Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Study Measures
2.2.1. Imaging Data
2.2.2. Diet Data
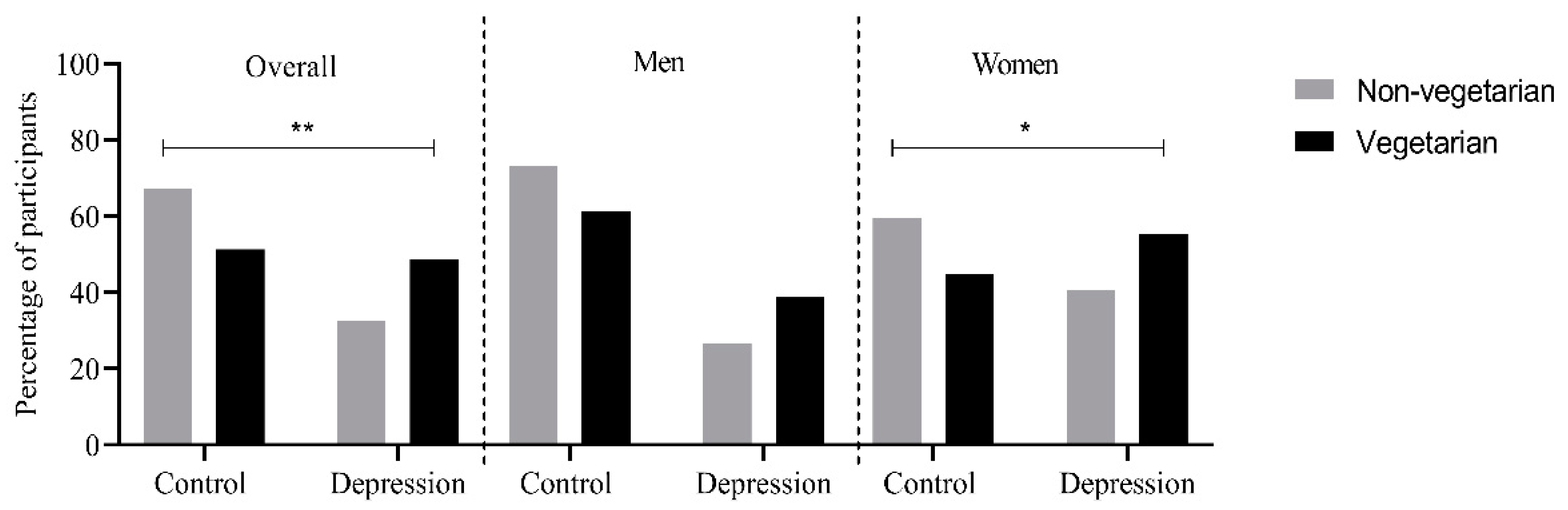
2.2.3. Depression and Dietary Groups
2.3. Study Covariates
2.4. Statistical Analyses
3. Results
3.1. Mean Intakes of Vitamin B6, B12 and Folate Intake Stratified by Depression Status and Diet Groups
3.2. Association of Nutrient Intake, Depression and Diet Status with Brain Volumes
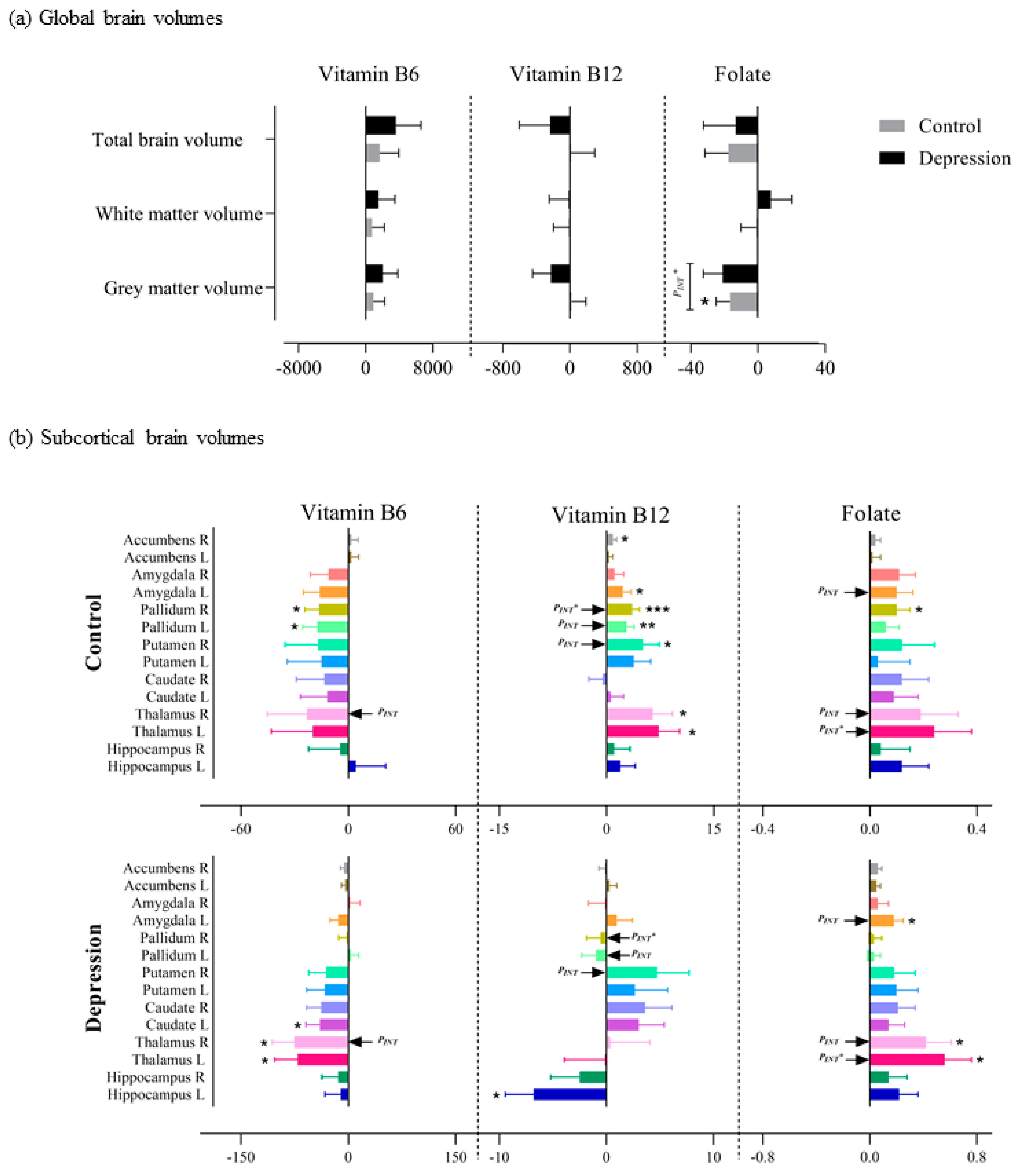
3.3. Association of Vitamin B6, B12 and Folate Intake with Brain Volumes Based on Depression Status
3.3.1. Global Volumes
3.3.2. Subcortical Volumes
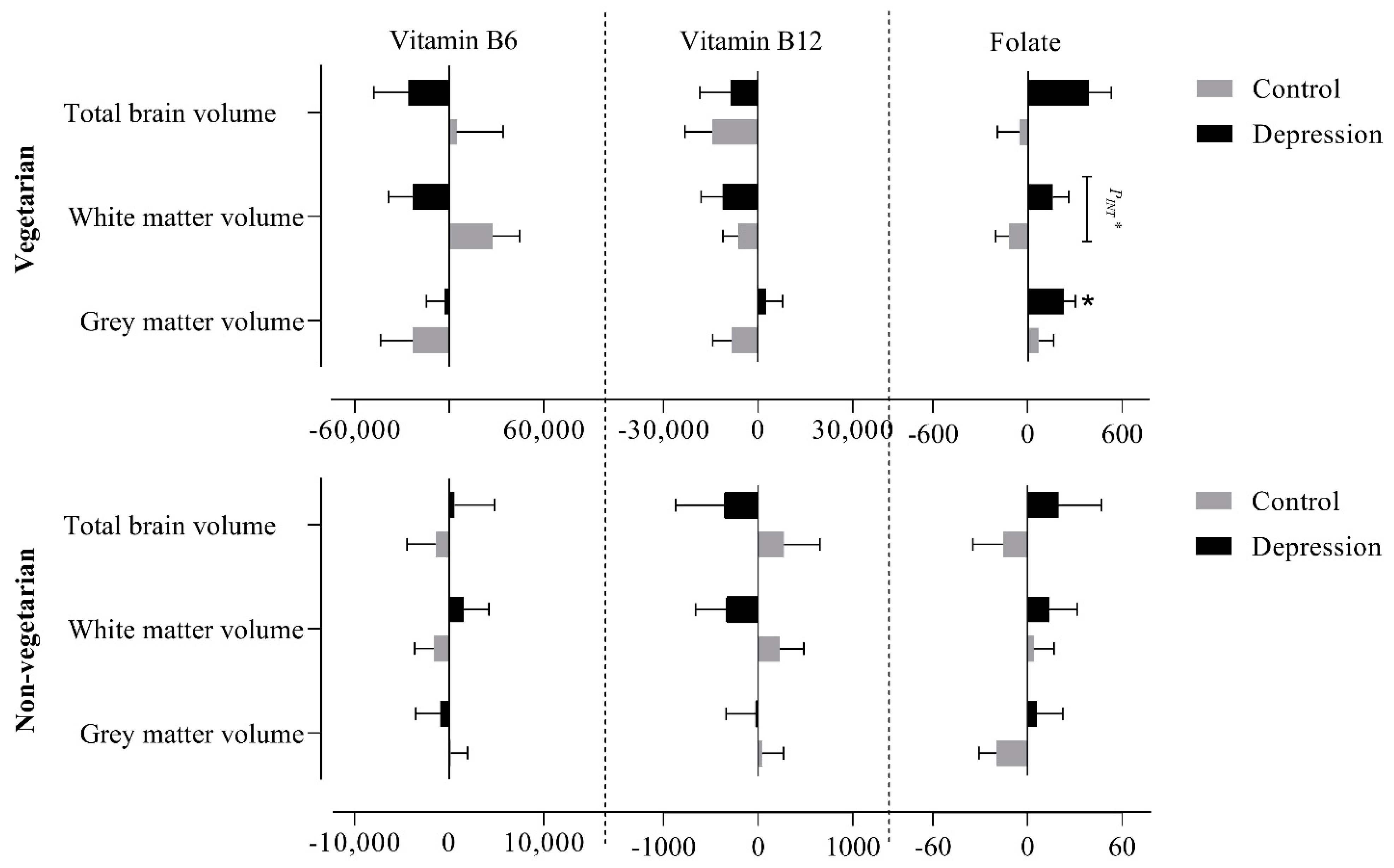
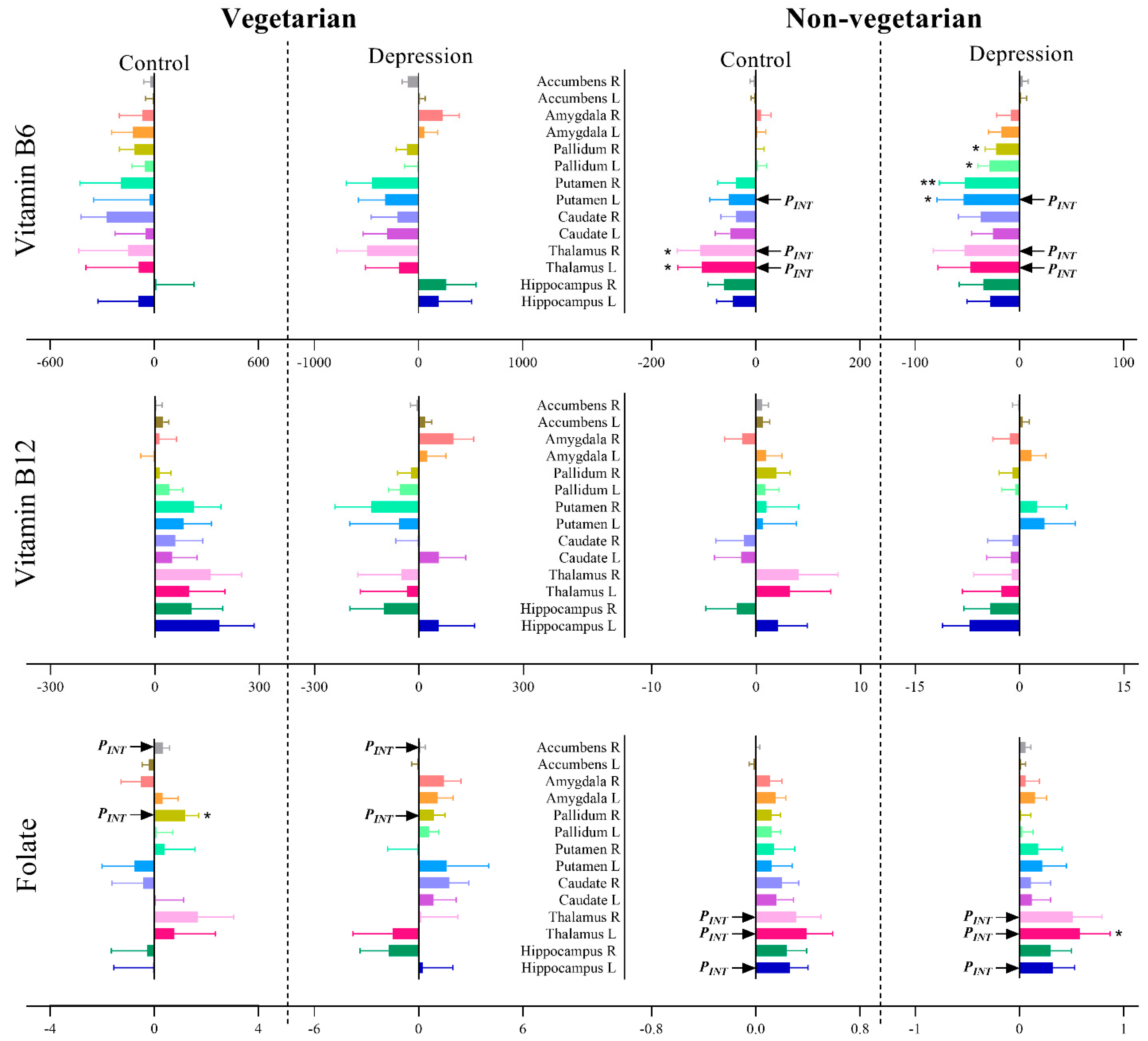
3.4. Association of Vitamin B6, B12 and Folate Intake with Brain Volumes after Stratifying for Depression Status and Diet Groups
3.4.1. Global Volumes
3.4.2. Subcortical Volumes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Executive Board. Global Burden of Mental Disorders and the Need for a Comprehensive, Coordinated Response from Health and Social Sectors at the Country Level: Report by the Secretariat; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Berk, M. Depression, diet and exercise. Med. J. Aust. 2012, 1, 21–23. [Google Scholar] [CrossRef]
- Meesters, A.N.R.; Maukonen, M.; Partonen, T.; Mannisto, S.; Gordijn, M.C.M.; Meesters, Y. Is There a Relationship between Vegetarianism and Seasonal Affective Disorder? A Pilot Study. Neuropsychobiology 2016, 74, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Northstone, K.; Evans, J.; Golding, J. Vegetarian diets and depressive symptoms among men. J. Affect. Disord. 2018, 225, 13–17. [Google Scholar] [CrossRef]
- Forestell, C.A.; Nezlek, J.B. Vegetarianism, depression, and the five factor model of personality. Ecol. Food Nutr. 2018, 57, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Vegan Society Poll. Available online: https://www.ipsos.com/ipsos-mori/en-uk/vegan-society-poll (accessed on 13 May 2020).
- Find out How Many Vegans There are in Great Britain. 2016. Available online: https://www.vegansociety.com/whats-new/news/find-out-how-many-vegans-there-are-great-britain. (accessed on 29 April 2020).
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European Prospective Investigation into Cancer and Nutrition–Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B(1)(2) and omega-3 fatty acids on brain function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef]
- Moorthy, D.; Peter, I.; Scott, T.M.; Parnell, L.D.; Lai, C.Q.; Crott, J.W.; Ordovas, J.M.; Selhub, J.; Griffith, J.; Rosenberg, I.H.; et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J. Nutr. 2012, 142, 1554–1560. [Google Scholar] [CrossRef]
- Kalita, J.; Misra, U.K. Benefit of vitamin B-12 supplementation in asymptomatic elderly: A matter of endpoints. Am. J. Clin. Nutr. 2015, 102, 529–530. [Google Scholar] [CrossRef][Green Version]
- Hooshmand, B.; Polvikoski, T.; Kivipelto, M.; Tanskanen, M.; Myllykangas, L.; Erkinjuntti, T.; Mäkelä, M.; Oinas, M.; Paetau, A.; Scheltens, P.; et al. Plasma homocysteine, Alzheimer and cerebrovascular pathology: A population-based autopsy study. Brain 2013, 136, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D. The Worldwide Challenge of the Dementias: A Role for B Vitamins and Homocysteine? Food Nutr. Bull. 2008, 29, S143–S172. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Herrmann, W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and Recommendations about Total Homocysteine Determinations: An Expert Opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef]
- Skarupski, K.A.; Tangney, C.; Li, H.; Ouyang, B.; Evans, D.A.; Morris, M.C. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am. J. Clin. Nutr. 2010, 92, 330–335. [Google Scholar] [CrossRef]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kareholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Muhlmann, M.; Ertl-Wagner, B.; et al. Association of Vitamin B12, Folate, and Sulfur Amino Acids with Brain Magnetic Resonance Imaging Measures in Older Adults: A Longitudinal Population-Based Study. JAMA Psychiatry 2016, 73, 606–613. [Google Scholar] [CrossRef]
- Pelletier, A.; Barul, C.; Feart, C.; Helmer, C.; Bernard, C.; Periot, O.; Dilharreguy, B.; Dartigues, J.F.; Allard, M.; Barberger-Gateau, P.; et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimer. Dement. 2015, 11, 1023–1031. [Google Scholar] [CrossRef]
- Erickson, K.I.; Suever, B.L.; Prakash, R.S.; Colcombe, S.J.; McAuley, E.; Kramer, A.F. Greater intake of vitamins B6 and B12 spares gray matter in healthy elderly: A voxel-based morphometry study. Brain Res. 2008, 1199, 20–26. [Google Scholar] [CrossRef]
- Gilsing, A.M.J.; Crowe, F.L.; Lloyd-Wright, Z.; Sanders, T.A.B.; Appleby, P.N.; Allen, N.E.; Key, T.J. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: Results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur. J. Clin. Nutr. 2010, 64, 933–939. [Google Scholar] [CrossRef]
- Vudhivai, N.; Ali, A.; Pongpaew, P.; Changbumrung, S.; Vorasanta, S.; Kwanbujan, K.; Charoenlarp, P.; Migasena, P.; Schelp, F.P. Vitamin B1, B2 and B6 status of vegetarians. J. Med. Assoc. Thail. Chotmaihet Thangphaet 1991, 74, 465–470. [Google Scholar]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Almagro, F.; Jenkinson, M.; Bangerter, N.K.; Andersson, J.L.R.; Griffanti, L.; Douaud, G.; Sotiropoulos, S.N.; Jbabdi, S.; Hernandez-Fernandez, M.; Vallee, E.; et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 2018, 166, 400–424. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Smith, S. A Global Optimisation Method for Robust Affine Registration of Brain Images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 2011, 56, 907–922. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Tong, T.Y.; Key, T.J. Dietary Intake of High-Protein Foods and Other Major Foods in Meat-Eaters, Poultry-Eaters, Fish-Eaters, Vegetarians, and Vegans in UK Biobank. Nutrients 2017, 9, 1317. [Google Scholar] [CrossRef]
- Rukh, G.; Dang, J.; Olivo, G.; Ciuculete, D.-M.; Rask-Andersen, M.; Schiöth, H.B. Personality, lifestyle and job satisfaction: Causal association between neuroticism and job satisfaction using Mendelian randomisation in the UK biobank cohort. Transl. Psychiatry 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Sable, P.; Dangat, K.; Kale, A.; Joshi, S. Altered brain neurotrophins at birth: Consequence of imbalance in maternal folic acid and vitamin B12 metabolism. Neuroscience 2011, 190, 127–134. [Google Scholar] [CrossRef]
- Albert, P. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W.; Goodwin, F.K.; Chrousos, G.P. Clinical and Biochemical Manifestations of Depression. N. Engl. J. Med. 1988, 319, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.L.; Longhurst, J.G.; Mazure, C.M. Childhood Sexual Abuse as a Risk Factor for Depression in Women: Psychosocial and Neurobiological Correlates. Am. J. Psychiatry 1999, 156, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.Y.; Huang, Y.L.; Chang, C.; Kang, H.Y. Deficiency in Androgen Receptor Aggravates the Depressive-Like Behaviors in Chronic Mild Stress Model of Depression. Cells 2019, 8, 1021. [Google Scholar] [CrossRef]
- Government Dietary Recommendations. Government recommendations for energy and nutrients for males and females aged 1–18 years and 19+ years. Public Health Engl. 2016, 1–12. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, O.B.V. and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US) CTI: Washington, DC, USA, 1998.
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring Study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, D.; Wang, K.; Kwok, T. High serum folate is associated with brain atrophy in older diabetic people with vitamin B12 deficiency. J. Nutr. Health Aging 2017, 21, 1065–1071. [Google Scholar] [CrossRef]
- Selhub, J.; Rosenberg, I.H. Excessive folic acid intake and relation to adverse health outcome. Biochimie 2016, 126, 71–78. [Google Scholar] [CrossRef]
- Selhub, J.; Morris, M.S.; Jacques, P.F.; Rosenberg, I.H. Folate–vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am. J. Clin. Nutr. 2009, 89, 702S–706S. [Google Scholar] [CrossRef]
- Sobczyńska-Malefora, A.; Harrington, D.J.; Voong, K.; Shearer, M.J. Plasma and Red Cell Reference Intervals of 5-Methyltetrahydrofolate of Healthy Adults in Whom Biochemical Functional Deficiencies of Folate and Vitamin B12Had Been Excluded. Adv. Hematol. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.F.; Rosenberg, I.H. The Effect of Folic Acid Fortification on Plasma Folate and Total Homocysteine Concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Tindell, A.J.; Aldridge, J.W.; Berridge, K.C. Ventral pallidum roles in reward and motivation. Behav. Brain Res. 2009, 196, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Vorel, S.R.; Tranguch, A.J.; Kenny, E.T.; Mazzoni, P.; Van Gorp, W.G.; Kleber, H.D. Anhedonia After a Selective Bilateral Lesion of the Globus Pallidus. Am. J. Psychiatry 2006, 163, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef]
- Hebert, J.R.; Hurley, T.G.; Chiriboga, D.E.; Barone, J. A comparison of selected nutrient intakes derived from three diet assessment methods used in a low-fat maintenance trial. Public Health Nutr. 1998, 1, 207–214. [Google Scholar] [CrossRef]
- Posner, B.M.; Martin-Munley, S.S.; Smigelski, C.; Cupples, L.A.; Cobb, J.L.; Schaefer, E.; Miller, D.R.; D’Agostino, R.B. Comparison of techniques for estimating nutrient intake: The Framingham Study. Epidemiology 1992, 3, 171–177. [Google Scholar] [CrossRef]
- Yoshino, K.; Nishide, M.; Inagawa, M.; Yokota, K.; Moriyama, Y.; Ikeda, A.; Noda, H.; Yamagishi, K.; Tanigawa, T.; Iso, H. Validity of brief food frequency questionnaire for estimation of dietary intakes of folate, vitamins B6and B12, and their associations with plasma homocysteine concentrations. Int. J. Food Sci. Nutr. 2010, 61, 61–67. [Google Scholar] [CrossRef]
- Cummings, D.; Dowling, K.; Silverstein, N.; Tanner, A.; Eryilmaz, H.; Smoller, J.; Roffman, J. A Cross-Sectional Study of Dietary and Genetic Predictors of Blood Folate Levels in Healthy Young Adults. Nutrients 2017, 9, 994. [Google Scholar] [CrossRef]
- Bingham, S.A. Limitations of the Various Methods for Collecting Dietary Intake Data. Ann. Nutr. Metab. 1991, 35, 117–127. [Google Scholar] [CrossRef]
- van ‘t Veer, P.; Kardinaal Af Fau-Bausch-Goldbohm, R.A.; Bausch-Goldbohm Ra Fau-Kok, F.J.; Kok, F.J. Biomarkers for validation. Eur. J. Clin. Nutr. 1993, 47, S58–S63. [Google Scholar]
- Jacques, P.F.; Sulsky, S.I.; Sadowski, J.A.; Phillips, J.C.; Rush, D.; Willett, W.C. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am. J. Clin. Nutr. 1993, 57, 182–189. [Google Scholar] [CrossRef] [PubMed]
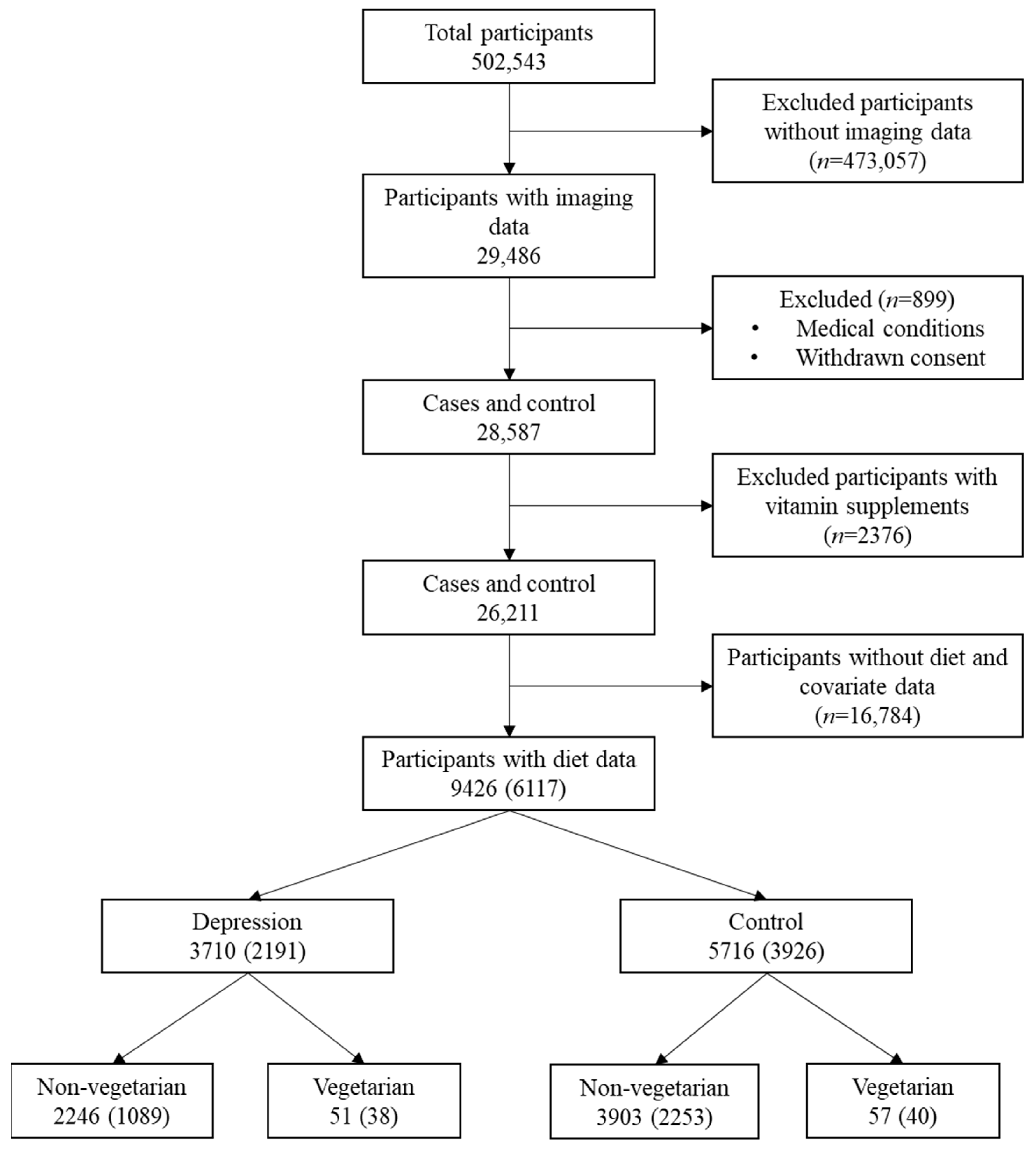
| Characteristics (n) | Total (6117) | Depression (2191) | Control (3926) | ||
|---|---|---|---|---|---|
| Non-Vegetarian (1089) | Vegetarian (38) | Non-Vegetarian (2253) | Vegetarian (40) | ||
| Age (Year) | 62.38 ± 7.44 | 61.62 ± 7.46 | 60.84 ± 7.09 | 62.57 ± 7.34 | 58.75 ± 6.71 |
| Sex | |||||
| Male | 3124 (48.9%) | 514 (47.15%) | 12 (31.58%) | 1409 (62.51%) | 19 (47.50%) |
| Female | 2993 (51.1%) | 575 (52.85%) | 26 (68.42%) | 845 (37.49%) | 21 (52.50%) |
| Body Mass Index | 26.03 ± 4.05 | 26.51 ± 4.27 | 25.66 ± 4.89 | 26.04 ± 3.91 | 25.55 ± 3.90 |
| Smoking | |||||
| Never | 4044 (66.1%) | 689 (63.24%) | 21 (55.26%) | 1535 (68.10%) | 32 (80.00%) |
| Previous | 1878 (30.7%) | 359 (33.00%) | 15 (39.47%) | 643 (28.53%) | 7 (17.50%) |
| Current | 195 (3.2%) | 41 (3.77%) | 2 (5.26%) | 76 (3.37%) | 1 (2.50%) |
| Alcohol | |||||
| Daily | 1163 (19.0%) | 225 (20.68%) | 8 (21.05%) | 448 (19.88%) | 5 (12.50%) |
| 3–4 times a week | 1931 (31.6%) | 314 (28.86%) | 6 (15.79%) | 758 (33.63%) | 7 (17.50%) |
| 1–2 times a week | 1631 (26.7%) | 268 (24.63%) | 12 (31.58%) | 636 (28.22%) | 14 (35.00%) |
| 1–3 times a month | 634 (10.4%) | 119 (10.94%) | 3 (7.89%) | 205 (9.09%) | 6 (15.00%) |
| Special occasions | 480 (7.8%) | 109 (10.02%) | 4 (10.53%) | 135 (5.99%) | 5 (12.50%) |
| Never | 278 (4.5%) | 54 (4.87%) | 5 (13.16%) | 72 (3.19%) | 3 (7.50%) |
| Townsend derivative index | −2.26 ± 2.44 | −2.14 ± 2.46 | −1.72 ± 3.01 | −2.39 ± 2.37 | −1.62 ± 2.70 |
| Dietary variation | |||||
| No | 2353 (38.5%) | 391 (35.85%) | 15 (39.47%) | 891 (39.55%) | 15 (37.50%) |
| Yes | 3763 (61.5%) | 698 (64.15%) | 23 (60.53%) | 1362 (60.45%) | 25 (62.50%) |
| Vitamin B6 intake | 2.18 ± 0.63 | 2.17 ± 0.62 | 1.87 ± 0.75 | 2.21 ± 0.63 | 1.77 ± 0.50 |
| Vitamin B12 intake | 6.3 ± 3.51 | 6.33 ± 3.51 | 2.44 ± 1.14 | 6.41 ± 3.44 | 2.75 ± 1.60 |
| Folate intake | 303.15 ± 94.97 | 298.34 ± 93.97 | 340.89 ± 131.67 | 303.42 ± 93.47 | 327.90 ± 98.34 |
| Vitamin B6 (mg) | Vitamin B12 (µg) | Folate (µg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Men | Women | Overall | Men | Women | Overall | Men | Women | |
| Diet classification | |||||||||
| Sample | 3420 | 1953 | 1467 | 3420 | 1953 | 1467 | 3420 | 1953 | 1467 |
| Non-vegetarian | 2.19 ± 0.62 | 2.26 ± 0.64 | 2.10 ± 0.59 | 6.37 ± 3.46 | 6.55 ± 3.55 | 6.14 ± 3.32 | 301.76 ± 93.65 | 311.73 ± 94.99 | 288.28 ± 90.09 |
| Vegetarian | 1.81 ± 0.63 | 1.91 ± 0.56 | 1.75 ± 0.67 | 2.60 ± 1.39 | 2.55 ± 1.45 | 2.62 ± 1.37 | 334.23 ± 115.20 | 351.61 ± 111.1 | 322.76 ± 117.57 |
| p-Value | 1.36 × 10−7 | 0.002 | 6.0 × 10−5 | 1.2 × 10−21 | 4.8 × 10−10 | 8.0 × 10−13 | 0.002 | 0.021 | 0.01 |
| Depression status | |||||||||
| Sample | 6117 | 3124 | 2993 | 6117 | 3124 | 2993 | 6117 | 3124 | 2993 |
| Controls | 2.19 ± 0.63 | 2.26 ± 0.64 | 2.09 ± 0.6 | 6.34 ± 3.49 | 6.49 ± 3.55 | 6.14 ± 3.38 | 304.37 ± 95.01 | 312.80 ± 95.3 | 293.23 ± 93.5 |
| Cases | 2.16 ± 0.62 | 2.27 ± 0.65 | 2.09 ± 0.59 | 6.21 ± 3.55 | 6.43 ± 3.74 | 6.06 ± 3.43 | 300.96 ± 94.86 | 317.67 ± 98.37 | 289.55 ± 90.68 |
| p-Value | 0.15 | 0.61 | 0.87 | 0.18 | 0.68 | 0.55 | 0.17 | 0.2 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkins, S.; Schiöth, H.B.; Rukh, G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients 2021, 13, 1790. https://doi.org/10.3390/nu13061790
Berkins S, Schiöth HB, Rukh G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients. 2021; 13(6):1790. https://doi.org/10.3390/nu13061790
Chicago/Turabian StyleBerkins, Samuel, Helgi Birgir Schiöth, and Gull Rukh. 2021. "Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes" Nutrients 13, no. 6: 1790. https://doi.org/10.3390/nu13061790
APA StyleBerkins, S., Schiöth, H. B., & Rukh, G. (2021). Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients, 13(6), 1790. https://doi.org/10.3390/nu13061790







