mass Index (BMI) and the child’s birth weight and body composition parameters [12,13]. On the other hand, adequate weight gain during pregnancy promotes a better balance in the production of maternal adiponectin and leptin, important adipokines involved in the neonate’s metabolic programming for adiposity [13,14].
Opportunities to prevent and control obesity, and the chronic diseases associated with being overweight, occur during multiple stages of life. However, early interventions provide better chances of prevention, including prenatal and postnatal care, maternal nutrition, and the reduction of environmental exposure to risk factors even in the intrauterine period, a critical stage of development for health throughout life [15,16].
Evidence suggests that preconception maternal excessive body weight is associated with greater adiposity in children [17]. Recent studies have investigated the developmental origins of health and disease [18,19,20,21,22,23,24,25], with the adiposity of the newborn being a significant predictor of morbidity in the neonatal period and throughout life [26]. This review aimed to assess the effect of lifestyle interventions in pregnant women with overweight or obesity on neonatal adiposity. Additionally, birth weight, neonatal fat-free mass, and gestational weight gain were evaluated as secondary outcomes.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Tables S1 and S2) [27] and Cochrane Handbook for Systematic Reviews of Interventions, v6.1, UK [28], were used to develop the present study. The study was registered on the International Prospective Registry of Systematic Reviews—PROSPERO database (CRD42020152489). The following guiding question was considered: What is the effect of nutritional interventions, combined or not with the practice of physical activity, on the adiposity of the newborns of pregnant women with overweight or obesity?
2.1. Eligibility Criteria
2.1.1. Type of Study
Original articles covering lifestyle intervention studies in pregnant women with overweight or obesity and the effect on neonatal adiposity, published in English, Portuguese or Spanish, published up to 28 October 2019, were considered eligible. Non-randomized clinical trials and quasi-experimental studies, prospective or retrospective observational cohort studies, cross-sectional studies, cases and case series, and experimental studies involving animals were not considered eligible for inclusion, as well as unpublished articles, abstracts or dissertations and theses.
2.1.2. Study Population
Pregnant women ≥18 years of age, preconception BMI ≥ 25 kg/m2, singleton pregnancy, gestational age at recruitment up to 20 weeks, follow-up period up to 36 weeks and 6 days. Studies that included women with type 1 or type 2 diabetes prior to pregnancy or that had an early diagnosis of gestational diabetes prior to recruitment were excluded.
2.1.3. Intervention
Nutritional interventions based on dietary guidelines combined or not with the incentive of physical activity were considered. Further details about the design and the intervention were obtained from all included studies.
2.1.4. Outcomes
Primary Outcome
The direct or indirect measurement of the infants’ body fat was considered, expressed in absolute (mass) or relative (percentage) values, performed up to 28 days after the birth. Fat mass and fat-free mass are considered to be more sensitive markers of intrauterine growth compared to indirect measures of adiposity such as body mass index and absolute values of anthropometric measures [29]. Furthermore, the neonatal period corresponds to fetal exposure to prenatal growth factors and the first peak of body fat accumulation [30].
In the qualitative analysis of the studies included, possible confounding factors were considered in the expression of the results. Additionally, in the quantitative analysis, it was verified whether there was a change in the effect on the outcome according to the method of measuring adiposity.
Secondary Outcomes
Secondary outcomes considered were birth weight; neonatal fat-free mass, expressed as mass or relative percentage; and maternal gestational weight gain.
2.2. Information Sources and Search
The databases PubMed (MEDLINE), Embase, Web of Science, Scopus and the Latin American and Caribbean Literature in Health Sciences (LILACS) were searched to identify eligible articles published up to 28 October 2019. The options “Advance Search” and “All fields” were selected: (((((Pregnancy OR Pregnancies OR Gestation OR Gravidity OR Pregnant) AND (Overweight OR Obesity OR Obese)))) AND ((Diet OR Diets OR Dietary OR Nutrition OR “Prenatal Nutritional Physiological Phenomena” OR “Life Style” OR “Life Styles” OR Lifestyle OR Lifestyles))) AND (((Adiposity OR “Body Composition” OR “Body Compositions”) AND (“Infant, Newborn” OR Newborns OR Newborn OR Neonate OR Neonates OR Offspring))). The search strategy adapted for each database is available in full as Supplementary File S1.
2.3. Study Selection
The Rayyan® reference manager software (Qatar Computing Research Institute, HBKU, Doha, Qatar, v.1.0) [31] was used for the storage and management of the publications. After eliminating duplicate articles, the titles and abstracts were read by two researchers independently (Naiara Franco Baroni and Nayara Ragi Baldoni), minimizing possible bias in the selection and exclusion of the studies. In this stage, contact was made via e-mail with the authors to formally request the publications in full [32,33]. In the eligibility stage, a third researcher (Lívia Castro Crivellenti.) assessed the possible conflicts and, by consensus, the articles to be included in the review were defined. Finally, the list of references of the included articles was consulted as an additional source of eligible studies.
2.4. Data Extraction
Data extraction was performed separately by two researchers (Naiara Franco Baroni and Nayara Ragi Baldoni) using a standardized form relating the coding of variables that were organized in the respective Excel spreadsheets, namely: identification data (title, authors, date of publication and place of investigation); study model, sample size and characteristics of the study population (preconception BMI, age, ethnicity and gestational age at delivery); details of the intervention (type, method, strategy, professional that performed it, frequency and period of follow-up in gestational weeks); primary outcome assessment method (neonatal adiposity); birth weight; neonatal fat-free mass; and gestational weight gain.
2.5. Risk of Bias Assessment
The risk of bias in each study was assessed in duplicate (Naiara Franco Baroni and Nayara Ragi Baldoni.) with the aid of a standardized form [34] and the RevMan® software (Copenhagen, Denmark) v5.3 [35]. The Cochrane Handbook for Systematic Reviews of Interventions tool [Risk of bias 2 (RoB 2)] [28] was used as a reference.
2.6. Synthesis of the Data
After reading and including the studies, the methods used were evaluated and the data were extracted and synthesized from the meta-analysis. The meta-analysis was calculated using the inverse variance for continuous data expressed as mean difference (MD), using the random effect model with a 95% confidence interval (CI). For the analysis of the main outcome, data were also considered according to the measurement method. The quantitative synthesis was performed using the RevMan® software v5.3 [35]. The heterogeneity or inconsistency of the studies, estimated through I², of up to 50% were considered [28]. Secondary outcomes were assessed and presented as Supplementary Materials.
The outcomes were submitted to Grading of Recommendations Assessment, Development and Evaluation (GRADE) [36], considering five factors (reporting bias, inconsistency, indirectness of evidence, imprecision and other considerations such as publication bias) to assess the quality of the body of evidence, using the GRADE Profiler® software Hamilton, ON, Canada, v1 [37]. Decisions to decrease confidence in effect estimates were justified using footnotes and comments of importance for each outcome assessed. The evaluation of heterogeneity and risk of bias was used, with the evidence quality only lowered if risk of bias was detected.
3. Results
3.1. Search Results
All publications identified were in the English language. One thousand seven hundred ninety-nine publications were screened after removing duplicates. The selection of studies consisted of reading the titles and abstracts, with a total of 14 studies screened (Reports sought for retrieval). Of these, nine were eligible (Reports assessed for eligibility). After reading them in full, five were excluded. In total, four studies were included in the qualitative and quantitative synthesis [37,38,39,40]. The process of identification and selection of articles included is shown in Figure 1.
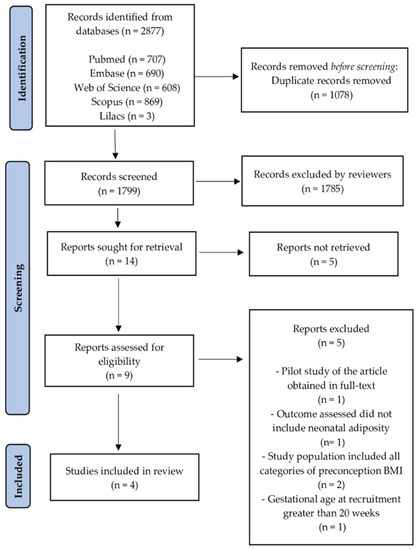
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram: identification and selection of studies process. BMI: body mass index.
3.2. Characteristics of the Studies
All studies included were conducted in developed countries: Dodd et al. [38] in Australia; Gallagher et al. [39] and van Horn et al. [40] in the USA; and van Poppel et al. [41], a multicentric study, in the United Kingdom, Ireland, Netherlands, Austria, Poland, Italy, Spain, Denmark and Belgium. Of these, three used a 1:1 proportion allocation between the treatment groups [38,39,40], and one employed a 3:1 proportion [41]. The articles were published between 2016 and 2019. Table 1 summarizes the characteristics of the included studies.

Table 1.
Characteristics of the included studies.
Dodd et al. [38], Gallagher et al. [39] and van Horn et al. [40] included pregnant women with overweight or obesity in the study population (preconception BMI ≥ 25 kg/m2). In the study by van Poppel et al. [41] only pregnant women with obesity (preconception BMI ≥ 30 kg/m2) were included. The mean age of pregnant women at the baseline ranged from 24.2–37.8 years in the intervention group (IG) and 24.6–38.5 years in the control group (CG). The terms to designate ethnicity varied, however, it was possible to suggest a higher frequency of white women (IG 46 to 90% CG 48 to 91.3%).
Among the studies included, interventions based on dietary guidelines combined with regular practice of physical activity were considered, mentioned as lifestyle interventions in this review. In the study by van Horn et al. [40], the content of the intervention also included guidance on increasing sleep time. All clinical trials included applied strategies that aimed to stimulate the empowerment and autonomy of the pregnant women to achieve the goals and were monitored by the researchers.
Two studies used individualized interventions associated with the incentive to participate in groups: Gallagher et al. [39] and van Horn et al. [40], derived from the Lifestyle Intervention for Two (LIFT) study and the Maternal-Offspring Metabolics: Family Intervention Trial (MOMFIT) study, respectively.
Two other studies carried out individualized interventions with remote monitoring: Dodd et al. [38] and van Poppel et al. [41], derived from the Limiting weight gain during pregnancy (LIMIT Study) and Vitamin D and Lifestyle Intervention for Gestational Diabetes Mellitus Prevention (DALI Study), respectively. It should be noted that all studies included adopted appropriate gestational weight gain as the primary outcome.
In three studies [38,39,40], the pregnant women in the intervention group received an individualized food plan with lifestyle change goals determined from healthy eating guidelines. A nutritionist was present in at least one session during the follow-up period. While in one of the studies [41] the pregnant women in the intervention group received lifestyle counseling through key messages applied by a coach. In all the studies included, the first intervention session was conducted at the beginning of the second gestational trimester, however, there was no information about the mean gestational age at the time. Only two studies discussed adherence to the intervention [39,40]. The treatment of pregnant women in the control group consisted of the usual prenatal care of the health services [38,41] only, or complemented with a general approach of the research team on healthy habits during pregnancy without the establishment of weight gain goals [39,40].
Two methods of measuring neonatal adiposity were identified: infant air displacement plethysmography (ADP) estimated by the PEA POD® system (COSMED USA, Inc., Concord, CA, USA) used by Gallagher et al. [39] and van Horn et al. [40] (n = 389); and the anthropometric model for estimating children’s body composition proposed by Deierlein et al. [42] adopted by Dodd et al. [38] and van Poppel et al. [41] (n = 1105).
Additional outcomes of birth weight (n = 607) and gestational weight gain (n = 651) were assessed by Gallagher et al. [39], van Horn et al. [40] and van Poppel et al. [41]; a third additional outcome was the newborn’s fat-free mass (n = 1296) assessed by Dodd et al. [38], Gallagher et al. [39] and van Poppel et al. [41].
3.3. Risk of Bias Assessment
The overall risk assessment consisted of a high risk of bias. Five domains were evaluated in each study (Table 2): Randomization process: “Some concerns” were attributed to one study [39], because not enough information was reported. Deviations from the intended intervention: “Some concerns” were attributed to one study [38], because no details on blinding were reported. Missing outcome data: Considering per-protocol analysis, a “low” risk of bias was attributed to all studies. Measurement of the outcome: Some concerns were attributed to the studies [38,41], because they did not explain the blinding in the outcome assessment. It should be highlighted that two studies [39,40] were evaluated as “high” risk of bias, because they did not consider smoking or exposure to tobacco during pregnancy as an adjustment factor to statistically determine the results. Selection of the reported results: All studies were attributed a “low” risk of bias [38,39,40,41].

Table 2.
Sources of bias and risk classification in each included study.
3.4. Effect of the Interventions
The lifestyle interventions with pregnant women had no effect on neonatal adiposity [I2 = 56%, n = 1494, MD = −0.21, CI = (−0.92, 0.50)]. Although the results suggest a positive effect trend, since the percentage of body adiposity was lower in the newborns in the intervention groups, the differences compared to the control groups were not significant (Figure 2). The analysis stratified by the outcome measurement method indicated the same result (Figure 3). Accordingly, the results of the meta-analyses are equal with a moderate degree of heterogeneity.
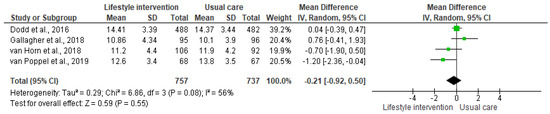
Figure 2.
Effect of the lifestyle interventions in pregnant women with excessive body weight on neonatal adiposity [38,39,40,41]. SD = standard deviation; CI = confidence interval; Tau2 = Tau-squared test; Chi2 = Chi-squared test; Df = difference; P = p value; I2 = heterogeneity; Z = Z test.
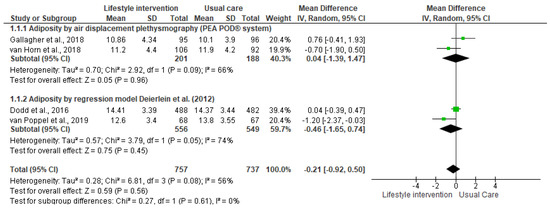
Figure 3.
Effect of the lifestyle interventions in pregnant women with excessive body weight on neonatal adiposity according to the assessment method [38,39,40,41]. SD = standard deviation; CI = confidence interval; Tau2 = Tau-squared test; Chi2 = Chi-squared test; Df = difference; P = p value; I2 = heterogeneity; Z = Z test.
The analysis of secondary outcomes showed a positive effect of the intervention in reducing gestational weight gain considering the mean difference between the treatment groups [I2 = 0%, n = 651, MD = −1.97 CI = (−2.7, −1.24)] (Supplementary Figure S1) however, no effect of the intervention was observed regarding the neonatal outcomes of birth weight [I2 = 55%, n = 607, MD = 23.26, CI = (−102.73, 149.24)] (Supplementary Figure S2) and the fat-free mass [I2 = 50%, n = 1296, MD = −0.68, CI = (−72.87, 71.51)] (Supplementary Figure S3).
The GRADE evaluation indicated low confidence in the evidence regarding the effect of the intervention on neonatal adiposity, with a very serious risk of bias. However, moderate confidence was observed in relation to the secondary outcomes, with a serious risk of bias (Supplementary Figure S4).
4. Discussion
The present systematic review study with meta-analysis evaluated randomized clinical trials of lifestyle interventions in pregnant women with overweight or obesity, and the effects on neonatal adiposity. Four studies were included in the analyses, all conducted in developed countries with similar population characteristics and performance scenarios. There was no effect of the interventions on neonatal adiposity with low confidence, according to GRADE evaluation. It is possible that new studies could modify the results found.
Previous systematic reviews of interventions in the first 1000 days of life, between conception and 24 months, have evaluated excessive body weight as the outcome among children and adolescents aged six months to 18 years. These studies showed a high degree of heterogeneity due to the variability of the types of intervention and methods of anthropometric measurements [43,44]. Therefore, there is no evidence regarding the influence of interventions during pregnancy and the prevention of obesity in children. However, the researchers stress the importance of future studies operating at a systemic and environmental level to prevent childhood obesity.
A systematic review of observational studies suggested that exposure at the first 1000 days of life to preconception maternal BMI, excessive gestational weight gain, prenatal tobacco exposure, high birth weight, and accelerated infant weight gain are relevant risk factors for obesity in late childhood [45]. Important to note that two studies included in the present review [39,40] did not consider smoking or exposure to tobacco as a confounder in the analyses. In addition, the secondary outcomes of birth weight and fat-free mass did not differ between the treatment groups, with low confidence in the results. However, there was less weight gain among the pregnant women in the intervention group when compared to the control group, with moderate confidence in the results. However, it is not possible to affirm that it was clinically significant since the mean weight gain between the groups was considered, and not the proportion of women with adequate weight gain according to the Institute of Medicine (IOM) recommendation [46].
A systematic review and meta-analysis of observational studies assessed the dose-response association between maternal preconception BMI and childhood obesity in children aged between one and 18 years. The evidence showed significantly increased chances of childhood obesity with an increase in maternal BMI, where the association was stronger among women with preconception obesity than among those that were overweight prior to conception, 264% and 89%, respectively [47].
A meta-analysis of clinical trials of lifestyle interventions with overweight or obese pregnant women showed that even with a significant reduction in weight gain in the intervention group, no impact was found on other outcomes considered as risks for childhood obesity, including birth weight [48]. Preconception BMI ≥ 30 kg/m2 may be more relevant than excessive weight gain during pregnancy, as it favors greater neonatal adiposity and, in the long term, childhood obesity and the emergence of chronic non-communicable diseases. In light of epigenetics, this is due to an inverse relationship between the increase in preconception BMI and insulin sensitivity, which allows the fetus a greater availability of energy nutrients, causing a greater impact on neonatal outcomes [49,50,51,52,53]. Accordingly, using mixed categories of BMI in the same population may have interfered with the outcome results of the present review.
It is considered that initiating interventions prior to conception in women of reproductive age who have obesity is the most appropriate strategy for metabolic control before, during, and after pregnancy. With this, there is a greater chance of breaking with the intergenerational cycle of obesity, enabling better maternal and fetal health outcomes in the short and long terms [54,55,56,57,58,59]. In addition, for greater effectiveness of the interventions, the adoption is recommended of expanded approaches beyond the health sector, applied to the ethnic-socio-cultural context of different populations [60,61,62,63].
The global epidemic of obesity has led to an increase in women of reproductive age with obesity. The maternal and child double burden of malnutrition in low-income and middle-income countries (LMICs) encompasses both undernutrition and a growing problem with overweight and obesity [64]. Excessive body weight is frequent among pregnant women from LMICs in Europe, the eastern Mediterranean region, and the Americas. In Africa, obesity rates among pregnant women ranged from 0.7% to 26.8% [65]. It is assumed that using mixed categories of BMI in the study population may have interfered with the findings of this systematic review. In addition, the characteristics of the population and study scenarios may have influenced the results, with studies conducted in low and medium development countries and at other levels of health care possibly producing different results from those observed.
Methods of Measuring the Newborn’s Body Composition
Two methods of measuring for estimative the newborn’s body composition were identified: air displacement plethysmography (ADP, PEA POD® system), used by Gallagher et al. [39] and van Horn et al. [40], and the anthropometric model proposed by Deierlein et al. [42], adopted by Dodd et al. [38] and van Poppel et al. [41]. The meta-analysis stratified by the measurement method also did not indicate any effect of the intervention on neonatal adiposity.
There are many methods available to assess body composition at birth and all present limitations. The ADP is a densitometry technique. By definition, the density of the whole body is body mass divided by body volume. ADP measurement procedures are performed in air, compressed under isothermal conditions, using gas Boyle’s Law and Poisson’s Law to determine body volume. Body density is then used in a two-compartment model, fixed density of fat (0.9007 g/mL), to calculate the percentage of fat, fat mass, and fat-free mass, age, and sex-specific density values. It is considered the gold standard, that is, the reference method, with its main advantage being high accuracy. However, it also has a high cost [66,67,68,69,70,71].
Deierlein et al. [42] determined an anthropometric model, using backward stepwise linear regression, including infant gender/sex, age, race/ethnicity, weight, and skinfold thickness measurements showing 81% agreement in the estimates in relation to the PEA POD® system (ADP) in a multi-ethnic population of term infants at 1–3 days post-delivery. The final statistical model that predicted neonatal fat mass (kg) was: (−0.012 − 0.064 × gender + 0.024 × day of measurement post-delivery − 0.150 × weight (kg) + 0.055 × weight (kg)2 + 0.046 × ethnicity + 0.020 × sum of three skinfold thicknesses (triceps, subscapular, and thigh)).
The authors highlighted the following limitations of the model: it estimates only the amount of subcutaneous adipose tissue in the central and peripheral regions, not being possible to obtain information on the amount of visceral fat, and has a limited ability to predict the amount of fat mass in small and large for gestational age newborns. On the other hand, it is the only anthropometric model for estimating the newborn’s body composition that considers ethnicity as one of the predictor variables. It should be emphasized that, regarding the genetic aspect, ethnicity can influence the amount of fat-free mass of the newborn, and the ratio between fat mass and fat-free mass of body fat at birth is an important characteristic to assess potential metabolic risks in childhood and throughout life [72].
In general, anthropometric models has low accuracy, however, greater applicability and low cost, being the most feasible in population studies. Although the need for an alternative to ADP is recognized, the performance of the predictive model compared to this technology is negatively evaluated, as it can overestimate the fat mass values in the newborn [73,74].
When adopting the predictive model, Dodd et al. [38] clearly reported the time elapsed between the birth and the measurements. In addition, they calculated the intra-observer variation (0.55–0.88), which can be considered a way to reduce the method’s limitation regarding possible variability. The study by van Poppel et al. [41] was the only one to present any significant effect of the intervention on neonatal adiposity, since the neonates in the intervention group had a lower percentage of body fat mass compared to the newborns in the control group. However, it was observed that no technique was adopted to reduce the limitations of the method in a population of unrepresentative size.
The use of predictive models to estimate neonatal adiposity in population studies is considered valid, emphasizing the need to apply techniques that reduce their limitations. Therefore, to advance the understanding of how early life exposures affect obesity and metabolic risk, improvement in the accuracy and standardization of technologies to measure infant body composition are greatly needed [75].
5. Potentials and Limitations
Limitations at the level of the studies were characterized regarding the performance scenario, all conducted in developed countries. In addition, the use of mixed BMI categories in the study population made it impossible to analyze by subgroup. The use of different methods for measuring neonatal adiposity was considered a limitation related to the outcome, as it may have interfered with the findings. Accordingly, to minimize this limitation, it was decided to present the meta-analysis stratified by the method of measuring body composition. Moreover, it is possible that new studies could modify the results found since the search was conducted at the end of 2019.
The development of a comprehensive search strategy and the use of the PRISMA and Cochrane review guidelines were considered strengths of this review. However, the period elapsed after the last search carried out in the databases and the non-inclusion of gray literature as a source of information, especially the registration databases of clinical trial protocols, characterizing a limitation related to the risk of publication bias.
6. Conclusions
It was concluded that there is no evidence for the effect of the lifestyle interventions in pregnant women with overweight or obesity on neonatal adiposity, fat-free mass, and birth weight. While there was a positive effect of the intervention on the mean gestational weight gain in the study population. Further studies are needed, especially in countries of low and medium socioeconomic development with different ethnic-racial populations, aimed at encouraging the consumption of a healthy diet and the regular practice of physical activity in women of reproductive age before, during, and after pregnancy. Studies should present the outcome according to the category of preconception BMI, avoiding confusion bias. New studies could contribute to the definition of effective interventions in the prevention of childhood obesity and, consequently, support public health actions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13061903/s1. Supplementary File S1: Search strategy generated in the databases from the terms and between constituent terms of PICO. Table S1: PRISMA 2020 Checklist. Table S2: PRISMA Abstract Checklist. Figure S1: Effect of the lifestyle intervention with overweight or obese pregnant women on gestational weight gain; Figure S2: Effect of the lifestyle interventions in pregnant women with excessive body weight on birth weight; Figure S3: Effect of the lifestyle interventions in pregnant women with excessive body weight on the fat-free mass of the newborn; Figure S4: Summary of findings: The effect of the lifestyle intervention with overweight/obese pregnant women in relation to the neonatal adiposity.
Author Contributions
N.F.B. was responsible for the design, acquisition, analysis and interpretation of the data. N.R.B., G.C.S.A., L.C.C. and G.C.B. were responsible for the acquisition, analysis and interpretation of the data. N.F.B., N.R.B., G.C.S.A., L.C.C. and G.C.B. wrote the first draft of the manuscript. D.S.S. was responsible for the for the design, interpretation of the data, and critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordination for the Improvement of Higher Education Personnel (CAPES). The São Paulo Research Foundation (FAPESP 2017/18980-2). The National Council for Scientific and Technological Development (CNPq 406000/2018-2). The funding agencies provided financial support through scholarships to the authors N.F.B. (CAPES), L.C.C. (FAPESP), and for the development of the study (CNPq).
Institutional Review Board Statement
PROSPERO (CRD42020152489): https://www.crd.york.ac.uk/prospero/#searchadvanced (accessed on 12 May 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets arising from the study might be available upon reasonable request from the corresponding author Daniela S. Sartorelli.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Report of the Commission on Ending Childhood Obesity—Implementation Plan: Executive Summary. Available online: https://www.who.int/end-childhood-obesity/publications/echo-plan-executive-summary/en/ (accessed on 23 May 2021).
- World Health Organization (WHO); UNICEF; The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2018: Edition of the Joint Child Malnutrition Estimates. Available online: https://www.who.int/nutgrowthdb/estimates2017/en/ (accessed on 24 May 2021).
- Global Nutrition Report. Action on Equity to End Malnutrition. Available online: https://globalnutritionreport.org/reports/2020-global-nutrition-report/ (accessed on 24 May 2021).
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 May 2021).
- World Health Organization (WHO). Taking Action on Childhood Obesity Report. Available online: https://www.who.int/end-childhood-obesity/publications/taking-action-childhood-obesity-report/en/ (accessed on 23 May 2021).
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries Series: Maternal and Child Nutrition 1. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Rivera, J.Á.; Cossío, T.G.; Pedraza, L.S.; Aburto, T.C.; Sánchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in Latin America: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef]
- Lobstein, T.; Baur, L.; Uauy, R. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 1, 4–104. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines: Assessing and Managing Children at Primary Health-Care Facilities to Prevent Over-Weight and Obesity in the Context of the Double Burden of Malnutrition. Available online: https://www.who.int/publications/i/item/9789241550123 (accessed on 24 May 2021).
- Toro-Ramos, T.; Paley, C.; Pi-Sunyer, F.X.; Gallagher, D. Body composition during fetal development and infancy through the age of 5 years. Eur. J. Clin. Nutr. 2015, 69, 1279–1289. [Google Scholar] [CrossRef]
- Pereira-Freire, J.A.; Lemos, J.O.; Sousa, A.F.; Meneses, C.C.; Rondó, P.H. Association between weight at birth and body composition in childhood: A Brazilian cohort study. Early Hum. Dev. 2015, 91, 445–449. [Google Scholar] [CrossRef]
- Carlsen, E.M.; Renault, K.M.; Nørgaard, K.; Nilas, L.; Jensen, J.E.; Hyldstrup, L.; Michaelsen, K.F.; Cortes, D.; Pryds, O. New-born regional body composition is influenced by maternal obesity, gestational weight gain and the birthweight standard score. Acta Paediatr. 2014, 103, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.P.; Euclydes, V.V.; Simões, F.A.; Vaz-de-Lima, L.R.; Brito, C.A.; Luzia, L.A.; Devakumar, D.; Rondó, P.H. The Relationship between Maternal Plasma Leptin and Adiponectin Concentrations and Newborn Adiposity. Nutrients 2017, 9, 182. [Google Scholar] [CrossRef]
- Euclydes, V.L.; Castro, N.P.; Lima, L.R.; Brito, C.; Ribeiro, L.; Simões, F.A.; Requena, G.; Luzia, L.A.; Rondó, P.H. Cord blood concentrations of leptin, zinc-α2-glycoprotein, and adiponectin, and adiposity gain during the first 3 months of life. Nutrition 2018, 54, 89–93. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Available online: https://www.who.int/publications/i/item/9789241506236 (accessed on 24 May 2021).
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Castillo-Laura, H.; Santos, I.S.; Quadro, L.C.; Matijasevich, A. Maternal obesity and offspring body composition by indirect methods: A systematic review and meta-analysis. Cadernos de Saúde Pública 2015, 31, 2073–2092. [Google Scholar] [CrossRef]
- Dodd, J.M.; Turnbull, D.A.; McPhee, A.J.; Wittert, G.; Crowther, C.A.; Robinson, J.S. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: The LIMIT randomised controlled trial. BMC Pregnancy Childbirth 2011, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Jelsma, J.G.; van Poppel, M.N.; Galjaard, S.; Desoye, G.; Corcoy, R.; Devlieger, R.; van Assche, A.; Timmerman, D.; Jans, G.; Harreiter, J.; et al. DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: An European multicenter, randomised trial—Study protocol. BMC Pregnancy Childbirth 2013, 13, 142. [Google Scholar] [CrossRef]
- Dodd, J.M.; Cramp, C.; Sui, Z.; Yelland, L.N.; Deussen, A.R.; Grivell, R.M.; Moran, L.J.; Crowther, C.A.; Turnbull, D.; McPhee, A.J.; et al. LIMIT Randomised Trial Group. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: The LIMIT randomised trial. BMC Med. 2014, 12, 161. [Google Scholar] [CrossRef]
- Briley, A.L.; Barr, S.; Badger, S.; Bell, R.; Croker, H.; Godfrey, K.M.; Holmes, B.; Kinnunen, T.I.; Nelson, S.M.; Oteng-Ntim, E.; et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2014, 14, 74. [Google Scholar] [CrossRef]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicenter, randomized controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef]
- Patel, N.; Godfrey, K.M.; Pasupathy, D.; Levin, J.; Flynn, A.C.; Hayes, L.; Briley, A.L.; Bell, R.; Lawlor, D.A.; Oteng-Ntim, E.; et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int. J. Obes. 2017, 41, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Sartorelli, D.S.; Crivellenti, L.C.; Manochio-Pina, M.G.; Baroni, N.F.; Carvalho, M.R.; Diez-Garcia, R.W.; Franco, L.J. Study Protocol effectiveness of a nutritional intervention based on encouraging the consumption of unprocessed and minimally processed foods and the practice of physical activities for appropriate weight gain in overweight, adult, pregnant women: A randomized controlled trial. BMC Pregnancy Childbirth 2020, 20, 24. [Google Scholar]
- Au, C.P.; Raynes-Greenow, C.H.; Turner, R.M.; Carberry, A.E.; Jeffery, H. Fetal and maternal factors associated with neonatal adiposity as measured by air displacement plethysmography: A large cross-sectional study. Early Hum. Dev. 2013, 89, 839–843. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Catalano, P.M.; Thomas, A.; Huston-Presley, L.; Amini, S.B. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am. J. Obstet. Gynecol. 2003, 189, 1698–1704. [Google Scholar] [CrossRef]
- Wells, J.C. The evolution of human fatness and susceptibility to obesity: An ethological approach. Biol. Rev. Camb. Philos. Soc. 2006, 81, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan: A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Vijai, V.; Petrella, E.; Zoccoli, S.G.; Pignatti, L.; Di Cerbo, L.; Neri, I. Food glycemic index changes in over-weight/obese pregnant women enrolled in a lifestyle program: A randomized controlled trial. Am. J. Obstet. Gynecol. 2019, 1, 100030. [Google Scholar]
- Josefson, J.L.; Peaceman, A.M.; Kwasny, M.J.; Gernhofer, N.; Vincent, E.; van Horn, L. Glucose tolerance and neonatal adiposity in women enrolled in a randomized diet and lifestyle clinical trial to prevent excess gestational weight gain. Diabetes 2017, 66, A388. [Google Scholar]
- Brasil Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Ciência e Tecnologia. Diretrizes Metodológicas: Elaboração de Revisão Sistemática e Metanálise de Ensaios Clínicos Randomizados. Editora do Ministério da Saúde 012;92Metanálise de Ensaios Clínicos Randomizados. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/diretrizes metodologicas_elaboracao_sistematica.pdf (accessed on 24 May 2021).
- The Cochrane Collaboration. Review Manager; Version 5.3; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014; Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 24 May 2021).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; The GRADE Working Group: Hamilton, ON, Canada, 2013; Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 24 May 2021).
- Evidence Prime, Inc. GRADEpro GDT: GRADEpro Guideline Development Tool Software; McMaster University: Hamilton, ON, Canada, 2015; Available online: https://gradepro.org/ (accessed on 24 May 2021).
- Dodd, J.M.; Deussen, A.R.; Mohamad, I.; Rifas-Shiman, S.L.; Yelland, L.N.; Louise, J.; McPhee, A.J.; Grivell, R.M.; Owens, J.A.; Gillman, M.W.; et al. The effect of antenatal lifestyle advice for women who are overweight or obese on secondary measures of neonatal body composition: The LIMIT randomised trial. BJOG 2016, 123, 244–253. [Google Scholar] [CrossRef]
- Gallagher, D.; Rosenn, B.; Toro-Ramos, T.; Paley, C.; Gidwani, S.; Horowitz, M.; Crane, J.; Lin, S.; Thornton, J.C.; Pi-Sunyer, X. Greater Neonatal Fat-Free Mass and Similar Fat Mass Following a Randomized Trial to Control Excess Gestational Weight Gain. Obesity 2018, 26, 578–587. [Google Scholar] [CrossRef]
- Van Horn, L.; Peaceman, A.; Kwasny, M.; Vincent, E.; Fought, A.; Josefson, J.; Spring, B.; Neff, L.M.; Gernhofer, N. Dietary Approaches to Stop Hypertension Diet and Activity to Limit Gestational Weight: Maternal Offspring Metabolics Family In-tervention Trial, a Technology Enhanced Randomised Trial. Am. J. Prev. Med. 2018, 55, 603–614. [Google Scholar] [CrossRef]
- Van Poppel, M.N.M.; Simmons, D.; Devlieger, R.; van Assche, F.A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; et al. A reduction in sedentary behaviour in obese women during pregnancy reduces neonatal adiposity: The DALI randomised controlled trial. Diabetologia 2019, 62, 915–925. [Google Scholar] [CrossRef]
- Deierlein, A.L.; Thornton, J.; Hull, H.; Paley, C.; Gallagher, D. An anthropometric model to estimate neonatal fat mass using air displacement plethysmography. Nutr. Metab. 2012, 9, 21. [Google Scholar] [CrossRef]
- Blake-Lamb, T.L.; Locks, L.M.; Perkins, M.E.; Woo Baidal, J.A.; Cheng, E.R.; Taveras, E.M. Interventions for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; Martyni-Orenowicz, J.; Flynn, A.C.; Poston, L.; O’Keeffe, M. Can antenatal diet and lifestyle interventions influence childhood obesity? A systematic review. Matern. Child. Nutr. 2018, 14, e12628. [Google Scholar] [CrossRef]
- Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Institute of Medicine (US); National Research Council (US); Committee to Reexamine IOM. Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Peaceman, A.M.; Clifton, R.G.; Phelan, S.; Gallagher, D.; Evans, M.; Redman, L.M.; Knowler, W.C.; Joshipura, K.; Haire-Joshu, D.; Yanovski, S.Z.; et al. Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE-Moms Prospective Meta-Analysis. Obesity 2018, 26, 1396–1404. [Google Scholar] [CrossRef]
- Catalano, P.M.; Farrell, K.; Thomas, A.; Huston-Presley, L.; Mencin, P.; Mouzon, S.H.; Amini, S.B. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am. J. Clin. Nutr. 2009, 90, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, K.L.; Marshall, N. The placenta is the center of the chronic disease universe. Am. J. Obstet. Gynecol. 2015, 213, 14–20. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Catalano, P.M.; Mouzon, S.H. Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef]
- Waters, T.P.; Huston-Presley, L.; Catalano, P.M. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J. Clin. Endocrinol. Metab. 2012, 97, 3648–3654. [Google Scholar] [CrossRef]
- Siega-Riz, A.M. Prepregnancy Obesity: Determinants, consequences, and solutions. American Society for Nutrition. Adv. Nutr. 2012, 3, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.E.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Hanson, M.; Barker, M.; Dodd, J.M.; Kumanyika, S.; Norris, S.; Steegers, E.; Stephenson, J.; Thangaratinam, S.; Yang, H. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017, 5, 65–76. [Google Scholar] [CrossRef]
- Hanson, M.; Gluckman, P.; Bustreo, F. Obesity and the health of future generations. Lancet Diabetes Endocrinol. 2016, 4, 966–967. [Google Scholar] [CrossRef]
- Mitanchez, D.; Chavatte-Palmer, P. Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Pædiatrica 2018, 107, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Dutton, H.; Borengasser, S.J.; Gaudet, L.M.; Barbour, L.A.; Keely, E.J. Obesity in Pregnancy: Optimizing outcomes for mom and baby. Med. Clin. North Am. 2018, 102, 87–106. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Early Nutrition Project Systematic Review Group. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Yeo, S.; Walker, J.S.; Caughey, M.C.; Ferraro, A.M.; Asafu-Adjei, J.K. What characteristics of nutrition and physical activity interventions are key to effectively reducing weight gain in obese or overweight pregnant women? A systematic review and meta-analysis. Obes. Rev. 2017, 18, 385–399. [Google Scholar] [CrossRef]
- Chasan-Taber, L. Physical activity and dietary behaviors associated with weight gain and impaired glucose tolerance among pregnant Latinas. Adv. Nutr. 2012, 3, 108–118. [Google Scholar] [CrossRef]
- Headen, I.E.; Davis, E.M.; Mujahid, M.S.; Abrams, B. Racial-Ethnic Differences in Pregnancy-Related Weight. American Society for Nutrition. Adv. Nutr. 2012, 3, 83–94. [Google Scholar] [CrossRef]
- Ma, R.C.; Schmidt, M.I.; Tam, W.H.; McIntyre, H.D.; Catalano, P.M. Clinical management of pregnancy in the obese mother: Before conception, during pregnancy, and post partum. Lancet 2016, 4, 1037–1049. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Demerath, E.W.; Fields, D.A. Body Composition Assessment in the Infant. Am. J. Hum. Biol. 2014, 26, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; von Hurst, P.R.; McKinlay, C.J.; Cormack, B.E.; Conlon, C.A. Air displacement plethysmography (pea pod) in full-term and pre-term infants: A comprehensive review of accuracy, reproducibility, and practical challenges. Matern. Health Neonatol. Perinatol. 2018, 4, 12. [Google Scholar] [CrossRef]
- Fidanza, F.; Keys, A.; Anderson, J.T. Density of body fat in man and other mammals. J. Appl. Physiol. 1953, 6, 252–256. [Google Scholar] [CrossRef]
- Urlando, A.; Dempster, P.; Aitkens, S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr. Res. 2003, 53, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Nommsen-Rivers, L.; Dewey, K.; Urlando, A. Preliminary evaluation of a new pediatric air displacement plethysmograph for body composition assessment in infants. Acta Diabetol. 2003, 40, S55–S58. [Google Scholar] [CrossRef]
- Li, C.; McCargar, L.J.; Casey, L.M. Infant body composition in the PEA POD® era: What have we learned and where do we go from here? J. Dev. Orig. Health Dis. 2013, 4, 116–120. [Google Scholar] [CrossRef]
- Singh, K.A.; Huston-Presley, L.P.; Mencin, P.; Thomas, A.; Amini, S.B.; Catalano, P.M. Birth weight and body composition of neonates born to Caucasian compared with African-American mothers. Obstet. Gynecol. 2010, 115, 998–1002. [Google Scholar] [CrossRef]
- Wells, J.C. Body composition in infants: Evidence for developmental programming and techniques for measurement. Rev. Endocr. Metab. Disord. 2012, 13, 93–101. [Google Scholar] [CrossRef]
- Cauble, J.A.; Dewi, M.; Hull, H.R. Validity of anthropometric equations to estimate infant fat mass at birth and in early in-fancy. BMC Pediatr. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; Hernandez, T.L.; Reynolds, R.M.; Reece, M.S.; Chartier-Logan, C.; Anderso, M.K.; Kelly, T.; Friedman, J.E.; van Pelt, R.E. Striking differences in estimates of infant adiposity by new and old DXA Software, PEAPOD and skinfolds at two weeks and one year of life. Pediatr. Obes. 2016, 11, 264–271. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).