The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Metabolites and Polyphenol Detection and Identification
2.2. Anoxic Gut Bioreactors Mankai Experiment Performed at Biodesign Swette Center for Environmental Biotechnology, Arizona State University, USA
2.3. Polyphenols in Human Plasma and Urine. The 18-Month DIRECT-PLUS Clinical Trial, Performed at Ben Gurion University, Israel
2.3.1. Study Design
2.3.2. Randomization and Intervention
2.3.3. Human Samples and Polyphenol Analysis
2.3.4. Statistical Analysis
3. Results
3.1. Mankai Plant Metabolites
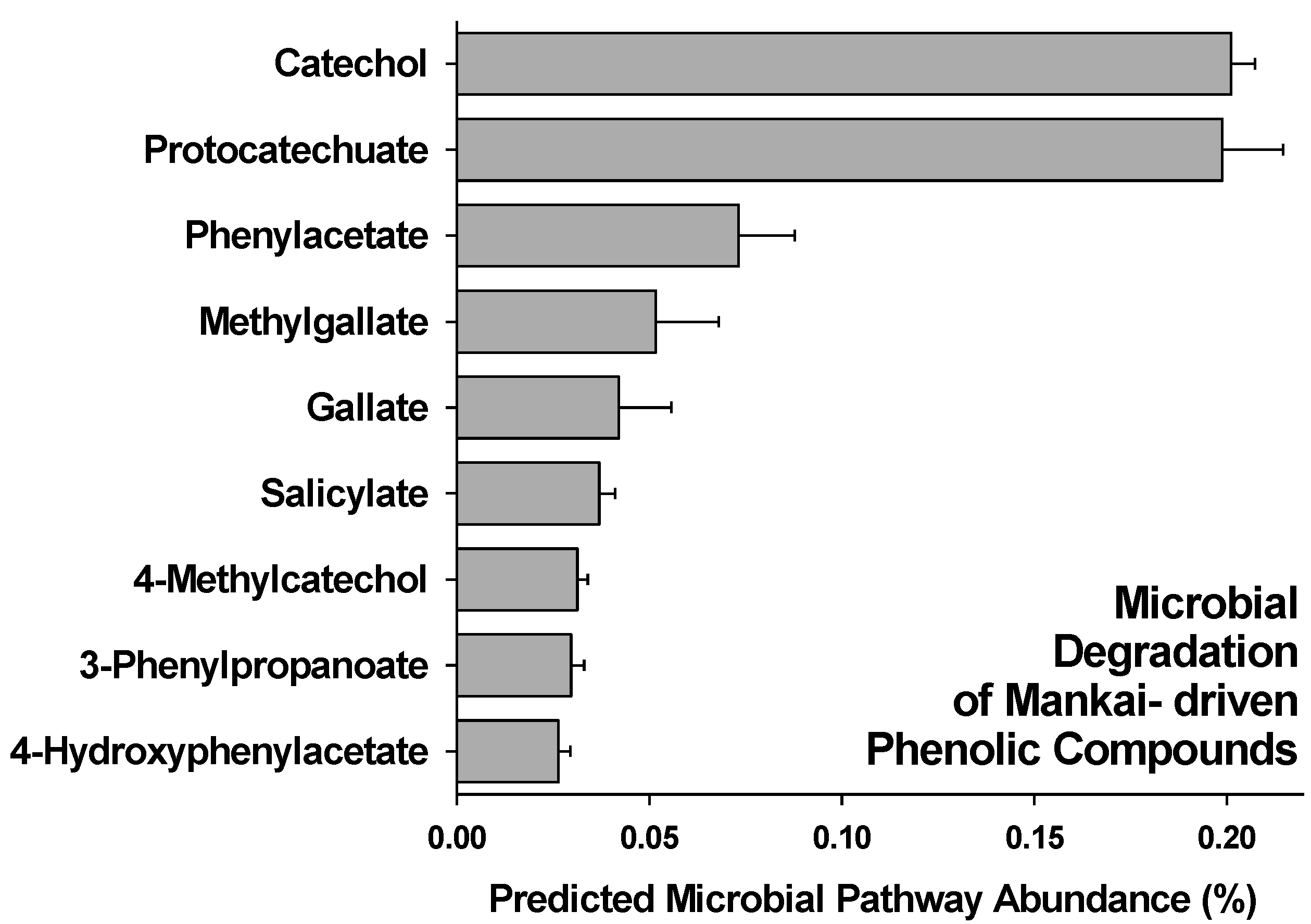
3.2. Gut Degradation Products
3.3. Polyphenols Detection in Human Plasma and Urine
3.3.1. DIRECT PLUS Baseline Characteristics
3.3.2. Plasma Polyphenols following 18 Months of DIRECT PLUS Weight Loss Intervention
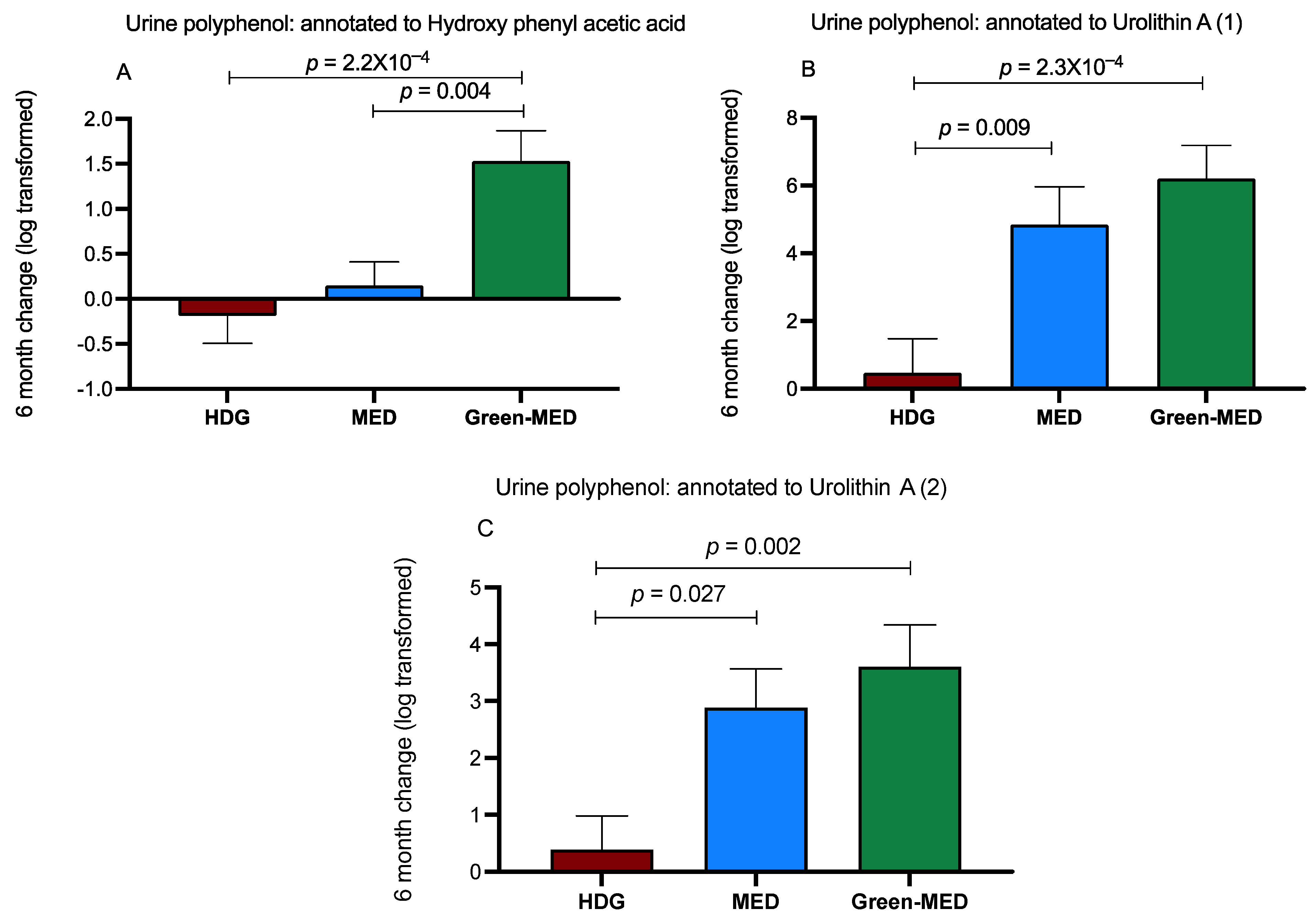
3.3.3. The Effect of the Intervention on Urine Polyphenols
3.3.4. Polyphenols and Health Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: From dietary sources to human microRNA modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.L.; Rooney, C.; McCall, D.; Young, I.S.; McCance, D.; McKinley, M.C.; Woodside, J.V.; McKeown, P.P. Beneficial effect of a polyphenol-rich diet on cardiovascular risk: A randomised control trial. Heart 2016, 102, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Khalili, M.; Haghighat, N.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Ebrahimpour-Koujan, S.; Eghtesadi, M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015, 11, 21. [Google Scholar]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean diet: From dietary sources to microRNA modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goni, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of US adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Cipolletti, M.; Solar Fernandez, V.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the antioxidant activity of dietary polyphenols in cancer: The modulation of estrogen receptors (ers) signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Trošt, K.; Ulaszewska, M.M.; Stanstrup, J.; Albanese, D.; De Filippo, C.; Tuohy, K.M.; Natella, F.; Scaccini, C.; Mattivi, F. Host: Microbiome co-metabolic processing of dietary polyphenols–An acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects. Food Res. Int. 2018, 112, 108–128. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Bog, M.; Ecker, J.; Boehm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; Kirmse, R. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef]
- Kawamata, Y.; Shibui, Y.; Takumi, A.; Seki, T.; Shimada, T.; Hashimoto, M.; Inoue, N.; Kobayashi, H.; Narita, T. Genotoxicity and repeated-dose toxicity evaluation of dried Wolffia globosa Mankai. Toxicol. Rep. 2020, 7, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Candreva, J.; Shi, H.; Ernst, E.; Martienssen, R.; Schwender, J.; Shanklin, J. Survey of the total fatty acid and triacylglycerol composition and content of 30 duckweed species and cloning of a Δ6-desaturase responsible for the production of γ-linolenic and stearidonic acids in Lemna gibba. BMC Plant. Biol. 2013, 13, 201. [Google Scholar] [CrossRef]
- Daduang, J.; Daduang, S.; Hongsprabhas, P.; Boonsiri, P. High phenolics and antioxidants of some tropical vegetables related to antibacterial and anticancer activities. Afr. J. Pharm. Pharmacol. 2011, 5, 608–615. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Tsaban, G.; Yaskolka Meir, A.; Rinott, E.; Kovsan, J.; Novack, L.; Thiery, J.; Ceglarek, U.; Burkhardt, R.; et al. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant—A randomized controlled trial. Clin. Nutr. 2019, 38, 2576–2582. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Rinott, E.; Kaplan, A.; Youngster, I.; Rudich, A.; Shelef, I.; Tirosh, A.; Brikner, D.; et al. A green-mediterranean diet, supplemented with mankai duckweed, preserves iron-homeostasis in humans and is efficient in reversal of anemia in rats. J. Nutr. 2019, 149. [Google Scholar] [CrossRef]
- Sela, I.; Yaskolka Meir, A.; Brandis, A.; Krajmalnik-Brown, R.; Zeibich, L.; Chang, D.; Dirks, B.; Tsaban, G.; Kaplan, A.; Rinott, E. Wolffia globosa–mankai plant-based protein contains bioactive vitamin B12 and is well absorbed in humans. Nutrients 2020, 12, 3067. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778. [Google Scholar] [CrossRef] [PubMed]
- Heinig, U.; Aharoni, A. Analysis of steroidal alkaloids and saponins in Solanaceae plant extracts using UPLC-qTOF mass spectrometry. In Plant Isoprenoids; Springer: Berlin/Heidelberg, Germany, 2014; pp. 171–185. [Google Scholar]
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The influence of storage on the “chemical age” of red wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Shahaf, N.; Franceschi, P.; Arapitsas, P.; Rogachev, I.; Vrhovsek, U.; Wehrens, R. Constructing a mass measurement error surface to improve automatic annotations in liquid chromatography/mass spectrometry based metabolomics. Rapid Commun. Mass Sp. 2013, 27, 2425–2431. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Development of a targeted method for twenty-three metabolites related to polyphenol gut microbial metabolism in biological samples, using SPE and UHPLC–ESI-MS/MS. Talanta 2014, 128, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tsaban, G.; Meir, A.Y.; Rinott, E.; Zelicha, H.; Kaplan, A.; Shalev, A.; Katz, A.; Rudich, A.; Tirosh, A.; Shelef, I. The effect of green Mediterranean diet on cardiometabolic risk; a randomised controlled trial. Heart 2020. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT plus randomised controlled trial. Gut 2021. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Meir, A.Y.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Shahaf, N.; Rogachev, I.; Heinig, U.; Meir, S.; Malitsky, S.; Battat, M.; Wyner, H.; Zheng, S.; Wehrens, R.; Aharoni, A. The WEIZMASS spectral library for high-confidence metabolite identification. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; El Mohsen, M.M.A.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef]
- Pagliuso, D.; Jara, C.E.P.; Grandis, A.; Lam, E.; Ferreira, M.J.P.; Buckeridge, M.S. Flavonoids from duckweeds: Potential applications in the human diet. RSC Adv. 2020, 10, 44981–44988. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; BakarMohamad, A. Antioxidant activity of coumarins. Syst. Rev. Pharm. 2017, 8, 24. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Anzoise, M.L.; Basso, A.R.; Del Mauro, J.S.; Carranza, A.; Ordieres, G.L.; Gorzalczany, S. Potential usefulness of methyl gallate in the treatment of experimental colitis. Inflammopharmacology 2018, 26, 839–849. [Google Scholar] [CrossRef]
- Wu, K.K. Aspirin and salicylate: An old remedy with a new twist. Circulation 2000, 102, 2022–2023. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, M.; Kourbali, Y.; Starzec, A.; Vassy, R.; Jozefonvicz, J.; Perret, G.; Crepin, M.; Kraemer, M. Sodium phenylacetate enhances the inhibitory effect of dextran derivative on breast cancer cell growth in vitro and in nude mice. Br. J. Cancer 2001, 85, 917–923. [Google Scholar] [CrossRef][Green Version]
- Franco, O.E.; Onishi, T.; Umeda, Y.; Soga, N.; Wakita, T.; Arima, K.; Yanagawa, M.; Sugimura, Y. Phenylacetate inhibits growth and modulates cell cycle gene expression in renal cancer cell lines. Anticancer Res. 2003, 23, 1637–1642. [Google Scholar] [PubMed]
- Asakawa, E.; Hirose, M.; Hagiwara, A.; Takahashi, S.; Ito, N. Carcinogenicity of 4-methoxyphenol and 4-methylcatechol in F344 rats. Int. J. Cancer 1994, 56, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Kowalska, S. Phenol and catechol induce prehemolytic and hemolytic changes in human erythrocytes. Toxicol. Lett. 2004, 152, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-K.; Alkon, D.L. Effects of 4-methylcatechol on spatial memory and depression. Neuroreport 2008, 19, 355–359. [Google Scholar] [CrossRef]
- Mulla, S.I.; Bharagava, R.N.; Belhaj, D.; Saratale, G.D.; Kumar, A.; Rajasekar, A.; Tallur, P.N.; Edalli, V.A.; Hu, A.; Yu, C.-P. Microbial degradation of phenolic compounds. In Microbes and Enzymes in Soil Health and Bioremediation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 305–320. [Google Scholar]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—from one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef]
- Zelicha, H.; Kaplan, A.; Meir, A.Y.; Tsaban, G.; Rinott, E.; Shelef, I.; Tirosh, A.; Brikner, D.; Pupkin, E.; Qi, L. The effect of wolffia globosa mankai, a green aquatic plant, on postprandial glycemic response: A randomized crossover controlled trial. Diabetes Care 2019, 42, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology 2020, 160, 158–173.e10. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Ristic, A.K.; Hollands, W.J. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Zheng, X.X.; Xu, Y.L.; Li, S.H.; Liu, X.X.; Hui, R.; Huang, X.H. Green tea intake lowers fasting serum total and LDL cholesterol in adults: A meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 2011, 94, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Jung, I.-C.; Lee, W.K.; Lee, Y.S.; Park, H.K.; Go, H.J.; Kim, K.; Lim, N.K.; Hong, J.T.; Ly, S.Y. A combination of green tea extract and l-theanine improves memory and attention in subjects with mild cognitive impairment: A double-blind placebo-controlled study. J. Med. Food 2011, 14, 334–343. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell, C.F.; Forster, J.S.; Veasey, R.C.; Kennedy, D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book Am. Soc. Plant. Biol. 2011, 9, e0156. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid. Based Complementary Altern. Med. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Tulipani, S.; Urpi-Sarda, M.; García-Villalba, R.; Rabassa, M.; López-Uriarte, P.; Bulló, M.; Jáuregui, O.; Tomás-Barberán, F.; Salas-Salvadó, J.; Espín, J.C. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J. Agric. Food Chem. 2012, 60, 8930–8940. [Google Scholar] [CrossRef]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- Loke, W.M.; Jenner, A.M.; Proudfoot, J.M.; McKinley, A.J.; Hodgson, J.M.; Halliwell, B.; Croft, K.D. A metabolite profiling approach to identify biomarkers of flavonoid intake in humans. J. Nutr. 2009, 139, 2309–2314. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M.; O’leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.-M.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.-P.; Cheynier, V.; Donovan, J.L.; Manach, C.; Morand, C.; Mila, I.; Lapierre, C.; Rémésy, C.; Scalbert, A. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J. Nutr. 2003, 133, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; Van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Bioactive peptides and dietary polyphenols: Two sides of the same coin. Molecules 2020, 25, 3443. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef] [PubMed]
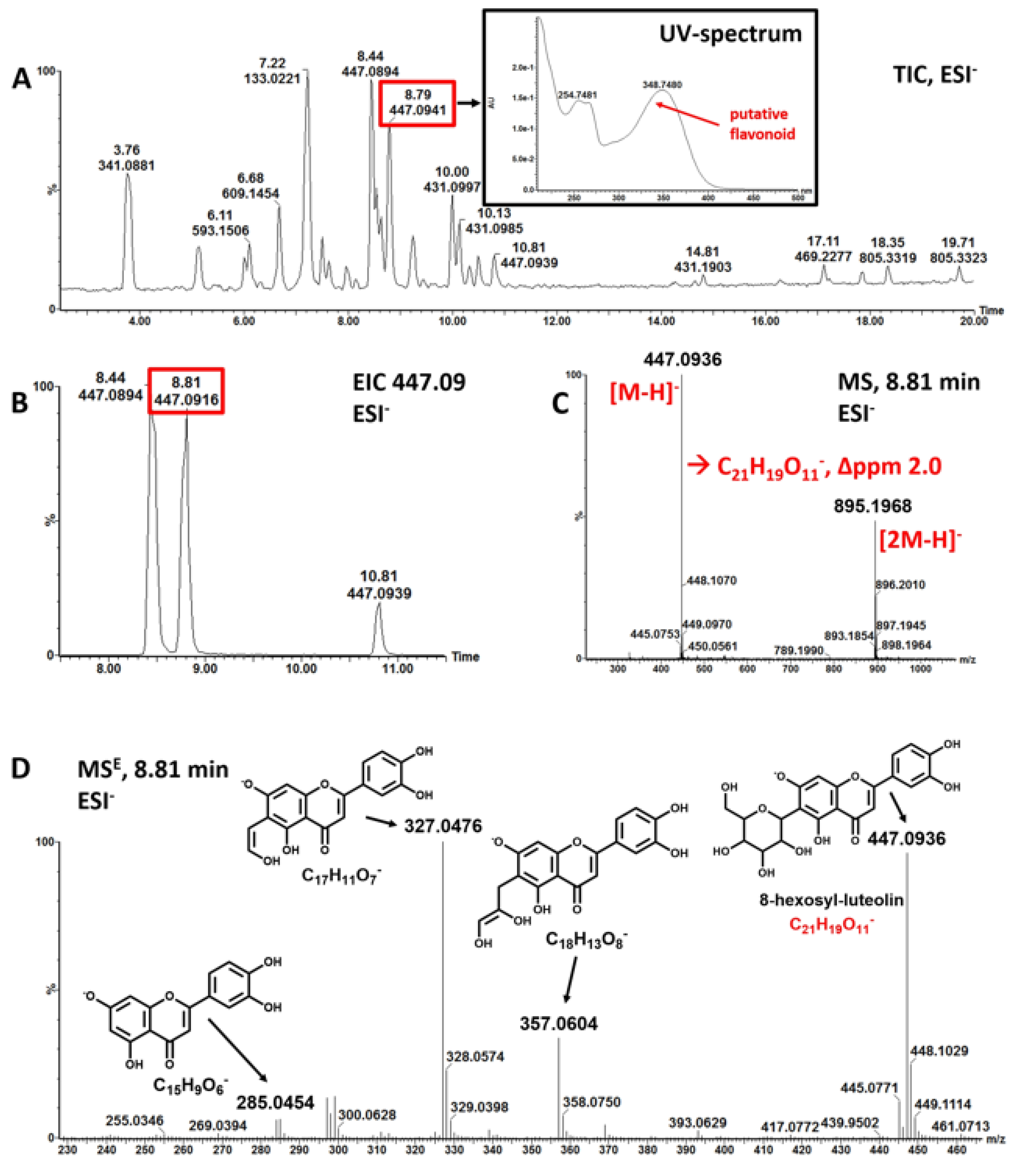
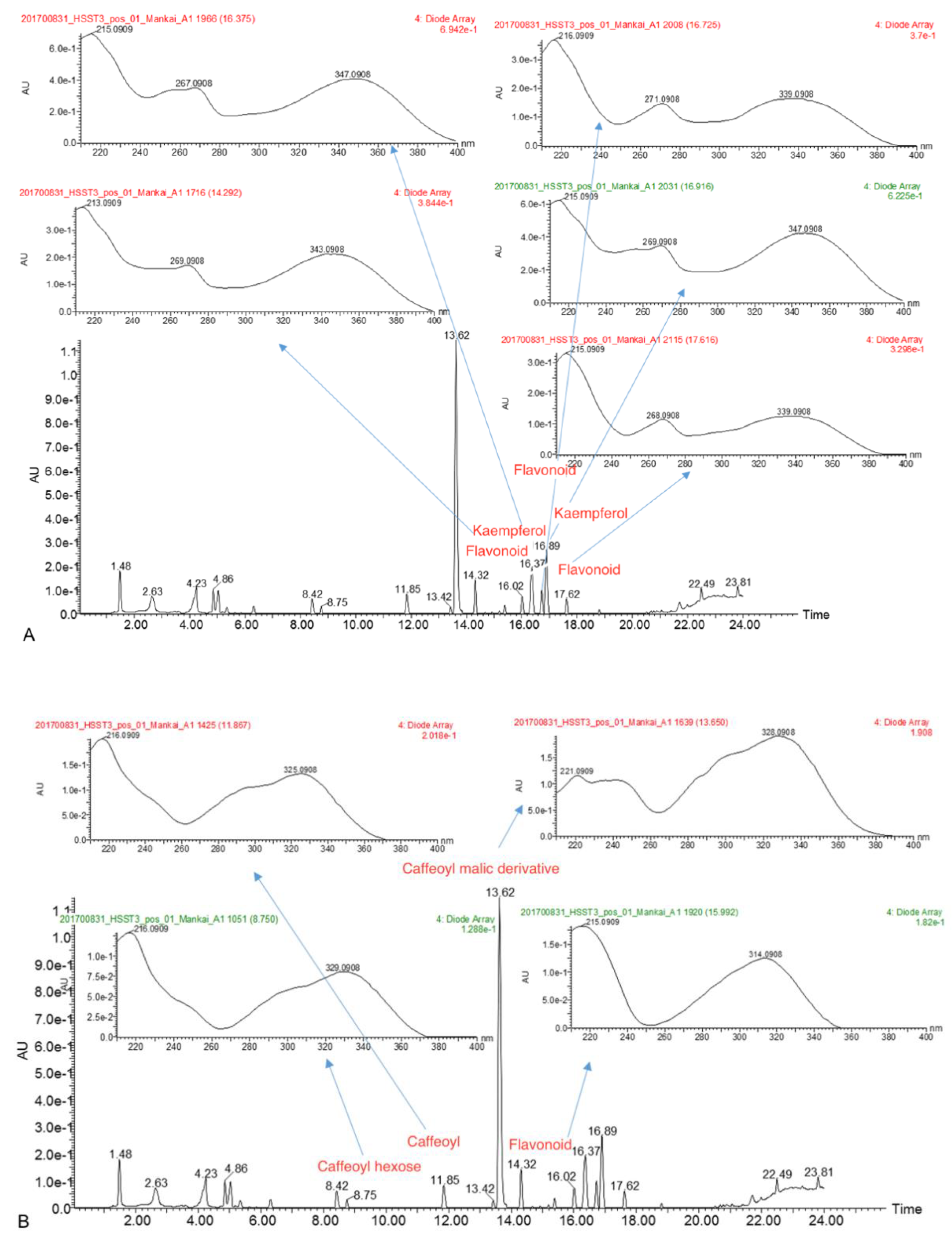
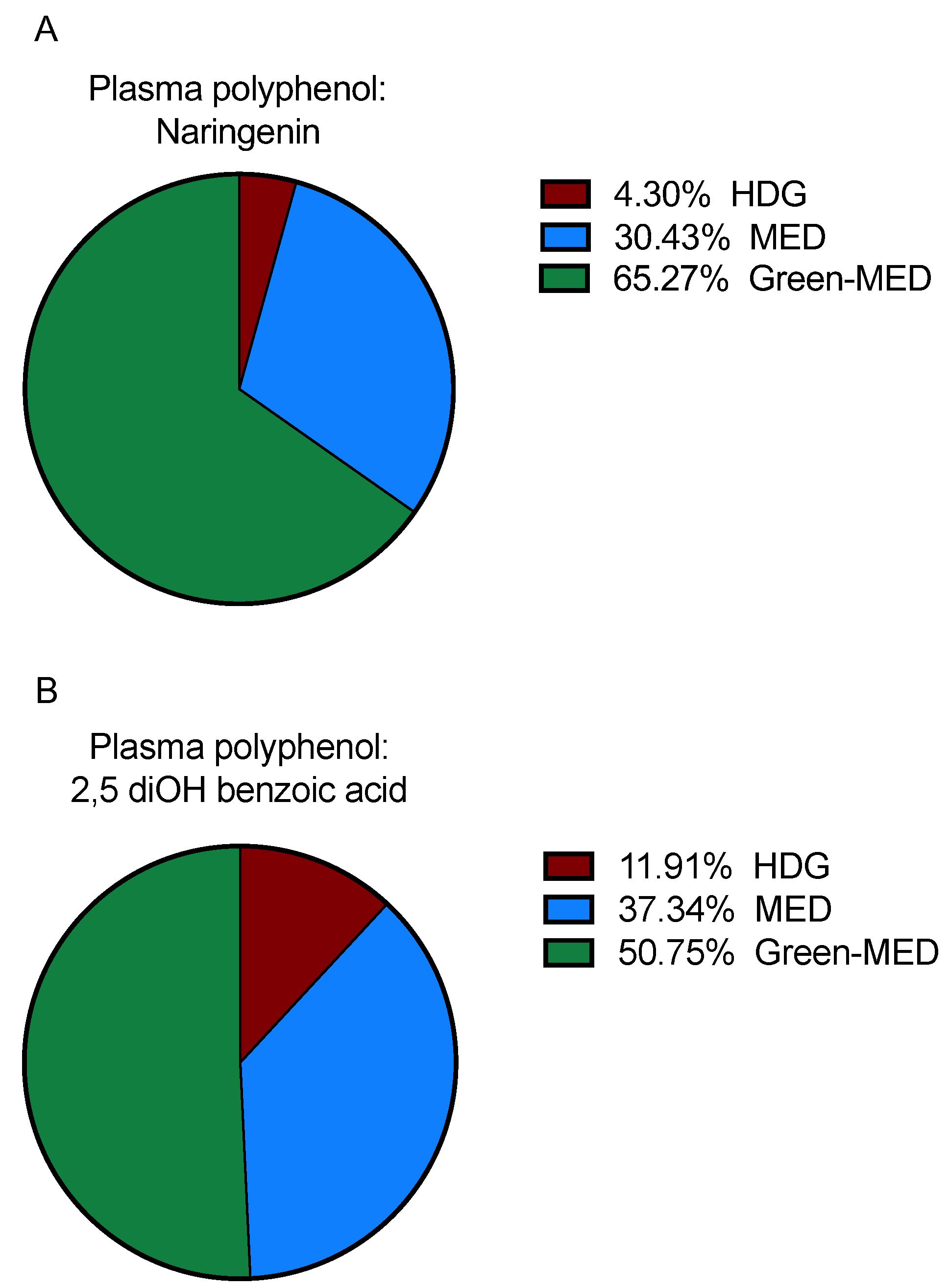
| Polyphenol Class 1 | Subclass 1 | Examples of Polyphenols Detected in the Mankai Plant (Mankai Polyphenol Metabolomic Experiment #) |
|---|---|---|
| Flavonoids | Flavonols | Quercetin (1,3,4,5), Rutin (1,4,5), Isorhamnetin (4,5), Kaempferol (3,4), Myricetin (4) |
| Flavones | Apigenin (3,4,5), Luteolin (3,4,5), Orientin (1,4), Syringetin (4,5), Laricitrin (4) | |
| Flavanols | Epicatechin (4,5) | |
| Flavanones | Naringenin (4,5), Taxifolin (4,5), Astilbin (1) | |
| Phenolic acids | Hydroxycinnamic acids | Caffeic acid (3,4,5), Ferulic acid (4,5), p-Coumaric acid (4,5), Sinapic acid (4) |
| Hydroxybenzoic acids | Benzoic acid (4,5), Ellagic acid (4,5), Ginkgoic acid (1,2), Gallic acid (4,5), Vanillic acid (4,5), 4-Methylsalicylic acid (2) | |
| Other polyphenols | Other polyphenols | Arbutin (4,5), Esculin (4,5) |
| Hydroxycoumarins | Daphnetin (2,3,4), Coumarin (3) | |
| Phenolic terpenes | Carnosol (4) | |
| Stilbenes | Stilbenes | cis-Resveratrol (4) |
| Unclassified | Olivetol (1,2) |
| Detected Polyphenols in Plasma, % 1 | Entire n = 294 | HDG n = 98 | MED n = 98 | Green-MED n = 98 | p between Groups 2 |
|---|---|---|---|---|---|
| Hippuric acid | 93.0 | 94.6 | 89.4 | 94.9 | 0.25 |
| m-hydroxyhippuric acid | 82.8 | 80.7 | 84.0 | 83.5 | 0.80 |
| Vanillin | 61.3 | 61.3 | 60.6 | 61.8 | 0.99 |
| 2,6 diOH benzoic acid | 45.4 | 47.3 | 43.6 | 45.4 | 0.88 |
| 3,4 hydroxyphenyl propionic acid | 33.0 | 27.2 | 29.4 | 42.1 | 0.06 |
| diOH isoferulic acid | 28.5 | 23.7 | 27.7 | 34.0 | 0.28 |
| Pyrogallol | 20.8 | 18.3 | 22.3 | 21.7 | 0.77 |
| Vanillic acid | 15.5 | 15.1 | 13.8 | 17.5 | 0.77 |
| 2,5 diOH benzoic acid | 9.2 | 6.5 | 8.5 | 10.3 | 0.63 |
| Naringenin | 0.35 | 0 | 0 | 1.03 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaskolka Meir, A.; Tuohy, K.; von Bergen, M.; Krajmalnik-Brown, R.; Heinig, U.; Zelicha, H.; Tsaban, G.; Rinott, E.; Kaplan, A.; Aharoni, A.; et al. The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols. Nutrients 2021, 13, 1866. https://doi.org/10.3390/nu13061866
Yaskolka Meir A, Tuohy K, von Bergen M, Krajmalnik-Brown R, Heinig U, Zelicha H, Tsaban G, Rinott E, Kaplan A, Aharoni A, et al. The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols. Nutrients. 2021; 13(6):1866. https://doi.org/10.3390/nu13061866
Chicago/Turabian StyleYaskolka Meir, Anat, Kieran Tuohy, Martin von Bergen, Rosa Krajmalnik-Brown, Uwe Heinig, Hila Zelicha, Gal Tsaban, Ehud Rinott, Alon Kaplan, Asaph Aharoni, and et al. 2021. "The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols" Nutrients 13, no. 6: 1866. https://doi.org/10.3390/nu13061866
APA StyleYaskolka Meir, A., Tuohy, K., von Bergen, M., Krajmalnik-Brown, R., Heinig, U., Zelicha, H., Tsaban, G., Rinott, E., Kaplan, A., Aharoni, A., Zeibich, L., Chang, D., Dirks, B., Diotallevi, C., Arapitsas, P., Vrhovsek, U., Ceglarek, U., Haange, S.-B., Rolle-Kampczyk, U., ... Shai, I. (2021). The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols. Nutrients, 13(6), 1866. https://doi.org/10.3390/nu13061866













