Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity
Abstract
1. Introduction
2. Dietary Intake of Selenium
2.1. Selenium in the Diet
2.2. Absorption of Se and Its Transport in the Body
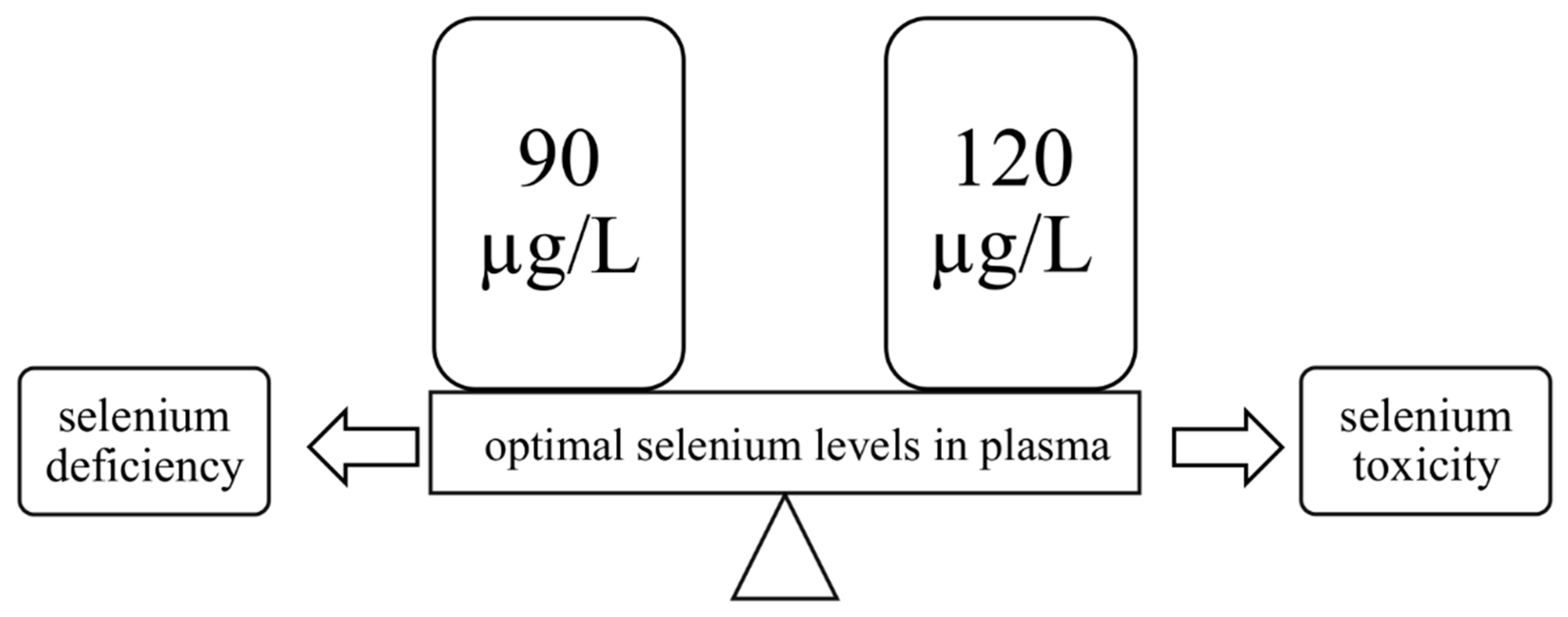
2.3. Selenium Deficiency
2.4. Selenium Overdose
3. Biological Activity of Selenium
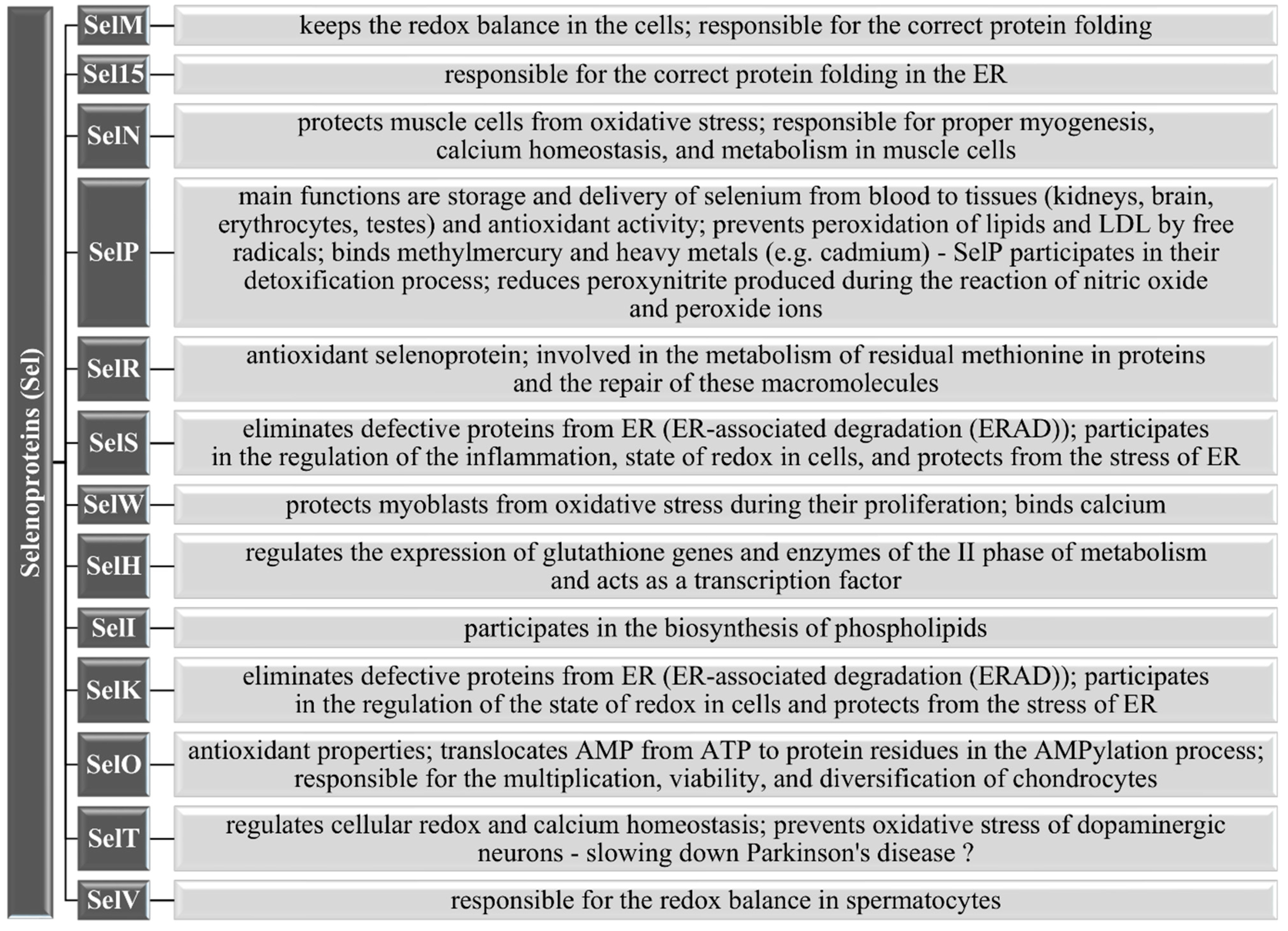
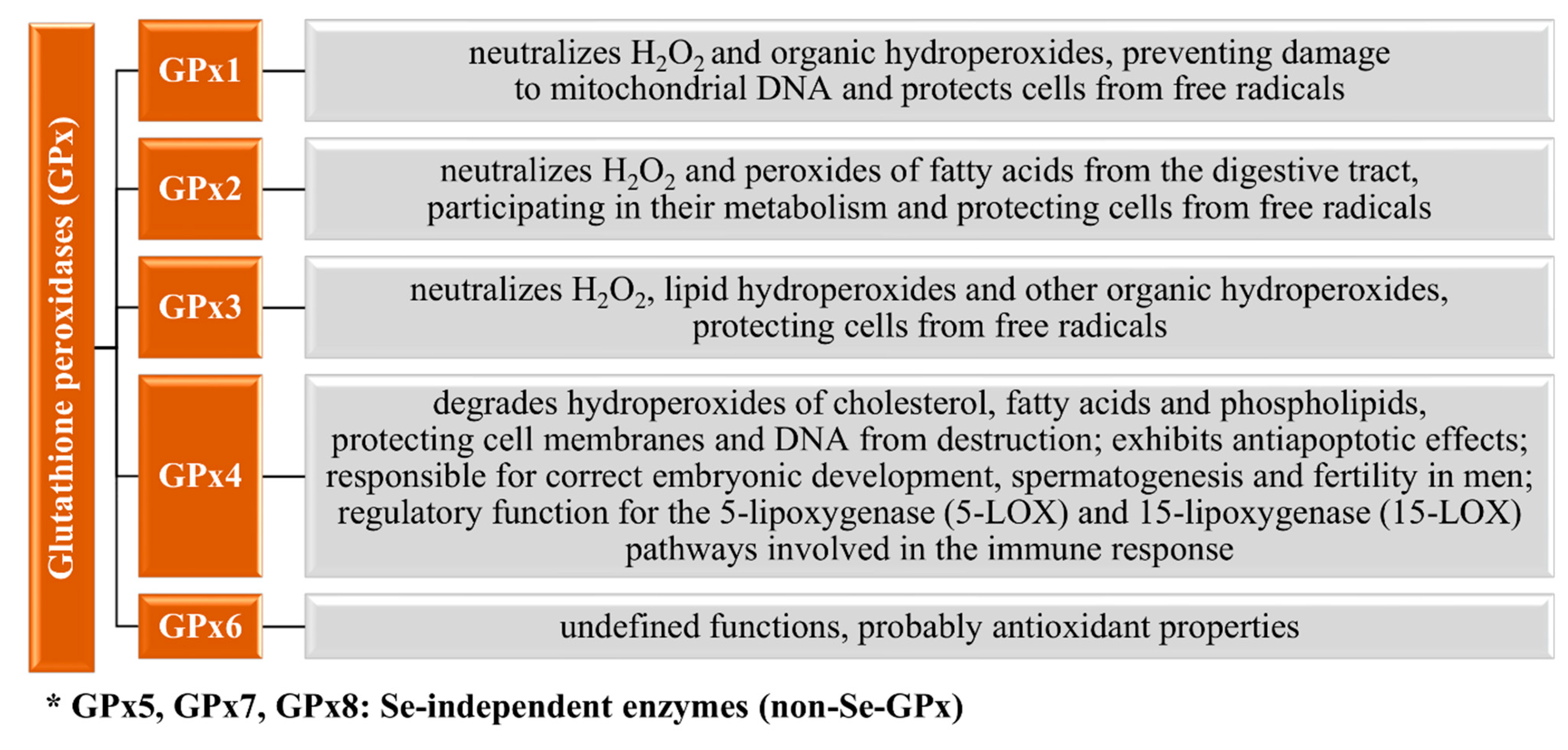
3.1. Selenoproteins in the Human Body
3.1.1. Glutathione Peroxidases
3.1.2. Thioredoxin Reductases
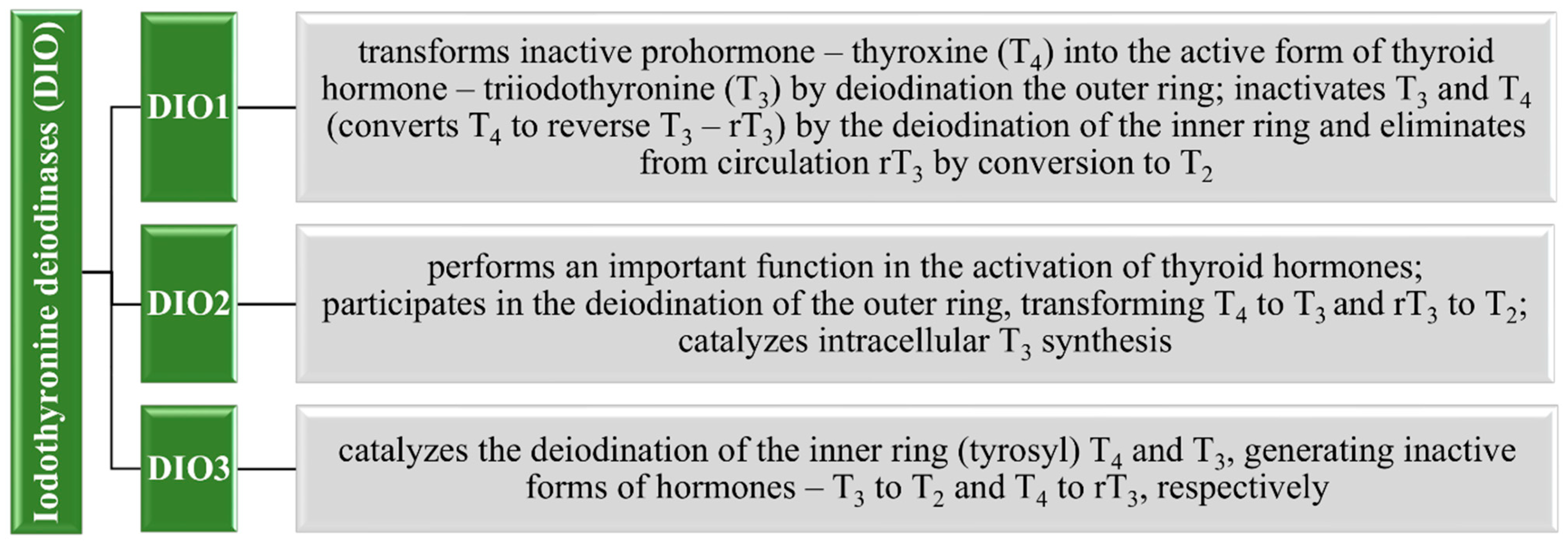
3.1.3. Thyroid Gland and Iodothyronine Deiodinases
3.1.4. Selenophosphate Synthetase 2
3.1.5. Other Selenoproteins
3.2. Effects of Se on Immune Response
3.3. Effects of Se on the Reproductive System and Other Body Functions
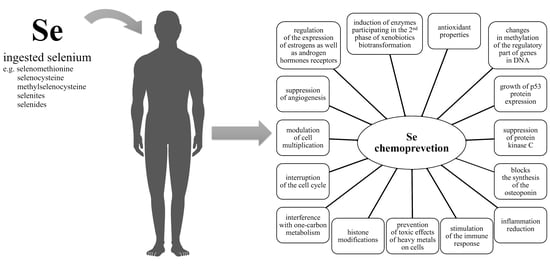
4. Selenium and Cancer Chemoprevention
4.1. Clinical Trials and Other Types Of Studies on the Chemopreventive Effects of Selenium
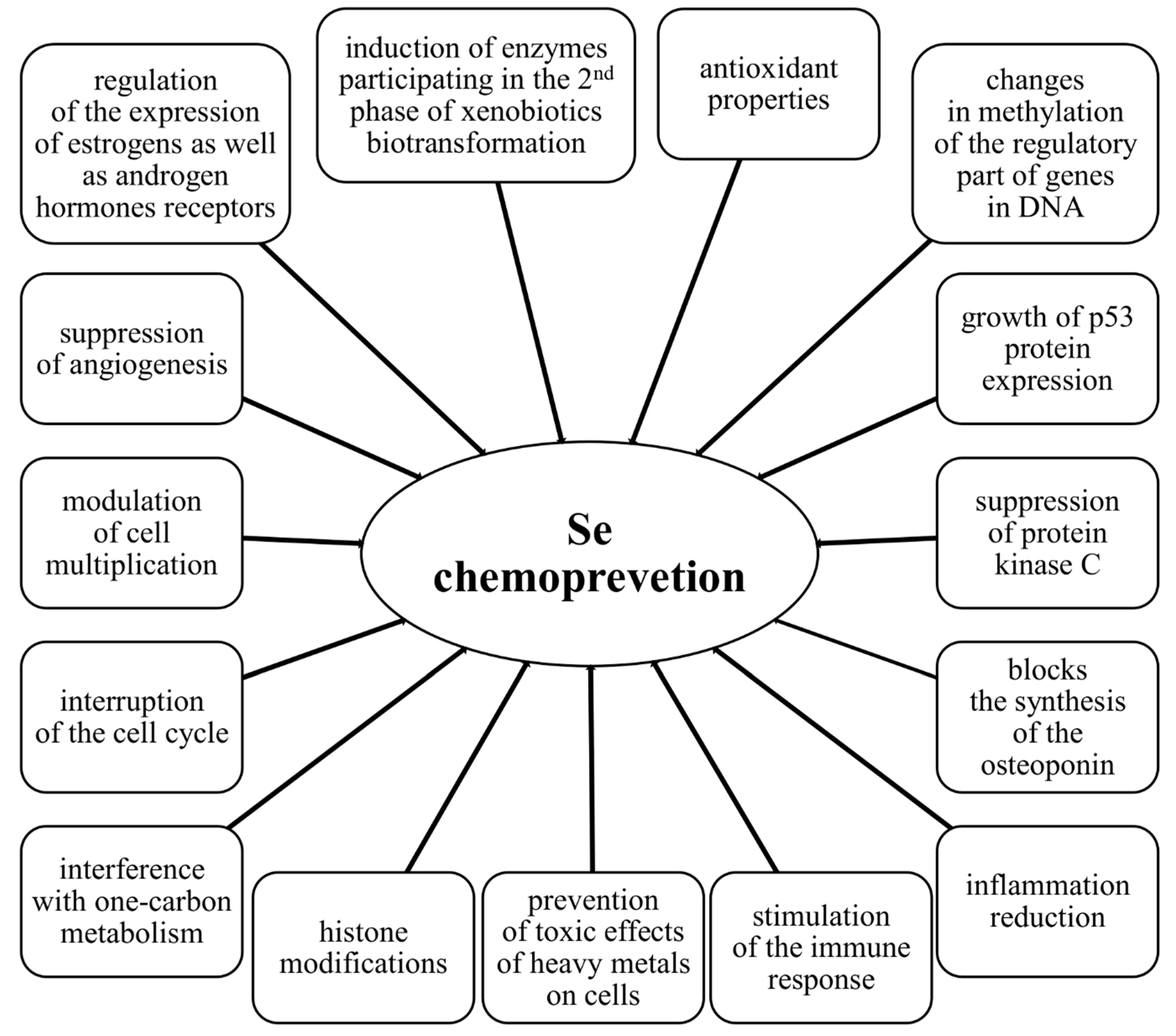
4.2. Molecular Mechanisms of the Chemopreventive Activity of Selenium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynologia Polska 2017, 68, 440–465. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F. Recent developments in trace element metabolism and function: Newer roles of selenium in nutrition. J. Nutr. 1989, 119, 1051–1054. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology: Impacts of the Natural Environment on Public Health; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Elsevier: London, UK, 2005; pp. 373–415. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000; p. 528. [Google Scholar] [CrossRef]
- Stockler-Pinto, M.B.; Lobo, J.; Moraes, C.; Leal, V.O.; Farage, N.E.; Rocha, A.V.; Boaventura, G.T.; Cozzolino, S.M.; Malm, O.; Mafra, D. Effect of Brazil nut supplementation on plasma levels of selenium in hemodialysis patients: 12 months of follow-up. J. Ren. Nutr. 2012, 22, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152. [Google Scholar] [CrossRef]
- Finley, J.W.; Ip, C.; Lisk, D.J.; Davis, C.D.; Hintze, K.J.; Whanger, P.D. Cancer-protective properties of high-selenium broccoli. J. Agric. Food Chem. 2001, 49, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Cappon, C.J.; Smith, J.C. Chemical form and distribution of mercury and selenium in edible seafood. J. Anal. Toxicol. 1982, 6, 10–21. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.-J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Smrkolj, P.; Pograjc, L.; Hlastan-Ribic, C.; Stibilj, V. Selenium content in selected Slovenian foodstuffs and estimated daily intakes of selenium. Food Chem. 2005, 90, 691–697. [Google Scholar] [CrossRef]
- Bierla, K.; Dernovics, M.; Vacchina, V.; Szpunar, J.; Bertin, G.; Lobinski, R. Determination of selenocysteine and selenomethionine in edible animal tissues by 2D size-exclusion reversed-phase HPLC-ICP MS following carbamidomethylation and proteolytic extraction. Anal. Bioanal. Chem. 2008, 390, 1789–1798. [Google Scholar] [CrossRef]
- Waegeneers, N.; Thiry, C.; De Temmerman, L.; Ruttens, A. Predicted dietary intake of selenium by the general adult population in Belgium. Food Addit. Contam. Part A 2013, 30, 278–285. [Google Scholar] [CrossRef]
- Pappa, E.C.; Pappas, A.C.; Surai, P.F. Selenium content in selected foods from the Greek market and estimation of the daily intake. Sci. Total Environ. 2006, 372, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lobinski, R.; Edmonds, J.; Suzuki, K.; Uden, P. Species-selective determination of selenium compounds in biological materials. Pure. Appl. Chem. 2000, 72, 447–461. [Google Scholar] [CrossRef]
- Guo, X.; Wu, L. Distribution of free seleno-amino acids in plant tissue of Melilotus indica L. grown in selenium-laden soils. Ecotoxicol. Environ. Saf. 1998, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hoac, T.; Lundh, T.; Önning, G.; Åkesson, B. Selenoproteins and Selenium Speciation in Food. In Selenoproteins and Mimics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 183–206. [Google Scholar] [CrossRef]
- Gawor, A.; Ruszczynska, A.; Czauderna, M.; Bulska, E. Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals 2020, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.; Nielsen, S.A. Comparison of whole blood selenium values and erythrocyte glutathione peroxidase activities of normal individuals on supplementation with selenate, selenite, L-selenomethionine, and high selenium yeast. Biol. Trace Elem. Res. 1988, 15, 125–138. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F.; Butler, J.A.; Whanger, P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br. J. Nutr. 1993, 69, 577–588. [Google Scholar] [CrossRef]
- Richie, J.P., Jr.; Muscat, J.E.; Ellison, I.; Calcagnotto, A.; Kleinman, W.; El-Bayoumy, K. Association of selenium status and blood glutathione concentrations in blacks and whites. Nutr. Cancer 2011, 63, 367–375. [Google Scholar] [CrossRef]
- Skalny, A.V.; Burtseva, T.I.; Salnikova, E.V.; Ajsuvakova, O.P.; Skalnaya, M.G.; Kirichuk, A.A.; Tinkov, A.A. Geographic variation of environmental, food, and human hair selenium content in an industrial region of Russia. Environ. Res. 2019, 171, 293–301. [Google Scholar] [CrossRef]
- Hintze, K.J.; Lardy, G.P.; Marchello, M.J.; Finley, J.W. Areas with high concentrations of selenium in the soil and forage produce beef with enhanced concentrations of selenium. J. Agric. Food Chem. 2001, 49, 1062–1067. [Google Scholar] [CrossRef]
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘Geographical and geological influences on nutrition’ Factors controlling the distribution of selenium in the environment and their impact on health and nutrition: Conference on ‘Over-and undernutrition: Challenges and approaches’. Proc. Nutr. Soc. 2010, 69, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Hardy, G. Can dietary selenium intake increase the risk of toxicity in healthy children? Nutrition 2016, 32, 149–150. [Google Scholar] [CrossRef]
- Strand, T.A.; Lillegaard, I.T.L.; Frøyland, L.; Haugen, M.; Henjum, S.; Løvik, M.; Stea, T.; Holvik, K. Assessment of Selenium Intake in Relation to Tolerable Upper Intake Levels. Eur. J. Nutr. Food Saf. 2018, 8, 155–156. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Betancor, M.; Caballero, M.J.; Hillestad, M.; Rasinger, J.; Hamre, K.; Sele, V.; Amlund, H.; Ørnsrud, R. Safe limits of selenomethionine and selenite supplementation to plant-based Atlantic salmon feeds. Aquaculture 2018, 495, 617–630. [Google Scholar] [CrossRef]
- Lee, S.; Nambi, R.W.; Won, S.; Katya, K.; Bai, S.C. Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 153–158. [Google Scholar] [CrossRef]
- Han, D.; Xie, S.; Liu, M.; Xiao, X.; Liu, H.; Zhu, X.; Yang, Y. The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2011, 17, e741–e749. [Google Scholar] [CrossRef]
- Hurst, R.; Collings, R.; Harvey, L.J.; King, M.; Hooper, L.; Bouwman, J.; Gurinovic, M.; Fairweather-Tait, S.J. EURRECA—Estimating Selenium Requirements for Deriving Dietary Reference Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 1077–1096. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Kazi Tani, L.S.; Dennouni-Medjati, N.; Toubhans, B.; Charlet, L. Selenium Deficiency—From Soil to Thyroid Cancer. Appl. Sci. 2020, 10, 5368. [Google Scholar] [CrossRef]
- Baum, M.K.; Shor-Posner, G.; Lai, S.; Zhang, G.; Lai, H.; Fletcher, M.A.; Sauberlich, H.; Page, J.B. High Risk of HIV-Related Mortality Is Associated with Selenium Deficiency. J. Acquir. Immune Defic. Syndr. 1997, 15, 370–374. [Google Scholar] [CrossRef]
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum selenium levels and the risk of progression of laryngeal cancer. PLoS ONE 2018, 13, e0184873. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, D.; Liu, C.; Liu, G. Serum selenium levels and prostate cancer risk: A MOOSE-compliant meta-analysis. Medicine 2017, 96, e5944. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef]
- Talebi, S.S.; Badfar, G.; Shohani, M.; Soleymani, A.; Azami, M. The Relationship between Selenium and Lung Cancer: An Updated Systematic Review and Meta-Analysis. Int. J. Cancer Manag. 2018, 11, e8370. [Google Scholar] [CrossRef]
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Rahimifar, P.; Bahrami, S.; Delpesheh, A.; Hemati, F.; Alizadeh, S. The relationship between selenium levels and breast cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2014, 159, 1–7. [Google Scholar] [CrossRef]
- Amaral, A.F.; Cantor, K.P.; Silverman, D.T.; Malats, N. Selenium and bladder cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2407–2415. [Google Scholar] [CrossRef]
- Gong, H.Y.; He, J.G.; Li, B.S. Meta-analysis of the association between selenium and gastric cancer risk. Oncotarget 2016, 7, 15600–15605. [Google Scholar] [CrossRef] [PubMed]
- Mark, S.D.; Qiao, Y.L.; Dawsey, S.M.; Wu, Y.P.; Katki, H.; Gunter, E.W.; Fraumeni, J.F., Jr.; Blot, W.J.; Dong, Z.W.; Taylor, P.R. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J. Natl. Cancer Inst. 2000, 92, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Steevens, J.; van den Brandt, P.A.; Goldbohm, R.A.; Schouten, L.J. Selenium status and the risk of esophageal and gastric cancer subtypes: The Netherlands cohort study. Gastroenterology 2010, 138, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Cai, W.-S.; Li, J.-L.; Feng, Z.; Cao, J.; Xu, B. The Association Between Serum Levels of Selenium, Copper, and Magnesium with Thyroid Cancer: A Meta-analysis. Biol. Trace Elem. Res. 2015, 167, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Pathophysiological relevance of selenium. J. Endocrinol. Investig. 2013, 36, 1–7. [Google Scholar]
- Kiremidjian-Schumacher, L.; Roy, M.A.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. Biol. Trace Elem. Res. 1994, 41, 115. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.-S.; Kim, J.-H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Bügel, S.; Larsen, E.H.; Sloth, J.J.; Flytlie, K.; Overvad, K.; Steenberg, L.C.; Moesgaard, S. Absorption, excretion, and retention of selenium from a high selenium yeast in men with a high intake of selenium. Food Nutr. Res. 2008, 52, 1642. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef]
- Chen, J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac. J. Clin. Nutr. 2012, 21, 320–326. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, C.; Wang, X.; Liu, Y. Preliminary quantitative proteomics analysis in chronic and latent Keshan disease by iTRAQ labeling approach. Oncotarget 2017, 8, 105761–105774. [Google Scholar] [CrossRef]
- Zha, X.; Gao, X. Ecological analysis of Kashin-Beck osteoarthropathy risk factors in Tibet’s Qamdo City, China. Sci. Rep. 2019, 9, 2471. [Google Scholar] [CrossRef]
- Guo, X.; Ma, W.J.; Zhang, F.; Ren, F.L.; Qu, C.J.; Lammi, M.J. Recent advances in the research of an endemic osteochondropathy in China: Kashin-Beck disease. Osteoarthr. Cartil. 2014, 22, 1774–1783. [Google Scholar] [CrossRef]
- Schepman, K.; Engelbert, R.H.; Visser, M.M.; Yu, C.; de Vos, R. Kashin Beck Disease: More than just osteoarthrosis: A cross-sectional study regarding the influence of body function-structures and activities on level of participation. Int. Orthop. 2011, 35, 767–776. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.M.; Jones, D.P.; Brown, L.A. Glutathione redox control of asthma: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 17, 375–408. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Franke, S.I.; Bordin, D.L.; Prá, D.; Henriques, J.A. Biological functions of selenium and its potential influence on Parkinson’s disease. An. Acad. Bras. Cienc. 2016, 88, 1655–1674. [Google Scholar] [CrossRef]
- Conner, T.S.; Richardson, A.C.; Miller, J.C. Optimal Serum Selenium Concentrations Are Associated with Lower Depressive Symptoms and Negative Mood among Young Adults. J. Nutr. 2014, 145, 59–65. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Ngatu, N.R.; Januka, K.; Huang, H.-L.; Nattadech, C.; Suzuki, T.; Wada, K.; Ikeda, S. Selenium supplementation in HIV-infected individuals: A systematic review of randomized controlled trials. Clin. Nutr. ESPEN 2019, 34, 1–7. [Google Scholar] [CrossRef]
- Lim, T.K. Lecythis ollaria. In Edible Medicinal and Non Medicinal Plants: Volume 3, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 138–140. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Selenium interactions and toxicity. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biotechnol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.B.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420. [Google Scholar] [PubMed]
- Köhrle, J.; Jakob, F.; Contempré, B.; Dumont, J.E. Selenium, the Thyroid, and the Endocrine System. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chung, H.S.; Choi, M.-K.; Roh, Y.K.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Yu, J.M.; Moon, S. Association between Serum Selenium Level and the Presence of Diabetes Mellitus: A Meta-Analysis of Observational Studies. Diabetes Metab. J. 2019, 43, 447–460. [Google Scholar] [CrossRef]
- Cherdwongchareonsuk, D.; Aguas, A.P.; Henrique, R.; Upatham, S.; Sousa Pereira, A. Toxic effects of selenium inhalation: Acute damage of the respiratory system of mice. Hum. Exp. Toxicol. 2003, 22, 551–557. [Google Scholar] [CrossRef]
- Sobolev, O.; Gutyj, B.; Petryshak, R.; Pivtorak, J.; Kovalskyi, Y.; Naumyuk, A.; Petryshak, O.; Semchuk, I.; Mateusz, V.; Shcherbatyy, A.; et al. Biological role of selenium in the organism of animals and humans. Ukr. J. Ecol. 2018, 8, 654–665. [Google Scholar] [CrossRef]
- Cubas-Gaona, L.L.; de Francisco, P.; Martín-González, A.; Gutiérrez, J.C. Tetrahymena Glutathione Peroxidase Family: A Comparative Analysis of These Antioxidant Enzymes and Differential Gene Expression to Metals and Oxidizing Agents. Microorganisms 2020, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhang, Y. Systems Biology of Selenium and Complex Disease. Biol. Trace Elem. Res. 2019, 192, 38–50. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Zhou, Z. Selenium and Selenoproteins, from Structure, Function to Food Resource and Nutrition. Food Sci. Technol. Res. 2017, 23, 363–373. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Prabhu, K.S. Special Issue of “Optimal Selenium Status and Selenoproteins in Health”. Biol. Trace Elem. Res. 2019, 192, 1–2. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Wei, P.-C.; Hsu, J.-L.; Su, F.-Y.; Lee, W.-H. NPGPx (GPx7): A novel oxidative stress sensor/transmitter with multiple roles in redox homeostasis. Am. J. Transl. Res. 2016, 8, 1626–1640. [Google Scholar] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Guo, S.; Wang, G. Glutathione peroxidases as oncotargets. Oncotarget 2017, 8, 80093–80102. [Google Scholar] [CrossRef]
- Chung, S.S.; Kim, M.; Youn, B.-S.; Lee, N.S.; Park, J.W.; Lee, I.K.; Lee, Y.S.; Kim, J.B.; Cho, Y.M.; Lee, H.K.; et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol. Cell Biol. 2009, 29, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ouled-Haddou, H.; Messaoudi, K.; Lopes Dos Santos, R.; Carola, C.; Demont, Y.; Caulier, A.; Vong, P.; Jankovsky, N.; Lebon, D.; Platon, J.; et al. A New Role of Glutathion Peroxydase 4 in Human Erythroblast Enucleation. Blood 2019, 134, 938. [Google Scholar] [CrossRef]
- Alkazemi, D.; Rahman, A.; Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 2021, 11, 3034. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Herbette, S.; Roeckel-Drevet, P.; Drevet, J.R. Seleno-independent glutathione peroxidases. More than simple antioxidant scavengers. FEBS J. 2007, 274, 2163–2180. [Google Scholar] [CrossRef]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, B.; Li, X.; Han, X.; Liu, R.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: An update. Med. Res. Rev. 2019, 39, 5–39. [Google Scholar] [CrossRef]
- Naghashpour, M.; Amani, R.; Nutr, R.; Nematpour, S.; Haghighizadeh, M.H. Riboflavin Status and Its Association with Serum hs-CRP Levels among Clinical Nurses with Depression. J. Am. Coll. Nutr. 2011, 30, 340–347. [Google Scholar] [CrossRef]
- Saccoccia, F.; Angelucci, F.; Boumis, G.; Carotti, D.; Desiato, G.; Miele, A.E.; Bellelli, A. Thioredoxin reductase and its inhibitors. Curr. Protein Pept. Sci. 2014, 15, 621–646. [Google Scholar] [CrossRef]
- Yang, J.; Hamid, S.; Liu, Q.; Cai, J.; Xu, S.; Zhang, Z. Gene expression of selenoproteins can be regulated by thioredoxin(Txn) silence in chicken cardiomyocytes. J. Inorg. Biochem. 2017, 177, 118–126. [Google Scholar] [CrossRef]
- Rundlöf, A.-K.; Arnér, E.S. Regulation of the Mammalian Selenoprotein Thioredoxin Reductase 1 in Relation to Cellular Phenotype, Growth, and Signaling Events. Antioxid. Redox Signal. 2004, 6, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.-O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Zhu, J.-H.; Cheng, W.-H. Nuclear selenoproteins and genome maintenance. IUBMB Life 2016, 68, 5–12. [Google Scholar] [CrossRef]
- Hanschmann, E.-M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-J.; Geng, W.-S.; Wang, Z.-Q.; Chen, L.; Zeng, X.-S. The role of thioredoxin system in cancer: Strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and thioredoxin target proteins: From molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef]
- Williams, D.L.; Bonilla, M.; Gladyshev, V.N.; Salinas, G. Thioredoxin glutathione reductase-dependent redox networks in platyhelminth parasites. Antioxid. Redox Signal. 2013, 19, 735–745. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Brandstaedter, C.; Fritz-Wolf, K.; Weder, S.; Fischer, M.; Hecker, B.; Rahlfs, S.; Becker, K. Kinetic characterization of wild-type and mutant human thioredoxin glutathione reductase defines its reaction and regulatory mechanisms. FEBS J. 2018, 285, 542–558. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, X.; Ye, F.; Zhong, L. High-fat diet-induced changes in liver thioredoxin and thioredoxin reductase as a novel feature of insulin resistance. FEBS Open Bio 2014, 4, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; van Lith, M.; Mitchell, L.J.; Pringle, M.A.; Inaba, K.; Bulleid, N.J. The membrane topology of vitamin K epoxide reductase is conserved between human isoforms and the bacterial enzyme. Biochem. J. 2016, 473, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Xu, G.; Tu, W.; Qin, S. The relationship between deiodinase activity and inflammatory responses under the stimulation of uremic toxins. J. Transl. Med. 2014, 12, 239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alijani, E.; Abbasi, N. The Effect of Selenium on Hashimoto’s Thyroiditis; Systemic Review and Meta Analysis. J. Clin. Toxicol. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Milanesi, A.; Brent, G.A. Chapter 21—Thyroid Hormones. In Hormonal Signaling in Biology and Medicine; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 487–506. [Google Scholar] [CrossRef]
- Tsatsoulis, A. The role of iodine vs selenium on the rising trend of autoimmune thyroiditis in iodine sufficient countries. Endocrinol. Metab. Int. J. 2018, 6, 412–414. [Google Scholar] [CrossRef]
- Goemann, I.M.; Marczyk, V.R.; Romitti, M.; Wajner, S.M.; Maia, A.L. Current concepts and challenges to unravel the role of iodothyronine deiodinases in human neoplasias. Endocr. Relat. Cancer 2018, 25, R625–R645. [Google Scholar] [CrossRef]
- Bernal, J. Deiodinases. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 392–398. [Google Scholar] [CrossRef]
- Campos-Barros, A.; Meinhold, H.; Walzog, B.; Behne, D. Effects of selenium and iodine deficiency on thyroid hormone concentrations in the central nervous system of the rat. Eur. J. Endocrinol. 1997, 136, 316–323. [Google Scholar] [CrossRef]
- Panicker, V.; Cluett, C.; Shields, B.; Murray, A.; Parnell, K.S.; Perry, J.R.; Weedon, M.N.; Singleton, A.; Hernandez, D.; Evans, J.; et al. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J. Clin. Endocrinol. Metab. 2008, 93, 3075–3081. [Google Scholar] [CrossRef]
- Kazukauskiene, N.; Skiriute, D.; Gustiene, O.; Burkauskas, J.; Zaliunaite, V.; Mickuviene, N.; Brozaitiene, J. Importance of Thyroid Hormone level and Genetic Variations in Deiodinases for Patients after Acute Myocardial Infarction: A Longitudinal Observational Study. Sci. Rep. 2020, 10, 9169. [Google Scholar] [CrossRef]
- Sabatino, L.; Kusmic, C.; Iervasi, G. Modification of cardiac thyroid hormone deiodinases expression in an ischemia/reperfusion rat model after T3 infusion. Mol. Cell Biochem. 2020, 475, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Nunziata, C.; Polo, A.; Sorice, A.; Capone, F.; Accardo, M.; Guerriero, E.; Marino, F.Z.; Orditura, M.; Budillon, A.; Costantini, S. Structural analysis of human SEPHS2 protein, a selenocysteine machinery component, over-expressed in triple negative breast cancer. Sci. Rep. 2019, 9, 16131. [Google Scholar] [CrossRef]
- Tobe, R.; Mihara, H. Delivery of selenium to selenophosphate synthetase for selenoprotein biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2433–2440. [Google Scholar] [CrossRef]
- Mariotti, M.; Santesmasses, D.; Capella-Gutierrez, S.; Mateo, A.; Arnan, C.; Johnson, R.; D’Aniello, S.; Yim, S.H.; Gladyshev, V.N.; Serras, F.; et al. Evolution of selenophosphate synthetases: Emergence and relocation of function through independent duplications and recurrent subfunctionalization. Genome Res. 2015, 25, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.A.N.; Wang, T.; Pohl, T.; Sweetman, J.; Martin, S.A.M.; Secombes, C.J. Four selenoprotein P genes exist in salmonids: Analysis of their origin and expression following Se supplementation and bacterial infection. PLoS ONE 2018, 13, e0209381. [Google Scholar] [CrossRef]
- Mostert, V.; Lombeck, I.; Abel, J. A Novel Method for the Purification of Selenoprotein P from Human Plasma. Arch. Biochem. Biophys. 1998, 357, 326–330. [Google Scholar] [CrossRef]
- Kurokawa, S.; Bellinger, F.P.; Hill, K.E.; Burk, R.F.; Berry, M.J. Isoform-specific binding of selenoprotein P to the β-propeller domain of apolipoprotein E receptor 2 mediates selenium supply. J. Biol. Chem. 2014, 289, 9195–9207. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, S.; Eriksson, S.; Rose, K.L.; Wu, S.; Motley, A.K.; Hill, S.; Winfrey, V.P.; McDonald, W.H.; Capecchi, M.R.; Atkins, J.F.; et al. Sepp1(UF) forms are N-terminal selenoprotein P truncations that have peroxidase activity when coupled with thioredoxin reductase-1. Free Radic. Biol. Med. 2014, 69, 67–76. [Google Scholar] [CrossRef]
- Chen, P.; Wang, C.; Ma, X.; Zhang, Y.; Liu, Q.; Qiu, S.; Liu, Q.; Tian, J.; Ni, J. Direct Interaction of Selenoprotein R with Clusterin and Its Possible Role in Alzheimer’s Disease. PLoS ONE 2013, 8, e66384. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.M. Selenoproteins of the Human Prostate: Unusual Properties and Role in Cancer Etiology. Biol. Trace Elem. Res. 2019, 192, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Selenoprotein P as a significant regulator of pancreatic β cell function. J. Biochem. 2019, 167, 119–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Roh, Y.J.; Han, S.-J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.-R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Ujjawal, H.G.; Tejo, P.N.; Prabhu, K.S. Selenoproteins and their Role in Oxidative Stress and Inflammation. Curr. Chem. Biol. 2013, 7, 65–73. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Novoselov, V.I. Colocalization of selenium-containing protein V (SelV) and its partners in mammalian cells. Mol. Biol. 2012, 46, 735–737. [Google Scholar] [CrossRef]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol. Trace Elem. Res. 1996, 52, 227–239. [Google Scholar] [CrossRef]
- Latorre, A.O.; Greghi, G.F.; Netto, A.S.; Fukumasu, H.; Balieiro, J.C.; Côrrea, L.B.; Zanetti, M.A. Selenium and vitamin E enriched diet increases NK cell cytotoxicity in cattle. Pesqui. Vet. Bras. 2014, 34, 1141–1145. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Zhu, Y.J.; Li, W.G. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol. Trace Elem. Res. 1997, 56, 117–124. [Google Scholar] [CrossRef]
- Jayawardena, R.; Sooriyaarachchi, P.; Chourdakis, M.; Jeewandara, C.; Ranasinghe, P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. 2020, 14, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020, 37, 101715. [Google Scholar] [CrossRef]
- Bermano, G.; Méplan, C.; Mercer, D.K.; Hesketh, J.E. Selenium and viral infection: Are there lessons for COVID-19? Br. J. Nutr. 2021, 125, 618–627. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020, 112, 447–448. [Google Scholar] [CrossRef]
- Haberland, A.; Neubert, K.; Kruse, I.; Behne, D.; Schimke, I. Consequences of long-term selenium-deficient diet on the prostacyclin and thromboxane release from rat aorta. Biol. Trace Elem. Res. 2001, 81, 71–78. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2021, 82, 111053. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- de Faria Coelho-Ravagnani, C.; Corgosinho, F.C.; Sanches, F.L.F.Z.; Prado, C.M.M.; Laviano, A.; Mota, J.F. Dietary recommendations during the COVID-19 pandemic. Nutr. Rev. 2020, 79, 382–393. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef]
- Lipinski, B. Selenium Supplementation in the Prevention of Coronavirus Infections. J. Drug. Metab. Toxicol. 2020, 11, 246. [Google Scholar] [CrossRef]
- Morbat, M.M.; Hadi, A.M.; Hadri, D.H. Effect of Selenium in Treatment of Male Infertility. Exp. Tech. Urol. Nephrol. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.-J.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef]
- Jobeili, L.; Rousselle, P.; Béal, D.; Blouin, E.; Roussel, A.-M.; Damour, O.; Rachidi, W. Selenium preserves keratinocyte stemness and delays senescence by maintaining epidermal adhesion. Aging 2017, 9, 2302–2315. [Google Scholar] [CrossRef]
- Favrot, C.; Beal, D.; Blouin, E.; Leccia, M.T.; Roussel, A.M.; Rachidi, W. Age-Dependent Protective Effect of Selenium against UVA Irradiation in Primary Human Keratinocytes and the Associated DNA Repair Signature. Oxid. Med. Cell Longev. 2018, 2018, 5895439. [Google Scholar] [CrossRef]
- Jankovic, A.; Saso, L.; Korac, A.; Korac, B. Relation of Redox and Structural Alterations of Rat Skin in the Function of Chronological Aging. Oxid. Med. Cell Longev. 2019, 2019, 2471312. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 October 2020).
- Hatfield, D.L.; Gladyshev, V.N. The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol. Interv. 2009, 9, 18–21. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Soy, Selenium and Breast Cancer Risk. Available online: https://clinicaltrials.gov/ct2/show/NCT00555386 (accessed on 25 October 2020).
- ClinicalTrials.gov. Prevention of Female Cancers by Optimization of Selenium Levels in the Organism (SELINA). Available online: https://clinicaltrials.gov/ct2/show/NCT04014283 (accessed on 25 October 2020).
- ClinicalTrials.gov. Chemoprevention of Breast and Prostate Cancers in Shift Workers by Dietary Methylselenocysteine: Effects on Circadian Rhythm and Estrogen Receptor-B Cycling. Available online: https://clinicaltrials.gov/ct2/show/NCT01611038 (accessed on 25 October 2020).
- ClinicalTrials.gov. Colon Cancer Prevention Using Selenium. Available online: https://clinicaltrials.gov/ct2/show/NCT01211561 (accessed on 25 October 2020).
- ClinicalTrials.gov. S0000D: Effect of Vitamin E and/or Selenium on Colorectal Polyps in Men Enrolled on SELECT Trial SWOG-S0000 (ACP). Available online: https://clinicaltrials.gov/ct2/show/NCT00706121 (accessed on 25 October 2020).
- ClinicalTrials.gov. Antioxidant Supplement and Reduction of Metachronous Adenomas of the Large Bowel: A Double Blind Randomized Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT01437826 (accessed on 25 October 2020).
- ClinicalTrials.gov. Selenium in Preventing Tumor Growth in Patients with Previously Resected Stage I Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00008385 (accessed on 25 October 2020).
- ClinicalTrials.gov. Selenium in Preventing Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00978718 (accessed on 25 October 2020).
- ClinicalTrials.gov. Selenium and Prostate Cancer: Clinical Trial on Availability to Prostate Tissue and Effects on Gene Expression (SePros). Available online: https://clinicaltrials.gov/ct2/show/NCT00446901 (accessed on 25 October 2020).
- ClinicalTrials.gov. S0000 Selenium and Vitamin E in Preventing Prostate Cancer (SELECT). Available online: https://www.clinicaltrials.gov/ct2/show/NCT00006392 (accessed on 25 October 2020).
- ClinicalTrials.gov. S9917, Selenium in Preventing Cancer in Patients with Neoplasia of the Prostate. Available online: https://clinicaltrials.gov/ct2/show/NCT00030901 (accessed on 25 October 2020).
- ClinicalTrials.gov. Vitamin E, Selenium, and Soy Protein in Preventing Cancer in Patients with High-Grade Prostate Neoplasia. Available online: https://clinicaltrials.gov/ct2/show/NCT00064194 (accessed on 25 October 2020).
- ClinicalTrials.gov. Se-Methyl-Seleno-L-Cysteine or Selenomethionine in Preventing Prostate Cancer in Healthy Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT01497431 (accessed on 25 October 2020).
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Gutiérrez-González, E.; García-Esquinas, E.; de Larrea-Baz, N.F.; Salcedo-Bellido, I.; Navas-Acien, A.; Lope, V.; Gómez-Ariza, J.L.; Pastor, R.; Pollán, M.; Pérez-Gómez, B. Toenails as biomarker of exposure to essential trace metals: A review. Environ. Res. 2019, 179, 108787. [Google Scholar] [CrossRef]
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of Selenium Supplementation for Cancer Prevention in Patients with Carcinoma of the Skin: A Randomized Controlled Trial. JAMA 1996, 276, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Dunn, B.K. Selenium and prostate cancer prevention: Insights from the selenium and vitamin E cancer prevention trial (SELECT). Nutrients 2013, 5, 1122–1148. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, M.C.; Jung-Hynes, B.; Schmit, T.L.; Kumar, R.; Mukhtar, H.; Ahmad, N. Selenium and vitamin E for prostate cancer: Post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol. Med. 2011, 17, 134–143. [Google Scholar] [CrossRef]
- Dunn, B.K.; Richmond, E.; Anderson, D.E.; Greenwald, P. Chapter 40—Testing the Ability of Selenium and Vitamin E to Prevent Prostate Cancer in a Large Randomized Phase III Clinical Trial: The Selenium and Vitamin E Cancer Prevention Trial. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 567–582. [Google Scholar] [CrossRef]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C.; Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; FitzGerald, J.M.; Gleave, M.; Chambers, K. Intake of selenium in the prevention of prostate cancer: A systematic review and meta-analysis. Cancer Causes Control 2005, 16, 1125–3111. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Hooper, L.; Norat, T.; Lau, R.; Aune, D.; Greenwood, D.C.; Vieira, R.; Collings, R.; Harvey, L.J.; Sterne, J.A.; et al. Selenium and prostate cancer: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 111–122. [Google Scholar] [CrossRef]
- Fakhri, Y.; Kokhaei, P.; Moradi, M.; Dadar, M.; Amanidaz, N.; Zandsalimi, Y.; Moradi, B.; Amirhajeloo, L.R.; Keramati, H. Relationship between selenium and prostate cancer risk; systematic review and meta-analysis and meta-regression. Int. J. Med. Res. Health Sci. 2016, 5, 44–55. [Google Scholar]
- Sayehmiri, K.; Azami, M.; Mohammadi, Y.; Soleymani, A.; Tardeh, Z. The association between Selenium and Prostate Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2018, 19, 1431–1437. [Google Scholar] [CrossRef]
- Fritz, H.; Kennedy, D.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, A.; Sagar, S.; Wong, R.; Seely, D. Selenium and lung cancer: A systematic review and meta analysis. PLoS ONE 2011, 6, e26259. [Google Scholar] [CrossRef]
- Fakhri, Y.; Ghahremanfard, F.; Avazpour, M.; Moradi, M.; Amanidaz, N.; Zandsalimi, Y.; Moradi, B.; Amirhajeloo, L.R.; Keramati, H. Selenium and lung cancer: A systematic review, meta-analysis and meta-regression. Int. J. Pharm. Technol. 2016, 8, 13038–13056. [Google Scholar]
- Takata, Y.; Kristal, A.R.; King, I.B.; Song, X.; Diamond, A.M.; Foster, C.B.; Hutter, C.M.; Hsu, L.; Duggan, D.J.; Langer, R.D.; et al. Serum selenium, genetic variation in selenoenzymes, and risk of colorectal cancer: Primary analysis from the Women’s Health Initiative Observational Study and meta-analysis. Cancer Epidemiol. Biomar. Prev. 2011, 20, 1822–1830. [Google Scholar] [CrossRef]
- Ou, Y.; Jiang, B.; Wang, X.; Ma, W.; Guo, J. Selenium and Colorectal Adenomas Risk: A Meta-Analysis. Nutr. Cancer 2012, 64, 1153–1159. [Google Scholar] [CrossRef]
- Battin, E.E.; Perron, N.R.; Brumaghim, J.L. The central role of metal coordination in selenium antioxidant activity. Inorg. Chem. 2006, 45, 499–501. [Google Scholar] [CrossRef]
- de Rosa, V.; Erkekoğlu, P.; Forestier, A.; Favier, A.; Hincal, F.; Diamond, A.M.; Douki, T.; Rachidi, W. Low doses of selenium specifically stimulate the repair of oxidative DNA damage in LNCaP prostate cancer cells. Free Radic. Res. 2012, 46, 105–116. [Google Scholar] [CrossRef]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef]
- Zeng, H. Selenium as an essential micronutrient: Roles in cell cycle and apoptosis. Molecules 2009, 14, 1263–1278. [Google Scholar] [CrossRef]
- Lai, P.B.; Chi, T.Y.; Chen, G.G. Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis 2007, 12, 387–393. [Google Scholar] [CrossRef]
- Kapoor, S. Inhibition of osteopontin dependent carcinogenesis. J. Cancer Res. Clin. Oncol. 2008, 134, 927–928. [Google Scholar] [CrossRef]
- Gundimeda, U.; Schiffman, J.E.; Chhabra, D.; Wong, J.; Wu, A.; Gopalakrishna, R. Locally generated methylseleninic acid induces specific inactivation of protein kinase C isoenzymes: Relevance to selenium-induced apoptosis in prostate cancer cells. J. Biol. Chem. 2008, 283, 34519–34531. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bonorden, M.J.L.; Li, G.-X.; Lee, H.-J.; Hu, H.; Zhang, Y.; Liao, J.D.; Cleary, M.P.; Lü, J. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev. Res. 2009, 2, 484–495. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds-from toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef] [PubMed]
- Rikiishi, H. Apoptotic cellular events for selenium compounds involved in cancer prevention. J. Bioenerg. Biomembr. 2007, 39, 91–98. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Z.; Ganther, H.; Lü, J. Distinct Effects of Methylseleninic Acid versus Selenite on Apoptosis, Cell Cycle, and Protein Kinase Pathways in DU145 Human Prostate Cancer Cells. Mol. Cancer Ther. 2002, 1, 1059–1066. [Google Scholar]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug. Targets 2004, 4, 13–28. [Google Scholar] [CrossRef]
- Li, G.-x.; Lee, H.-J.; Wang, Z.; Hu, H.; Liao, J.D.; Watts, J.C.; Combs, G.F., Jr.; Lü, J. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 2008, 29, 1005–1012. [Google Scholar] [CrossRef]
- Zeng, H.; Briske-Anderson, M.; Wu, M.; Moyer, M.P. Methylselenol, a selenium metabolite, plays common and different roles in cancerous colon HCT116 cell and noncancerous NCM460 colon cell proliferation. Nutr. Cancer 2012, 64, 128–135. [Google Scholar] [CrossRef]
- Li, G.-X.; Hu, H.; Jiang, C.; Schuster, T.; Lü, J. Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int. J. Cancer 2007, 120, 2034–2043. [Google Scholar] [CrossRef]
- Shigemi, Z.; Manabe, K.; Hara, N.; Baba, Y.; Hosokawa, K.; Kagawa, H.; Watanabe, T.; Fujimuro, M. Methylseleninic acid and sodium selenite induce severe ER stress and subsequent apoptosis through UPR activation in PEL cells. Chem. Biol. Interact. 2017, 266, 28–37. [Google Scholar] [CrossRef]
- Gasparian, A.V.; Yao, Y.J.; Lü, J.; Yemelyanov, A.Y.; Lyakh, L.A.; Slaga, T.J.; Budunova, I.V. Selenium Compounds Inhibit IκB Kinase (IKK) and Nuclear Factor-κB (NF-κB) in Prostate Cancer Cells. Mol. Cancer Ther. 2002, 1, 1079–1087. [Google Scholar] [PubMed]
- Smith, M.L.; Lancia, J.K.; Mercer, T.I.; Ip, C. Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res. 2004, 24, 1401–1408. [Google Scholar] [PubMed]
- Cho, S.D.; Jiang, C.; Malewicz, B.; Dong, Y.; Young, C.Y.F.; Kang, K.-S.; Lee, Y.-S.; Ip, C.; Lü, J. Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol. Cancer Ther. 2004, 3, 605–612. [Google Scholar]


| Food | Typical Se Content (µg/g Fresh Weight) | References |
|---|---|---|
| Se-yeast | 3000 | [15] |
| Broccoli (Se-enriched) | 62.3 | [16] |
| Brazil nuts | 0.85–6.86 | [17] |
| Shellfish | 0.36–1.33 | [18] |
| Chicken | 0.57 | [19] |
| Beef | 0.35–0.47 | [20] |
| Lamb | 0.4 | [21] |
| Salmon | 0.21–0.27 | [22] |
| Eggs | 0.17 | [23] |
| Milk products | 0.1–0.55 | [17] |
| Age Group | Male (µg/d) | Female (µg/d) | Pregnancy (µg/d) | Lactation (µg/d) |
|---|---|---|---|---|
| Birth to 6 mths | 15 * | 15 * | ||
| 7 to 12 mths | 20 * | 20 * | ||
| 9 to 13 yrs | 40 | 40 | ||
| 14 to 18 yrs | 55 | 55 | 60 | 70 |
| 19 to 30 yrs | 55 | 55 | 60 | 70 |
| 31 to 50 yrs | 55 | 55 | 60 | 70 |
| 50 to 71 yrs | 55 | 55 | ||
| Above 71 yrs | 55 | 55 |
| NCT Identification Number | Study Title | Clinical Trial Status | Type of Cancer | Study Design | References |
|---|---|---|---|---|---|
| NCT00555386 | Soy, selenium, and breast cancer risk | Not applicable b | Breast Cancer | Randomized, double-blind (participant, investigator), placebo-controlled, parallel assignment | [158] |
| NCT04014283 | Prevention of female cancers by optimization of selenium levels in the organism (SELINA) | Not applicable c,d | Breast Cancer | Randomized, single-blind (investigator), placebo-controlled, parallel assignment | [159] |
| NCT01611038 | Chemoprevention of breast and prostate cancers in shift workers by dietary methylselenocysteine: effects on circadian rhythm and estrogen receptor-B cycling | Not applicable b | Breast and Prostate Cancer | Randomized, triple-blind (participant, investigator, outcomes assessor), placebo-controlled, parallel assignment | [160] |
| NCT01211561 | Colon cancer prevention using selenium | Unknown b,* | Colorectal Cancer | Randomized, double-blind (participant, investigator), placebo-controlled, single group assignment | [161] |
| NCT00706121 | S0000D: Effect of vitamin E and/or selenium on colorectal polyps in men enrolled on SELECT trial SWOG-S0000 (ACP) | Phase 3 completed b,+ | Colorectal Cancer | Randomized, quadruple-blind (participant, care provider, investigator, outcomes assessor), placebo-controlled, parallel assignment | [162] |
| NCT01437826 | Antioxidant supplement and reduction of metachronous adenomas of the large bowel: a double blind randomized trial | Phase 3 terminated a | Colorectal Cancer | Randomized, double-blind (participant, investigator), placebo-controlled, parallel assignment | [163] |
| NCT00008385 | Selenium in preventing tumor growth in patients with previously resected stage I non-small cell lung cancer | Phase 3 completed a,+ | Lung Cancer | Randomized, triple-blind (participant, care provider, investigator), placebo-controlled, parallel assignment | [164] |
| NCT00978718 | Selenium in preventing prostate cancer | Phase 3 Completed b | Prostate Cancer | Randomized, double-blind, placebo-controlled | [165] |
| NCT00446901 | Selenium and prostate cancer: clinical trial on availability to prostate tissue and effects on gene expression (SePros) | Not applicable b | Prostate Cancer | Randomized, quadruple-blind (participant, care provider, investigator, outcomes assessor), placebo-controlled, parallel assignment | [166] |
| NCT00006392 | S0000: Selenium and vitamin E in preventing prostate cancer (SELECT) | Phase 3 completed + | Prostate, Cancer | Randomized, quadruple-blind (participant, care provider, investigator, outcomes assessor), placebo-controlled, factorial assignment | [167] |
| NCT00030901 | S9917: Selenium in preventing cancer in patients with neoplasia of the prostate | Phase 3 completed a,+ | Prostate Cancer | Randomized, triple-blind (participant, care provider, investigator), placebo-controlled, parallel assignment | [168] |
| NCT00064194 | Vitamin E, selenium, and soy protein in preventing cancer in patients with high-grade prostate neoplasia | Phase 3 completed b | Prostate Cancer | Randomized, triple-blind (participant, care provider, investigator), placebo-controlled | [169] |
| NCT01497431 | Se-methyl-seleno-L-cysteine or selenomethionine in preventing prostate cancer in healthy participants | Phase 1 completed b | Prostate Cancer | Randomized, double-blind (participant, investigator), placebo-controlled, parallel assignment | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. https://doi.org/10.3390/nu13051649
Radomska D, Czarnomysy R, Radomski D, Bielawska A, Bielawski K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients. 2021; 13(5):1649. https://doi.org/10.3390/nu13051649
Chicago/Turabian StyleRadomska, Dominika, Robert Czarnomysy, Dominik Radomski, Anna Bielawska, and Krzysztof Bielawski. 2021. "Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity" Nutrients 13, no. 5: 1649. https://doi.org/10.3390/nu13051649
APA StyleRadomska, D., Czarnomysy, R., Radomski, D., Bielawska, A., & Bielawski, K. (2021). Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients, 13(5), 1649. https://doi.org/10.3390/nu13051649