Effects of Gestational Diabetes in Cognitive Behavior, Oxidative Stress and Metabolism on the Second-Generation Off-Spring of Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use Statement
2.2. Experimental Design and Animal Breeding
2.3. Cognitive Assessment
2.3.1. Anxious-Type Behavior Tests
Elevated Plus Maze Test
Open Field Test
2.3.2. Learning and Memory Tests
Spatial Learning Test
Spatial Working Memory Test
2.4. Tissue Preparation
2.5. Biochemical Determinations
2.5.1. Reactive Oxygen Species
2.5.2. Lipid-Peroxidation
2.5.3. Glutathione Measurement
2.5.4. Superoxide Dismutase Activity
2.5.5. Catalase Activity
2.6. Serum Parameters
2.7. Statistical Analysis
3. Results
3.1. Effect of GD on the Body Weight of Second-Generation Offspring
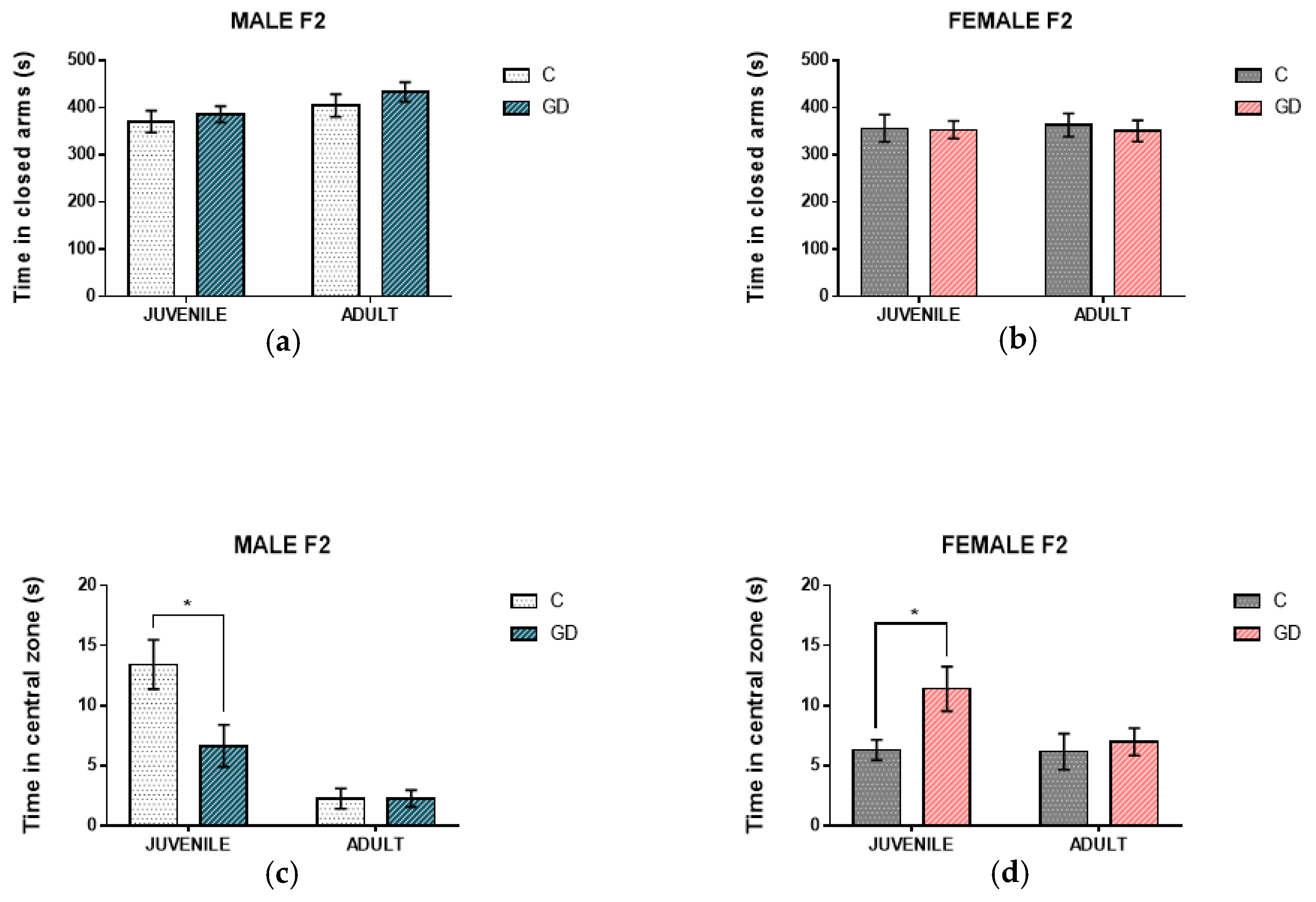
3.2. Effect of GD on the Anxious-Type Behavior of Second-Generation Offspring
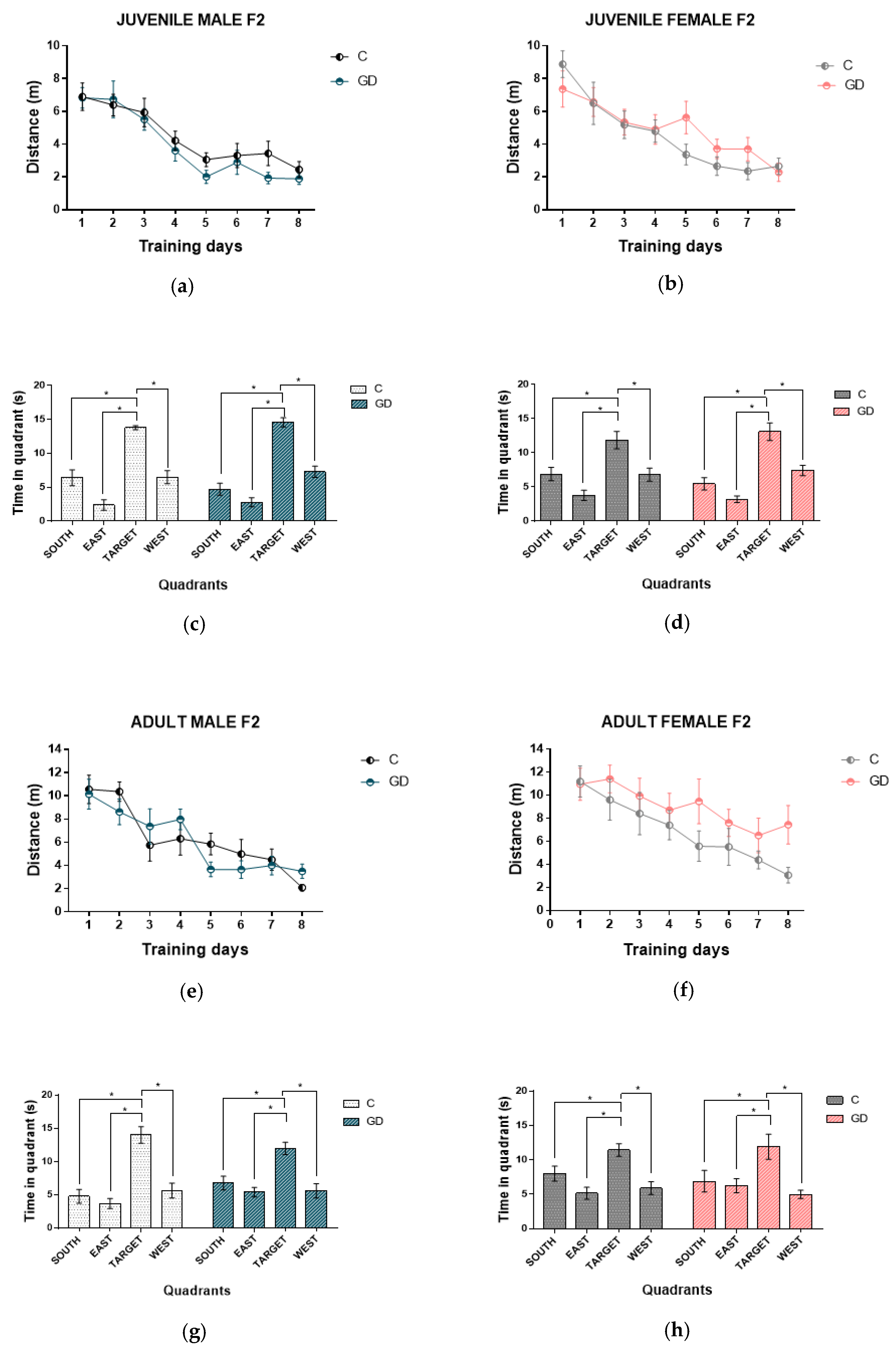
3.3. Effect of GD on the Spatial Learning of Second-Generation Offspring
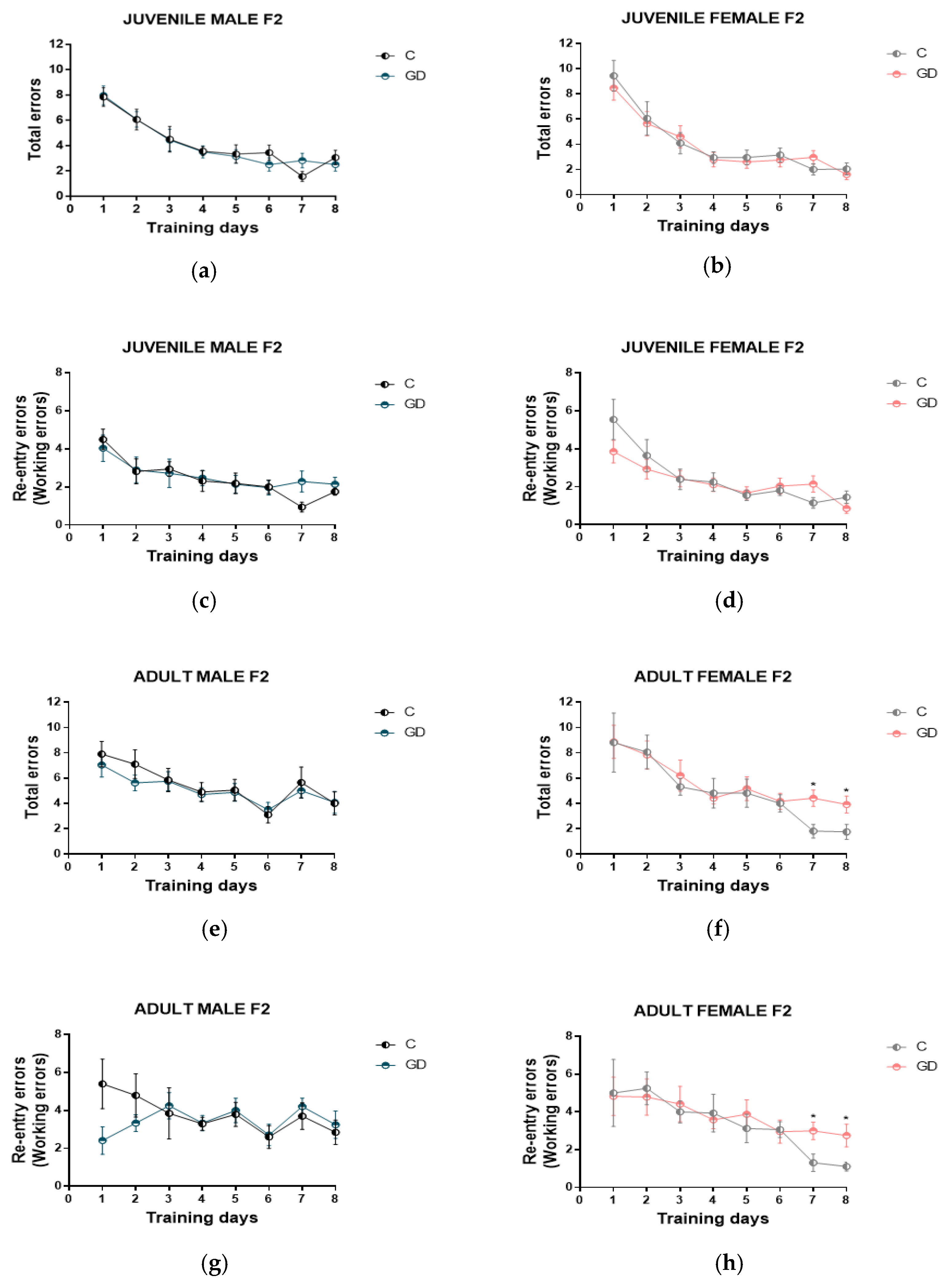
3.4. Effect of GD on the Spatial Working Memory of Second-Generation Offspring
3.5. Effecst of GD on Oxidative Stress Biomarkers of the Hippocampus and Second-Generation Cerebral Cortex
3.6. Effect of GD on Glutathione Levels of the Hippocampus and Second-Generation Cerebral Cortex
3.7. Effects of GD on the SOD and Catalase Enzimatic Activity of the Hippocampus and Second-Generation Cerebral Cortex
3.8. Effect of GD on the Serum Parameters of Second-Generation Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; pp. 1–150. Available online: http://www.diabetesatlas.org (accessed on 1 December 2020).
- Hay, W.W., Jr. Placental-fetal glucose exchange and fetal glucose metabolism. Trans. Am. Clin. Climatol. Assoc. 2006, 117, 321–340. [Google Scholar]
- Martin, F.I.; Heath, P.; Mountain, K.R. Pregnancy in women with diabetes mellitus. Fifteen years’ experience: 1970–1985. Med. J. Aust. 1987, 146, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, K.; Reiher, H.; Semmier, K.; Fischer, F.; Fischer, M.; Glockner, E. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care 1983, 6, 219–223. [Google Scholar] [CrossRef]
- Cowett, R.M.; Shwartz, R. The infant of the diabetic mother. NeoReviews 1982, 29, 1213–1231. [Google Scholar]
- Small, M.; Cameron, A.; Lunan, C.B.; MacCuish, A.C. Macrosomia in pregnancy complicated by insulin dependent diabetes mellitus. Diabetes Care 1987, 10, 594–599. [Google Scholar] [CrossRef]
- Pinney, S.E.; Simmons, R.A. Metabolic programming, epigenetics, and gestational diabetes mellitus. Curr. Diabetes Rep. 2012, 12, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Benyshek, D.C.; Johnston, C.S.; Martin, J.F. Glucose metabolism is altered in the adequately-nourished grand-offspring (F3 generation) of rats malnourished during gestation and perinatal life. Diabetologia 2006, 49, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, E.; Martinez-Samayoa, P.M.; Bautista, C.J.; Deas, M.; Guillen, L.; Rodriguez-Gonzalez, G.L.; Guzmán, C.; Larrea, F.; Nathanielsz, P.W. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J. Physiol. 2005, 566, 225–236. [Google Scholar] [CrossRef]
- Huang, Y.H.; Ye, T.T.; Liu, C.X.; Wang, L.; Chen, Y.W.; Dong, Y. Maternal high-fat diet impairs glucose metabolism, β-cell function and proliferation in the second generation of offspring rats. Nutr. Metab. 2017, 14, 2–7. [Google Scholar] [CrossRef]
- Aerts, L.; Holemans, K.; Van Assche, F.A. Maternal diabetes during pregnancy: Consequences for the offspring. Diabetes Metab. Rev. 1990, 6, 147–167. [Google Scholar] [CrossRef]
- Yamamoto, J.M.; Benham, J.L.; Dewey, D.; Sanchez, J.J.; Murphy, H.R.; Feig, D.S.; Donovan, D.E. Neurocognitive and behavioral outcomes in offspring exposed to maternal pre-existing diabetes: A systematic review and meta-analysis. Diabetologia 2019, 62, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Marks, D.J.; Grossman, B.; Yoon, M.; Loudon, H.; Stone, J.; Halperinet, J.F. Exposure to gestational diabetes mellitus and low socioeconomic status: Effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch. Pediatr. Adolesc. Med. 2012, 166, 337–343. [Google Scholar]
- Ornoy, A.; Ratzon, N.; Greenbaum, C.; Wolf, A.; Dulitzky, M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J. Pediatr. Endocrinol. Metab. 2001, 14, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.L.; Andersen, E.; Lundbye-Christensen, S. Maternal blood glucose in diabetic pregnancies and cognitive performance in offspring in young adulthood: A Danish cohort study. Diabet. Med. 2010, 27, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Kinney, B.A.; Rabe, M.B.; Jensen, R.A.; Steger, R.W. Maternal hyperglycemia leads to gender-dependent decits in learning and memory in offspring. Exp. Biol. Med. 2003, 228, 152–159. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sung, Y.H.; Lee, H.H.; Ko, I.G.; Kim, S.E.; Shin, M.S.; Kim, B.K. Postnatal treadmill exercise alleviates short-term memory impairment by enhancing cell proliferation and suppressing apoptosis in the hippocampus of rat pups born to diabetic rats. J. Exerc. Rehabil. 2014, 10, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chandna, A.R.; Kuhlmann, N.; Bryce, C.A.; Greba, Q.; Campanucci, V.A.; Howland, J.G. Chronic maternal hyperglycemia induced during mid-pregnancy in rats increases RAGE expression, augments hippocampal excitability, and alters behavior of the offspring. Neuroscience 2015, 303, 241–260. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Jiang, A.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef]
- Behl, C.; Trapp, T.; Skutella, T.; Holsboer, F. Protection against oxidative stress-induced neuronal cell death—A novel role for RU486. Eur. J. Neurosci. 1997, 9, 912–920. [Google Scholar] [CrossRef]
- Shelat, P.B.; Chalimoniuk, M.; Wang, J.H.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008, 10, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Huerta-Cervantes, M.; Peña-Montes, D.J.; Montoya-Pérez, R.; Trujillo, X.; Huerta, M.; López-Vázquez, M.Á.; Olvera-Cortés, M.E.; Saavedra-Molina, A. Gestational Diabetes Triggers Oxidative Stress in Hippocampus and Cerebral Cortex and Cognitive Behavior Modifications in Rat Offspring: Age-and Sex-Dependent Effects. Nutrients 2020, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Van Assche, F.A. Animal evidence for the transgenerational development of diabetes mellitus. Int. J. Biochem Cell. Biol. 2006, 38, 894–903. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989, 24, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E. Obesity and gestational diabetes mellitus pathways for programming in mouse, monkey, and man—where do we go next? The 2014 Norbert Freinkel Award Lecture. Diabetes Care 2015, 38, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.V.; Segabinazi, E.; de Meireles, A.L.F.; Mega, F.; de Figueiredo Spindler, C.; Augustin, O.A.; Salvalaggio, G.D.S.; Achaval, M.; Kruse, M.S.; Coirini, H. Severe Uncontrolled Maternal Hyperglycemia Induces Microsomia and Neurodevelopment Delay Accompanied by Apoptosis, Cellular Survival, and Neuroinflammatory Deregulation in Rat Offspring Hippocampus. Cell. Mol. Neurobiol. 2019, 39, 401–414. [Google Scholar] [CrossRef]
- Han, J.; Xu, J.; Long, Y.S.; Epstein, P.N.; Liu, Y.Q. Rat maternal diabetes impairs pancreatic β-cell function in the offspring. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E228–E236. [Google Scholar] [CrossRef]
- Seghieri, G.; Anichini, R.; De Bellis, A.; Alviggi, L.; Franconi, F.; Breschi, M.C. Relationship between gestational diabetes mellitus and low maternal birth weight. Diabetes Care 2002, 25, 1761–1765. [Google Scholar] [CrossRef]
- Sarti, C.; Gallagher, J. The metabolic syndrome: Prevalence, CHD risk, and treatment. J. Diabetes Compl. 2006, 20, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Guillemin, C.; Ergaz, Z.; Dimov, S.; Suderman, M.; Weinstein-Fudim, L.; Ornoy, A.; Szyf, M. Gestational diabetes alters offspring DNA methylation profiles in human and rat: Identification of key pathways involved in endocrine system disorders, insulin signaling, diabetes signaling, and ILK signaling. Endocrinology 2015, 156, 2222–2238. [Google Scholar] [CrossRef]
- Sarker, G.; Peleg-Raibstein, D. Maternal overnutrition induces long-term cognitive deficits across several generations. Nutrients 2019, 11, 7. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Li, Z.G.; Zhang, W.; Grunberger, G.; Sima, A.A. Hippocampal neuronal apoptosis in type 1 diabetes. Brain Res. 2002, 946, 221–231. [Google Scholar] [CrossRef]
- Lupien, S.B.; Bluhm, E.J.; Ishii, D.N. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J. Neurosci. Res. 2003, 74, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.; Moreira, P.I.; Proença, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.E.; Ferrante, R.J.; Beal, M.F. Oxidative stress in Huntington’s disease. Brain Pathol. 1999, 9, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Reynoso, M.A.; Ochoa-Hernández, A.B.; Juárez-Vázquez, C.I.; Barros-Núñez, P. Mecanismos epigenéticos en el desarrollo de la memoria y su implicación en algunas enfermedades neurológicas. Neurologia 2016, 31, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Glendining, K.A.; Fisher, L.C.; Jasoni, C.L. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology 2018, 96, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Warneke, W.; Klaus, S.; Fink, H.; Langley-Evans, S.C.; Voigt, J.P. The impact of cafeteria diet feeding on physiology and anxiety-related behaviour in male and female Sprague–Dawley rats of different ages. Pharmacol. Biochem. Behav. 2014, 116, 45–54. [Google Scholar] [CrossRef]
- Heisler, L.K.; Zhou, L.; Bajwa, P.; Hsu, J.; Tecott, L.H. Serotonin 5-HT2C receptors regulate anxiety-like behavior. Genes Brain Behav. 2007, 6, 491–496. [Google Scholar] [CrossRef]
- Thorré, K.; Chaouloff, F.; Sarre, S.; Meeusen, R.; Ebinger, G.; Michotte, Y. Differential effects of restraint stress on hippocampal 5-HT metabolism and extracellular levels of 5-HT in streptozotocin-diabetic rats. Brain Res. 1997, 772, 209–216. [Google Scholar] [CrossRef]
- Rebolledo-Solleiro, D.; Roldán-Roldán, G.; Díaz, D.; Velasco, M.; Larqué, C.; Rico-Rosillo, G.; Pérez de la Mora, M. Increased anxiety-like behavior is associated with the metabolic syndrome in non-stressed rats. PLoS ONE 2017, 12, e0176554. [Google Scholar]
- Sivanathan, S.; Thavartnam, K.; Arif, S.; Elegino, T.; McGowan, P.O. Chronic high fat feeding increases anxiety-like behaviour and reduces transcript abundance of glucocorticoid signalling genes in the hippocampus of female rats. Behav. Brain Res. 2015, 286, 265–270. [Google Scholar] [CrossRef]
- Sasaki, A.; De Vega, W.; Sivanathan, S.; St-Cyr, S.; McGowan, P.O. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 2014, 272, 92–101. [Google Scholar] [CrossRef]
| Body Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age | Male F2 Offspring | n | Female F2 Offspring | n | ||||
| C | GD | C | GD | C | GD | C | GD | |
| Birth | 7.26 ± 0.34 | 7.50 ± 0.08 | 16 | 23 | 7.00 ± 0.31 | 7.53 ± 0.11 | 19 | 19 |
| Weaning (21 days) | 45.33 ± 0.79 | 45.63 ± 1.39 | 12 | 19 | 42.89 ± 0.72 | 46.6 ± 1.32 | 20 | 20 |
| Juvenile (2 months) | 313.9 ± 2.38 | 331.2± 2.44 *▪ | 12 | 10 | 208.4 ± 4.76 | 218.2 ± 3.63 | 12 | 10 |
| Adult (6 months) | 497.0 ± 8.39 | 500.0 ± 5.79 | 10 | 10 | 282.0 ± 6.40 | 285.8 ± 6.12 | 10 | 10 |
| Cerebral Cortex | Juvenile-C n = 6 | Juvenile-GD n = 6 | Adult-C n = 6 | Adult-GD n = 6 |
|---|---|---|---|---|
| ROS (arbitrary units/mg protein) | 27.3 ± 4.71 | 29.0 ± 2.41 | 45.43 ± 2.27 | 51.65 ± 3.19 |
| Lipid peroxidation (nmoles TBARS/mg protein) | 263.6 ± 26.89 | 273 ± 28.48 | 203.4 ± 18.06 | 270.9 ± 44.75 |
| GSHt (µmoles/mg protein) | 0.31 ± 0.01 | 0.33 ± 0.01 | 0.50 ± 0.03 | 0.56 ± 0.04 |
| GSSG (µmoles/mg protein) | 0.12 ± 0.001 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.008 |
| GSH (µmoles/mg protein) | 0.18 ± 0.01 | 0.20 ± 0.03 | 0.39 ± 0.03 | 0.47 ± 0.003 |
| GSH/GSSG ratio | 1.90 ± 0.52 | 1.93 ± 0.54 | 4.86 ± 1.14 | 5.74 ± 0.39 |
| SOD activity (U/mg protein) | 16.02 ± 1.83 | 15.0 ± 1.90 | 12.24 ± 2.02 | 12.79 ± 1.27 |
| Catalase activity (U/mg protein) | 1.39 ± 0.09 | 1.10 ± 0.10 | 1.12 ± 0.07 | 0.94 ± 0.09 |
| Hippocampus | Juvenile-C n = 6 | Juvenile-GD n = 6 | Adult-C n = 6 | Adult-GD n = 6 |
| ROS (arbitrary units/mg protein) | 18.01 ± 1.39 | 32.32 ± 8.65 | 41.22 ± 2.40 | 47.55 ± 2.49 |
| Lipid peroxidation (nmoles TBARS/mg protein) | 283.3 ± 48.42 | 378.9 ± 46.48 | 221.5 ± 27.61 | 195.6 ± 23.15 |
| GSHt (µmoles/mg protein) | 0.33 ± 0.03 | 0.30 ± 0.01 | 0.51 ± 0.05 | 0.49± 0.06 |
| GSSG (µmoles/mg protein) | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 |
| GSH (µmoles/mg protein) | 0.23 ± 0.02 | 0.20 ± 0.01 | 0.40 ± 0.04 | 0.38 ± 0.06 |
| GSH/GSSG ratio | 2.63 ± 0.34 | 2.65 ± 0.47 | 4.34 ± 0.64 | 3.72 ± 0.44 |
| SOD activity (U/mg protein) | 13.19 ± 1.36 | 12.68 ± 2.23 | 13.49 ± 0.69 | 14.21 ± 0.52 |
| Catalase activity (U/mg protein) | 0.95 ± 0.11 | 0.90 ± 0.07 | 1.10 ± 0.05 | 1.13 ± 0.02 |
| Cerebral Cortex | Juvenile-C n = 5 | Juvenile-GD n = 5 | Adult-C n = 5 | Adult-C n = 5 |
|---|---|---|---|---|
| ROS (arbitrary units/mg protein) | 29.78 ± 5.22 | 28.1 ± 3.45 | 47.81 ± 4.59 | 51.66 ± 2.80 |
| Lipid peroxidation (nmoles TBARS/mg protein) | 149.9 ± 10.18 | 305.4 ± 13.02 *▪ | 158.3 ± 24.24 | 256.7 ± 24.14 *▪ |
| GSHt (µmoles/mg protein) | 0.31 ± 0.1 | 0.36 ± 0.01 | 0.52 ± 0.02 | 0.48 ± 0.03 |
| GSSG (µmoles/mg protein) | 0.11 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.01 *▪ |
| GSH (µmoles/mg protein) | 0.20 ± 0.02 | 0.26 ± 0.02 | 0.42 ± 0.01 | 0.34 ± 0.03 |
| GSH/GSSG ratio | 2.5 ± 0.6 | 4.9 ± 1.3 | 4.89 ± 0.74 | 2.76 ± 0.43 *▪ |
| SOD activity (U/mg protein) | 16.78 ± 1.91 | 16.05 ± 1.77 | 16.17 ± 1.71 | 14.44 ± 0.27 |
| Catalase activity (U/mg protein) | 1.10 ± 0.11 | 0.79 ± 0.07 *▪ | 1.12 ± 0.14 | 0.72 ± 0.08 *▪ |
| Hippocampus | Juvenile-C n = 5 | Juvenile-GD n = 5 | Adult-C n = 5 | Adult-C n = 5 |
| ROS (arbitrary units/mg protein) | 26.76 ± 2.38 | 25.48 ± 1.73 | 40.06 ± 2.97 | 48.07 ± 4.28 |
| Lipid peroxidation (nmoles TBARS/mg protein) | 377.2 ± 50.32 | 476.3 ± 44.34 | 208.0 ± 29.1 | 181.1 ± 16.79 |
| GSHt (µmoles/mg protein) | 0.33 ± 0.03 | 0.29 ± 0.01 | 0.40 ± 0.08 | 0.45 ± 0.01 |
| GSSG (µmoles/mg protein) | 0.10 ± 0.02 | 0.11± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.008 |
| GSH (µmoles/mg protein) | 0.22 ± 0.04 | 0.17 ± 0.02 | 0.29 ± 0.08 | 0.34 ± 0.01 |
| GSH/GSSG ratio | 3.49 ± 1.18 | 2.02 ± 0.50 | 2.57 ± 0.74 | 3.36 ± 0.39 |
| SOD activity (U/mg protein) | 16.19 ± 2.56 | 17.47 ± 1.54 | 17.93 ± 2.60 | 13.74 ± 1.90 |
| Catalase activity (U/mg protein) | 0.86 ± 0.08 | 0.75 ± 0.07 | 1.10 ± 0.12 | 1.02 ± 0.05 |
| Male Offspring | ||||
|---|---|---|---|---|
| Parameter | Juvenile-C n = 7 | Juvenile-GD n = 7 | Adult-C n = 10 | Adult-GD n = 10 |
| Glucose (mg/dL) | 95.43 ± 4.26 | 100.9 ± 2.96 | 88.0 ± 1.29 | 89.9 ± 2.71 |
| Insulin (ng/mL) | 0.33 ± 0.015 | 0.39 ± 0.020 *▪ | 0.39 ± 0.028 | 0.41 ± 0.022 |
| Total cholesterol (mg/dL) | 58.36 ± 4.00 | 80.23 ± 4.53 *▪ | 62.65 ± 2.86 | 78.78 ± 3.48 *▪ |
| HDL-cholesterol (mg/dL) | 50.73 ± 2.73 | 39.15 ± 1.72 *▪ | 54.84 ± 1.94 | 43.95 ± 2.39 *▪ |
| Triglycerides (mg/dL) | 43.93 ± 2.87 | 59.91 ± 5.74 *▪ | 29.37 ± 2.18 | 43.80 ± 5.04 *▪ |
| Female Offspring | ||||
| Parameter | Juvenile-C n = 8 | Juvenile-GD n = 8 | Adult-C n = 8 | Adult-GD n = 10 |
| Glucose (mg/dL) | 89.63 ± 2.57 | 91.63 ± 3.57 | 87.25 ± 2.44 | 91.1 ± 1.69 |
| Insulin (ng/mL) | 0.33 ± 0.006 | 0.42 ± 0.046 *▪ | 0.35 ± 0.016 | 0.52 ± 0.048 *▪ |
| Total cholesterol (mg/dL) | 72.68 ± 4.21 | 75.46 ± 2.96 | 68.1 ± 2.78 | 65.16 ± 4.67 |
| HDL-cholesterol (mg/dL) | 51.13 ± 2.99 | 49.99 ± 1.60 | 51.58 ± 2.78 | 50.85 ± 2.46 |
| Triglycerides (mg/dL) | 46.32 ± 2.72 | 66.93 ± 3.87 *▪ | 36.73 ± 3.65 | 57.57 ± 5.69 *▪ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Cervantes, M.; Peña-Montes, D.J.; López-Vázquez, M.Á.; Montoya-Pérez, R.; Cortés-Rojo, C.; Olvera-Cortés, M.E.; Saavedra-Molina, A. Effects of Gestational Diabetes in Cognitive Behavior, Oxidative Stress and Metabolism on the Second-Generation Off-Spring of Rats. Nutrients 2021, 13, 1575. https://doi.org/10.3390/nu13051575
Huerta-Cervantes M, Peña-Montes DJ, López-Vázquez MÁ, Montoya-Pérez R, Cortés-Rojo C, Olvera-Cortés ME, Saavedra-Molina A. Effects of Gestational Diabetes in Cognitive Behavior, Oxidative Stress and Metabolism on the Second-Generation Off-Spring of Rats. Nutrients. 2021; 13(5):1575. https://doi.org/10.3390/nu13051575
Chicago/Turabian StyleHuerta-Cervantes, Maribel, Donovan J. Peña-Montes, Miguel Ángel López-Vázquez, Rocío Montoya-Pérez, Christian Cortés-Rojo, María Esther Olvera-Cortés, and Alfredo Saavedra-Molina. 2021. "Effects of Gestational Diabetes in Cognitive Behavior, Oxidative Stress and Metabolism on the Second-Generation Off-Spring of Rats" Nutrients 13, no. 5: 1575. https://doi.org/10.3390/nu13051575
APA StyleHuerta-Cervantes, M., Peña-Montes, D. J., López-Vázquez, M. Á., Montoya-Pérez, R., Cortés-Rojo, C., Olvera-Cortés, M. E., & Saavedra-Molina, A. (2021). Effects of Gestational Diabetes in Cognitive Behavior, Oxidative Stress and Metabolism on the Second-Generation Off-Spring of Rats. Nutrients, 13(5), 1575. https://doi.org/10.3390/nu13051575







