3.1. Effect of Two-Week CBD Treatment on the n-6 and n-3 PUFA Ratio in the White and Red Skeletal Muscles As Well As Plasma of Rats Subjected to Standard and High-Fat Diets
Induction of obesity by feeding rats a HFD resulted in a significant reduction in the pool of the n-6 PUFAs, i.e., of linoleic acid (LA) (−16.9%,
p < 0.05), arachidonic acid (AA) (−10.1%,
p < 0.05), and also of n-3 PUFAs, i.e., of α-linolenic acid (ALA) (−16.0%,
p < 0.05) and eicosapentaenoic acid (EPA) (−17.5%,
p < 0.05) in the FFA fraction (
Table 1), in the white gastrocnemius muscle compared with the controls. We only observed an increase in the content of docosahexaenoic acid (DHA) (+32.9%,
p < 0.05;
Table 1) under the same conditions. Concomitantly, the rats fed both the standard and the HFD after the introduction of CBD exhibited a substantially elevated content of LA (+42.6% and +62.5%,
p < 0.05, vs. control group, respectively; +95.6%,
p < 0.05, vs. the HFD group;
Table 1), AA (+52.8% and +173.7%,
p < 0.05, vs. the control group, respectively; +204.5%,
p < 0.05, vs. the HFD group;
Table 1), ALA (+29.9%,
p < 0.05, vs. the control group; +30.7%,
p < 0.05, vs. the HFD group;
Table 1) and DHA (+52.8% and +161.1%,
p < 0.05, vs. the control group, respectively; +96.5%,
p < 0.05, vs. the HFD group;
Table 1) in the white skeletal muscle. However, compared to the control conditions, rats from the HFD group exhibited a considerably decreased EPA content after two-week CBD administration (−29.0%,
p < 0.05;
Table 1) in the same muscle type. In contrast, in the red gastrocnemius muscle, we noticed a significant increment in the fatty acid content of the FFA fraction, i.e., of LA (+76.0%), AA (+41.7%), ALA (+27.7%) and DHA (+75.1%) (
p < 0.05;
Table 2), in the rats subjected to a high-fat diet in comparison with the control rats, with the exception of EPA (
p > 0.05;
Table 2). Interestingly, the total intramuscular content of FFA fraction fatty acids in the rats fed either the standard or the high-fat diet was considerably greater in the chronic presence of CBD: LA (+39.2% and +63.5%,
p < 0.05, vs. the control group, respectively;
Table 1), AA (+41.7% and +99.3%,
p < 0.05, vs. the control group, respectively; +40.6%,
p < 0.05, vs. the HFD group;
Table 2), ALA (33.7% and 22.0%,
p < 0.05, vs. the control group, respectively;
Table 2), EPA (+33.6% and +20.1%,
p < 0.05, vs. the control group, respectively; +22.0%,
p < 0.05, vs. the HFD group;
Table 2) and DHA (+31.3% and +102.8%,
p < 0.05, vs. the control group, respectively; +15.8%,
p < 0.05, vs. the HFD group;
Table 2) in the oxidative muscle.
Concomitantly, in the DAG fraction, we observed that high-fat diet feeding caused a significant decrease of n-6 PUFAs (LA (−22.2%) and AA (−19.9%)) and n-3 PUFAs (ALA (−22.0%)), whereas the DHA content was markedly elevated (+42.0%) in the white gastrocnemius muscle in comparison with the control group (
p < 0.05;
Table 3). Interestingly, CBD treatment in the rats fed a standard chow resulted in a substantially reduced content of LA (−14.6%,
p < 0.05, vs. the control group;
Table 2) and increased AA (+32.5%,
p < 0.05, vs. the control group;
Table 3) in the white skeletal muscle. However, in the same muscle type, the HFD group after CBD administration was characterized by a significant increment in the pool of LA (+26.8%,
p < 0.05, vs. the HFD group;
Table 3), AA (+130.4%,
p < 0.05, vs. the control group; +187.5%,
p < 0.05, vs. the HFD group;
Table 3) and DHA (+107.9%,
p < 0.05, vs. the control group; +46.4%,
p < 0.05, vs. the HFD group;
Table 3), whereas the content of ALA and EPA was considerably reduced (−24.5% and −23.0%, respectively) in comparison with the controls (
p < 0.05;
Table 3). Conversely, in the red gastrocnemius muscle, changes in the total fatty acid content of the DAG fraction in the rats subjected to a high-fat diet were accompanied by a significant increase in the content of n-6 PUFAs (LA (+82.9%) and AA (+18.5%)) as well as all of n-3 PUFAs (ALA (+31.3%), EPA (+23.1%) and DHA (+93.6%)) in comparison with the rats fed a standard diet (
p < 0.05;
Table 4). CBD treatment in the rats fed the standard chow markedly enhanced the AA and EPA content (+29.9% and +39.7%,
p < 0.05, vs. the control group, respectively;
Table 4) with no change in LA, ALA and DHA levels (
p > 0.05;
Table 4) in the oxidative muscle. Similarly, we observed that the HFD-fed group after CBD administration showed a significantly greater accumulation of LA (+70%,
p < 0.05, vs. the control group;
Table 4), AA (+78.8%,
p < 0.05, vs. the control group; +50.9%,
p < 0.05, vs. the HFD group;
Table 4), ALA (+44.8%,
p < 0.05, vs. the control group; +10.2%,
p < 0.05, vs. the HFD group;
Table 4), EPA (+80.4%,
p < 0.05, vs. the control group; +46.5%,
p < 0.05, vs. the HFD group;
Table 4) and DHA (+131.3%,
p < 0.05, vs. the control group; +19.4%,
p < 0.05, vs. the HFD group;
Table 4) in the pool of the DAG fraction in the red gastrocnemius muscle.
As shown in
Table 5, the rats from the HFD group had an increased content of LA (+337.1%), AA (+64.8%), ALA (+138.1%) and DHA (+88.1%) compared to the control group in the white skeletal muscle’s TAG fraction (
p < 0.05;
Table 5) with no alterations in the EPA levels (
p > 0.05;
Table 5). Moreover, we noticed that two-week CBD treatment in the rats fed the standard chow considerably elevated only DHA levels (+50.0%,
p < 0.05, vs. the control group;
Table 5) of the TAG fraction in the white skeletal muscle. Concomitantly, in the same muscle type, we observed that CBD administration to rats after the HFD course substantially enhanced the levels of n-6 PUFAs, i.e., of LA and AA (+187.0% and +72.5%,
p < 0.05, vs. the control group, respectively;
Table 5) in the TAG fraction, whereas the LA content in the same experimental group compared to the corresponding untreated HFD group was decreased (−34.4%,
p < 0.05;
Table 5). Furthermore, we noticed a similar effect of two-week CBD treatment in the case of n-3 PUFAs such as ALA (+95.4%,
p < 0.05, vs. the control group;
Table 5) and DHA (+171.9%,
p < 0.05, vs. the control group; +43.9%,
p < 0.05, vs. the HFD group;
Table 5), although only the EPA content was significantly reduced (−37.5%,
p < 0.05, vs. the control group;
Table 5) in the white gastrocnemius muscle. With respect to the red skeletal muscle, high-fat feeding considerably intensified the accumulation of LA and DHA (+116.9% and +161.6%, respectively;
p < 0.05;
Table 6) in the TAG fraction in comparison with the rats fed the standard diet. Interestingly, compared to the control conditions, we did not observe any significant alterations in the PUFA composition of the TAG fraction in the CBD group in the same muscle type (
p > 0.05;
Table 6). However, the HFD group after CBD administration exhibited a substantially reduced level of LA (−37.7%,
p < 0.05, vs. the HFD group;
Table 6) along with a substantial rise in n-3 PUFAs EPA (+56.2%,
p < 0.05, vs. the HFD group;
Table 6) and DHA (+249.9%,
p < 0.05, vs. the control group; +33.7%,
p < 0.05, vs. the HFD group;
Table 6).
Our study demonstrated that the total fatty acid composition of PL was altered by the high-fat diet in both white and red gastrocnemius muscles. Regarding the glycolytic muscle, we noticed a pronounced decrease in the content of LA, ALA and EPA in the rats subjected to the HFD (−39.5%, −43.9% and −46.8%, respectively;
p < 0.05;
Table 7) compared to the control rats, whereas only the DHA content was increased (+12.7%,
p < 0.05;
Table 7) in the same conditions. CBD given to the rats fed a standard chow did not affect in a statistically significant manner the PUFA content in the white skeletal muscle in the PL fraction. However, when CBD was administered to rats after the HFD course, it considerably reduced the content of LA, ALA and EPA (−27.2%, −37.0% and −37.3%, respectively;
p < 0.05;
Table 7) and simultaneously increased the AA and DHA levels (+21.6% and +69.2%, respectively;
p < 0.05;
Table 7) in comparison with the control conditions. In the same experimental rats, compared to the HFD group alone, we observed a substantially increased accumulation of all PUFAs: LA, AA, ALA, EPA and DHA in the PL fraction (+20.3%, +19.9%, +12.3%, +17.7% and +50.1%, respectively;
p < 0.05;
Table 7). Concomitantly, in the PL fraction of the red gastrocnemius muscle, the HFD-fed rats were characterized by considerably elevated AA and DHA content (+21.5% and +25.6%, respectively;
p < 0.05;
Table 8) with a parallel decline in the levels of ALA and EPA (−42.4% and −30.1%, respectively;
p < 0.05;
Table 8) along with no changes in the LA content (
p > 0.05;
Table 8) compared to the controls. CBD administration while feeding rats with the standard chow resulted in a pronounced increase in n-6 PUFAs, that is, LA (+21.0%) and AA (+19.4%) as well as in n-3 PUFAs, that is, ALA (+11.1%) and DHA (+53.3%) (
p < 0.05;
Table 8) in comparison with the control group. Additionally, we observed a significant increase in the PUFA content of the PL fraction after prolonged CBD administration in the high-fat diet rats: LA (+27.2%,
p < 0.05, vs. the control group; +23.1%,
p < 0.05, vs. the HFD group), AA (+53.4%,
p < 0.05, vs. the control group; +26.3%,
p < 0.05, vs. the HFD group) and DHA (+78.7%,
p < 0.05, vs. the control group; +42.2%,
p < 0.05, vs. the HFD group) in the red skeletal muscle (
Table 8). These CBD effects were accompanied by a reduction in the ALA and EPA content (−32.7% and −25.9%, respectively;
p < 0.05;
Table 8) compared to the control rats in the red skeletal muscle.
In the plasma FFA fraction of the rats fed an HFD, we noticed significantly increased levels of n-6 PUFAs, i.e., of LA (+68.5%) and AA (+107.3%) and of n-3 PUFA DHA (+31.8%) (
p < 0.05;
Table 9). Interestingly, CBD administration to the animals fed a standard diet caused a pronounced elevation of all the PUFAs: LA, AA, ALA, EPA and DHA (+52.3%, +88.2%, +43.6%, +35.0% and +108.1%, respectively;
p < 0.05;
Table 9) in comparison with the corresponding control rats. Similarly, the high fat diet-fed rats after CBD administration presented a significantly elevated level of LA (+167.9%,
p < 0.05, vs. the control group; +59.0%,
p < 0.05, vs. the HFD group), AA (+90.5%,
p < 0.05, vs. the control group), ALA (+95.5%,
p < 0.05, vs. the control group; +67.2%,
p < 0.05, vs. the HFD group) and DHA (81.2%,
p < 0.05, vs. the control group; +37.5%,
p < 0.05, vs. the HFD group). The only fatty acid of plasma FFA PUFAs with the content reduced in the rats fed an HFD after two-week CBD treatment was EPA (−23.6%,
p < 0.05, vs. the control group;
Table 9). However, in comparison with the corresponding untreated HFD group, the EPA content was substantially enhanced (+33.5%,
p < 0.05;
Table 9). On the other hand, in the plasma TAG fraction, the high-fat diet resulted in a considerable decrease in fatty acids belonging to n-3 PUFAs: ALA (−46.6%), EPA (−65.8%) and DHA (−56.1%) (
p < 0.05, vs. the control group;
Table 10). Moreover, we observed a marked elevation of the DHA content in the plasma TAG fraction of animals fed a standard diet after prolonged CBD administration (+22.9%,
p < 0.05;
Table 10) compared to the controls. Consistently, CBD given to the rats after the HFD course significantly reduced the EPA and DHA content (−61.5% and −47.4%,
p < 0.05, vs. the control group;
Table 10). However, in comparison with the HFD group alone, we reported in the same experimental group a substantially declined content of AA in the chronic presence of CBD (−18.4%,
p < 0.05;
Table 10) and, conversely, a significantly greater content of ALA (+60.0%,
p < 0.05;
Table 10).
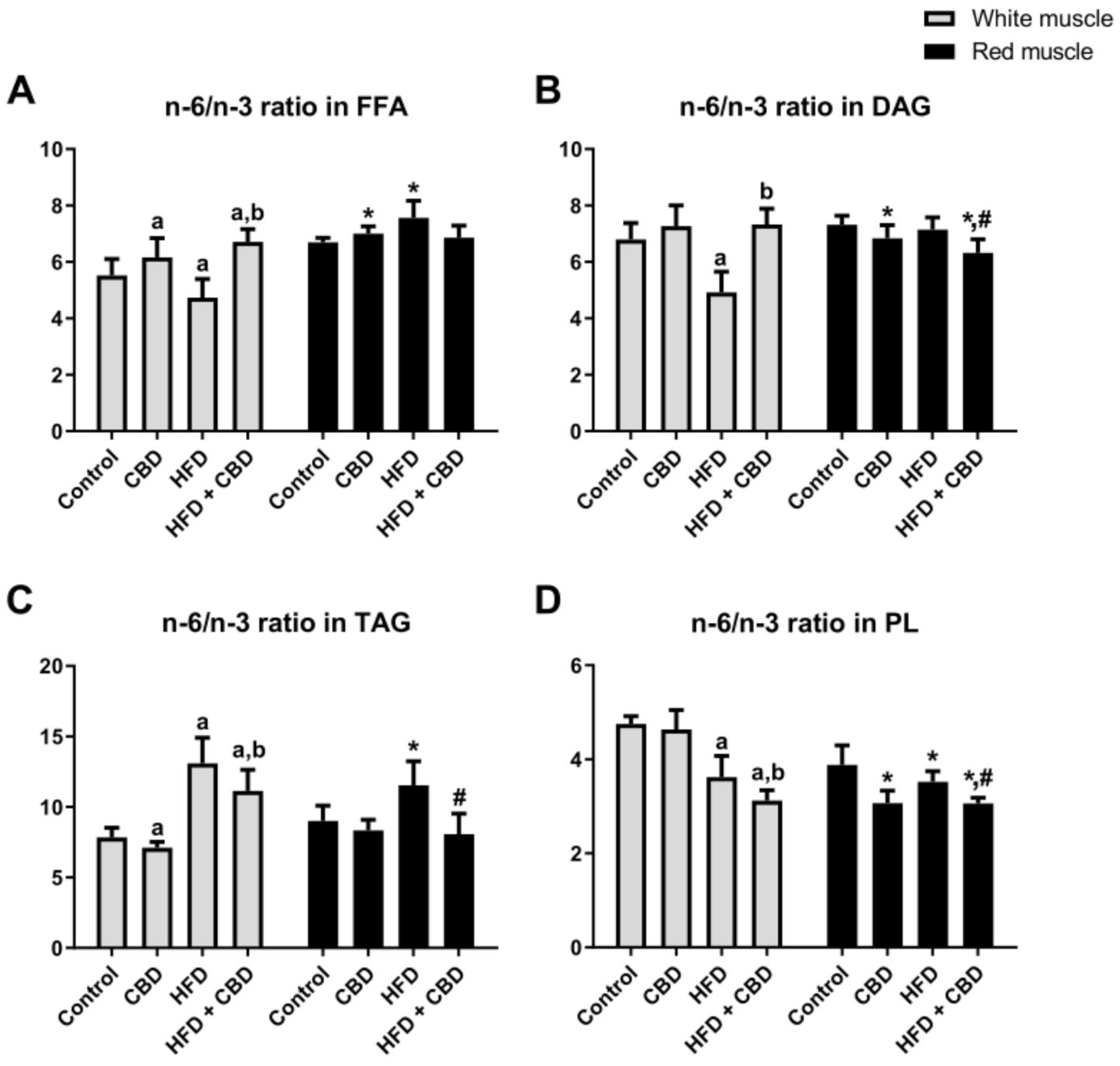
Our study demonstrated that the high-fat diet substantially reduced the n-6/n-3 PUFA ratio in the FFA and DAG fractions (−14.2% and −27.6%,
p < 0.05;
Figure 1A,B, respectively) in the white gastrocnemius muscle compared to the controls, whereas CBD administration considerably enhanced it (+41.7% and +49%.0%,
p < 0.05;
Figure 1A,B, respectively) in comparison with the HFD group alone. Regarding the FFA fraction, we also found that two-week CBD treatment significantly increased the n-6/n-3 PUFA ratio in the rats fed either the standard chow or the high-fat diet in the white gastrocnemius muscle (+11.6% and +21.6%,
p < 0.05;
Figure 1A) in comparison with the control group. On the other hand, in the red gastrocnemius muscle, we observed elevated n-6/n-3 PUFA ratio in the rats fed HFD in the FFA fraction (+12.9%,
p < 0.05, vs. the control group;
Figure 1A), whereas chronic CBD treatment resulted in a significant decrease in the same experimental group (−9.3%,
p < 0.05, vs. the HFD group;
Figure 1A). Interestingly, in the red gastrocnemius muscle, we observed a substantial reduction in the n-6/n-3 PUFA ratio in the rats subjected to standard chow or high-fat diet feeding after CBD administration in the DAG (−6.6% and −13.7%,
p < 0.05, vs. the control group, respectively; −11.6%,
p < 0.05, vs. the HFD group;
Figure 1B) and PL fractions (−21.0% and −21.2%,
p < 0.05, vs. the control group, respectively; −13.1%,
p < 0.05, vs. the HFD group;
Figure 1D). With respect to the TAG fraction (
Figure 1C) in the white and red gastrocnemius muscles, we observed that the HFD-fed groups exhibited a significantly increased n-6/n-3 PUFA ratio (+66.8% and +28.1%, respectively;
p < 0.05;
Figure 1C) in comparison with the control group. Concomitantly, two-week CBD treatment caused a substantial reduction in the n-6/n-3 PUFA ratio (−14.9% and −30.1%, respectively;
p < 0.05;
Figure 1C) compared to the HFD group alone. Moreover, the n-6/n-3 PUFA ratio in the TAG fraction only in the white gastrocnemius muscle was considerably reduced by CBD administration in the rats fed a standard chow (−9.5%,
p < 0.05;
Figure 1C) and, conversely, in the rats fed the HFD, it was significantly elevated (+42.0%,
p < 0.05;
Figure 1C) in comparison with the control group. Simultaneously, in the PL fraction, we observed that the HFD-fed groups exhibited a markedly decreased n-6/n-3 PUFA ratio in both the white (−23.8%,
p < 0.05;
Figure 1D) and the red (−9.3%,
p < 0.05;
Figure 1D) gastrocnemius muscle compared to the control conditions. Moreover, CBD administration to animals being on an HFD in the white gastrocnemius muscle resulted in a pronounced reduction of the n-6/n-3 PUFA ratio in the PL fraction (−13.9%,
p < 0.05, vs. the HFD group;
Figure 1D).
Induction of obesity by high-fat diet feeding resulted in a significantly increased n-6/n-3 PUFA ratio in both HFD-fed groups (untreated and treated with CBD) in the plasma FFA (+47.3% and +37.3%, respectively;
p < 0.05;
Figure 2A) and TAG fractions (+57.1% and +49.1%, respectively,
p < 0.05;
Figure 2B) compared to the control rats. Furthermore, in the plasma of the rats fed a standard diet and injected with CBD, we observed a markedly decreased n-6/n-3 PUFA ratio in the FFA (−7.4%,
p < 0.05;
Figure 2A) and TAG (−6.9%,
p < 0.05;
Figure 2B) fractions in comparison with the control group. Importantly, the n-6/n-3 PUFA ratio was substantially decreased in the chronic presence of CBD during the high-fat diet feeding course in the plasma FFA fraction (−6.8%,
p < 0.05, vs. the HFD group;
Figure 2A).
3.3. Effect of Two-Week CBD Treatment on the Total Intramuscular Expression of Proteins Involved in the Inflammatory Pathway in the White and Red Skeletal Muscles of Rats Subjected to Standard and High-Fat Diets
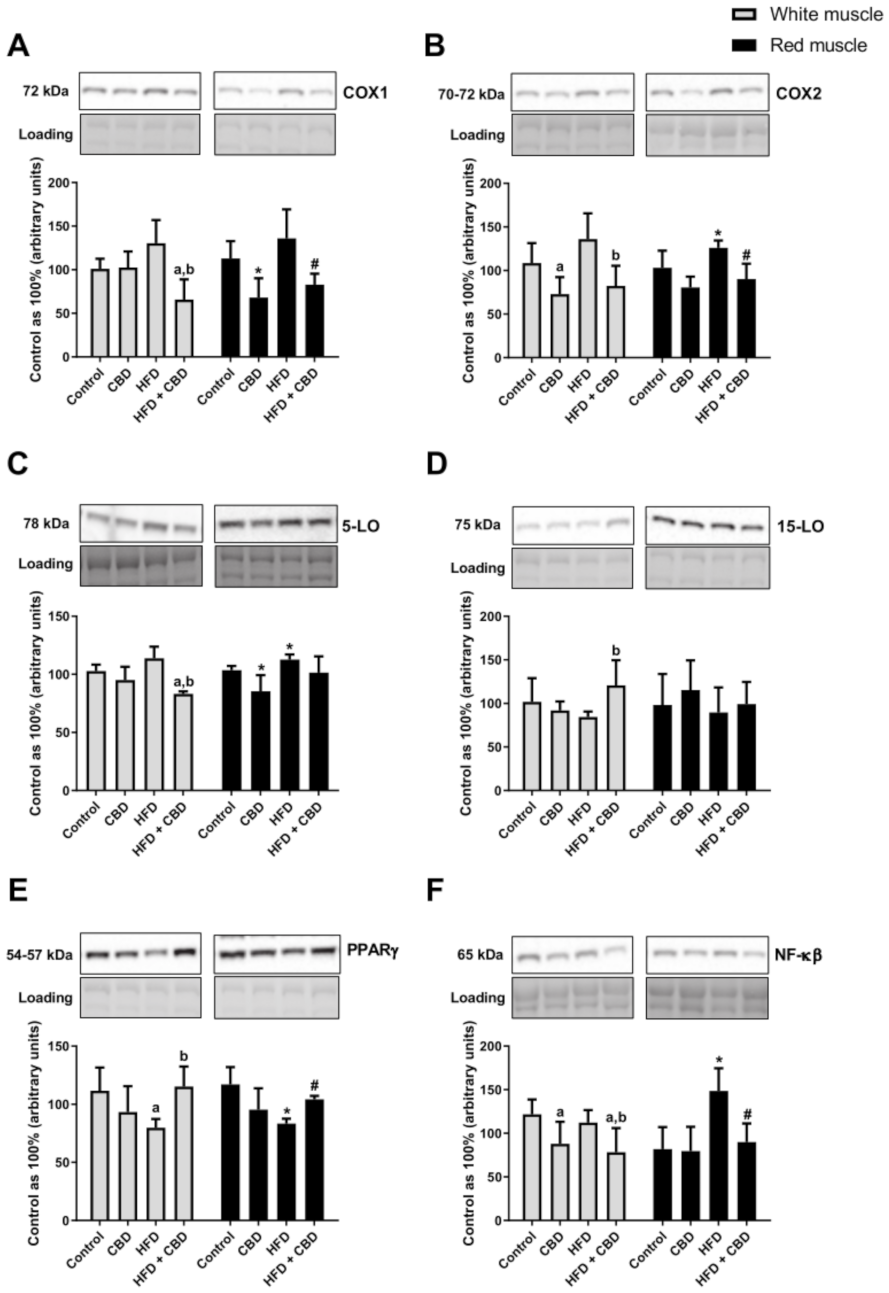
Compared to the control group, the rats fed a high-fat diet were characterized by a substantial increase in the total expression of COX2 and 5-LO in the red gastrocnemius muscle (+22.3% and +8.9%, respectively;
p < 0.05;
Figure 4B,C). Moreover, during HFD administration, we observed a trend towards an increase in the COX1 expression (
p = 0.0590, vs. the control group;
Figure 4A) in the white gastrocnemius muscle. Most importantly, two-week CBD injections in the high-fat diet group resulted in a considerable reduction of the total intramuscular expression of the proteins involved in the inflammatory pathway in both white and red gastrocnemius muscles, i.e., of COX1 (−49.5% and −39.0%, respectively;
p < 0.05;
Figure 4A) and COX2 (−39.5% and −28.4%, respectively;
p < 0.05;
Figure 4B) compared to the HFD alone. Similar effects of CBD treatment in the HFD-fed rats we reported in the case of the total intramuscular 5-LO expression in the white gastrocnemius muscle (−19.1%,
p < 0.05, vs. the control group; −26.9%,
p < 0.05, vs. the HFD group;
Figure 4C). Simultaneously, we noticed a markedly declined total expression of COX1 and 5-LO in the rats fed a standard chow with the chronic presence of CBD in the red skeletal muscle (−32.8% and −17.5%,
p < 0.05, vs. the control group;
Figure 4A,C). Concomitantly, in the white skeletal muscle, we observed that CBD treatment of the rats subjected to an HFD substantially increased the total expression of anti-inflammatory 15-LO (+43.2%,
p < 0.05;
Figure 4D) in comparison with the HFD group alone. As presented in
Figure 4, intramuscular PPARγ expression decreased considerably during the course of a high-fat diet in the white (−28.5%,
p < 0.05;
Figure 4E) and red (−28.8%,
p < 0.05;
Figure 4E) skeletal muscles in comparison with the control rats. However, prolonged CBD administration in the high-fat diet rats resulted in pronounced restoration of the total expression of PPARγ in both muscle types (+44.2% and +25.0%, respectively;
p < 0.05;
Figure 4E) compared to the HFD group. Furthermore, the group receiving a high-fat diet demonstrated a significant elevation in the total muscular expression of NF-κB in the red gastrocnemius muscle (+81.5%,
p < 0.05, vs. the control group;
Figure 4F). We also found that in the same muscle type, chronic CBD treatment in the HFD-fed rats substantially reduced the expression of NF-κB (−39.4%,
p < 0.05;
Figure 4F) and IL-6 (−17.2%,
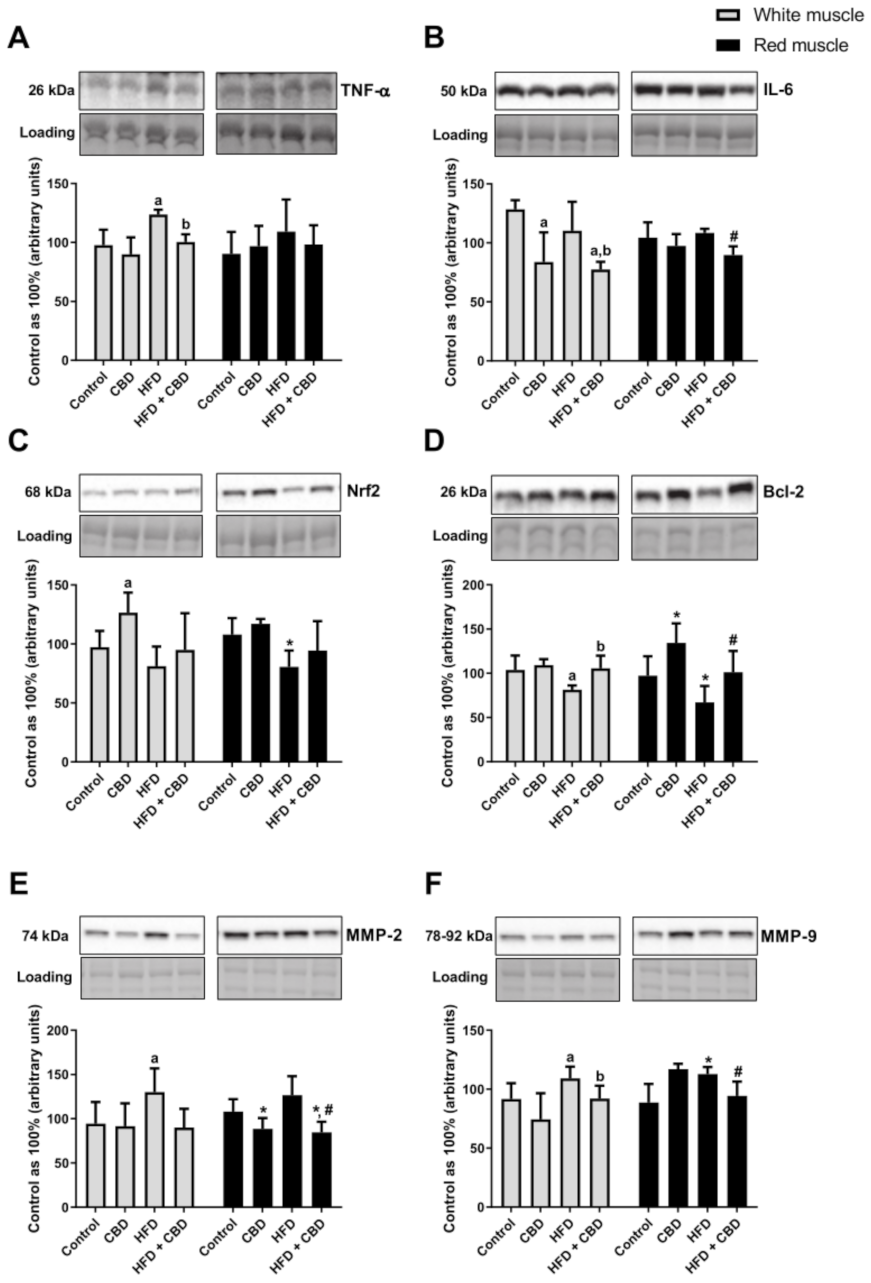
p < 0.05;
Figure 5B) in comparison with the HFD group. Similarly, in the white gastrocnemius muscle of the rats subjected to the standard or high-fat chow administration, we observed a significant decrease in the total expression of NF-κB after CBD injections (−27.8% and −35.8%, respectively,
p < 0.05, vs. the control group; −30.3%,
p < 0.05, vs. the HFD group;
Figure 4F) and IL-6 (−34.7% and −39.7%, respectively,
p < 0.05, vs. the control group; −29.9%,
p < 0.05, vs. the HFD group;
Figure 5B). In the case of TNF-α, we reported that the rats subjected to an HFD presented a substantially increased expression (+26.6%,
p < 0.05, vs. the control group;
Figure 5A), which was further decreased by CBD treatment (−18.7%,
p < 0.05, vs. the HFD group;
Figure 5A) in the white gastrocnemius muscle. Concomitantly, we did not notice any significant changes in TNF-α expression in the oxidative muscle (
p > 0.05;
Figure 5A). Moreover, we observed that high-fat diet feeding reduced the total expression of Nrf2 in the red skeletal muscle (−25.3%,
p < 0.05;
Figure 5C) and Bcl-2 in both muscle types (−21.5% and −31.3%, respectively;
p < 0.05;
Figure 5D) compared to the controls. Interestingly, Nrf2 expression considerably increased in the standard chow-fed group after two-week CBD injections (+30.1%,
p < 0.05, vs. the control group;
Figure 5C) in the white gastrocnemius muscle. Similar effects of CBD treatment compared to the control conditions were observed with respect to the total expression of Bcl-2 (+38.1%,
p < 0.05;
Figure 5D) in the red skeletal muscle. Concomitantly, we also noticed a substantial increase in the total Bcl-2 expression in the white and red gastrocnemius muscles of the HFD group after CBD administration (+29.5% and +50.9%, respectively;
p < 0.05;
Figure 5D) in comparison with the HFD group alone. Moreover, in both muscle types, we reported that high-fat diet feeding significantly increased the total expression of MMP-2 (+37.9%,
p < 0.05;
Figure 5E) and MMP-9 (+19.1% and +27.2%, respectively;
p < 0.05;
Figure 5F) compared to the respective controls. Simultaneously, in the red gastrocnemius muscle of the rats subjected to the standard or high-fat chow administration, we observed a significant decrease in the total expression of MMP-2 after CBD administration (−21.6% and −21.8%, respectively,
p < 0.05, vs. the control group; −33.2%,
p < 0.05, vs. the HFD group;
Figure 5E). Most importantly, CBD injections in the HFD-fed rats considerably decreased the expression of MMP-9 in both white (−21.5%,
p < 0.05;
Figure 5F) and red (−16.4%,
p < 0.05;
Figure 5F) gastrocnemius muscles in comparison with the HFD group.