Association between Sugar Intake and Intima Media Thickness as a Marker for Atherosclerosis: A Cross-Sectional Study in the Malmö Diet and Cancer Study (Sweden)
Abstract
1. Introduction
2. Materials and Methods
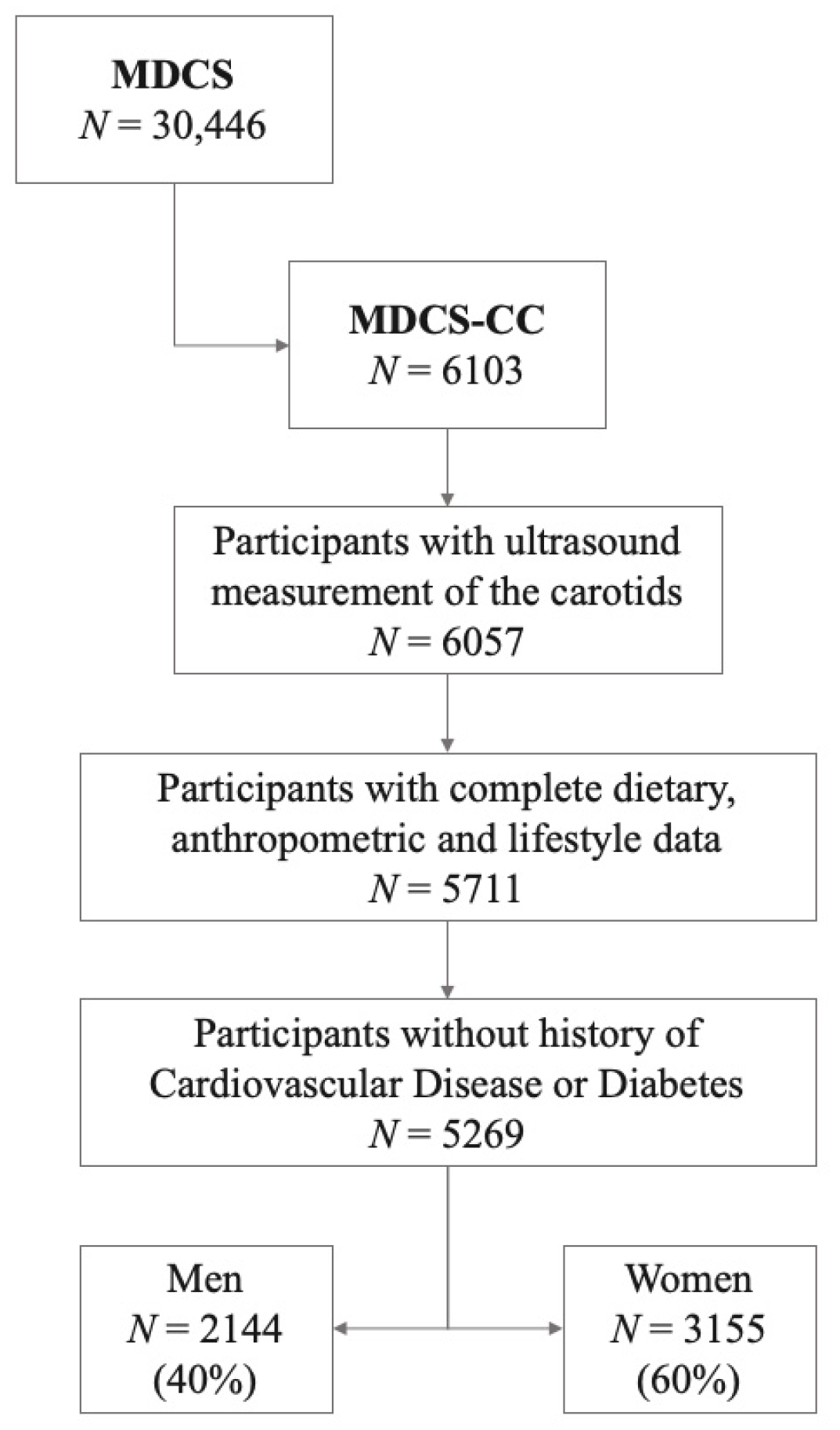
2.1. Subjects and Data Collection
2.2. Dietary Data Collection
2.3. Sugar Variables
2.4. Carotid Artery Measurements
2.5. Other Variables
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organisation: Geneva, Switzerland, 2011. [Google Scholar]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Polak, J.F.; Kavousi, M.; Mathiesen, E.B.; Völzke, H.; Tuomainen, T.P.; Sander, D.; Plichart, M.; Catapano, A.L.; Robertson, C.M.; et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet 2012, 379, 2053–2062. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K., Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Nutrition and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2499–2506. [Google Scholar] [CrossRef]
- Hosseini, B.; Saedisomeolia, A.; Skilton, M.R. Association between Micronutrients Intake/Status and Carotid Intima Media Thickness: A Systematic Review. J. Acad. Nutr. Diet. 2017, 117, 69–82. [Google Scholar] [CrossRef]
- Bhat, S.; Mocciaro, G.; Ray, S. The association of dietary patterns and carotid intima-media thickness: A synthesis of current evidence. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Clifton, P.M.; Keogh, J.B. The association between carotid intima media thickness and individual dietary components and patterns. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Janzi, S.; Ramne, S.; González-Padilla, E.; Johnson, L.; Sonestedt, E. Associations Between Added Sugar Intake and Risk of Four Different Cardiovascular Diseases in a Swedish Population-Based Prospective Cohort Study. Front. Nutr. 2020, 7, 603653. [Google Scholar] [CrossRef]
- Chun, S.; Choi, Y.; Chang, Y.; Cho, J.; Zhang, Y.; Rampal, S.; Zhao, D.; Ahn, J.; Suh, B.S.; Pastor-Barriuso, R.; et al. Sugar-sweetened carbonated beverage consumption and coronary artery calcification in asymptomatic men and women. Am. Heart. J. 2016, 177, 17–24. [Google Scholar] [CrossRef]
- Wang, D.; Karvonen-Gutierrez, C.A.; Jackson, E.A.; Elliott, M.R.; Appelhans, B.M.; Barinas-Mitchell, E.; Bielak, L.F.; Baylin, A. Prospective associations between beverage intake during the midlife and subclinical carotid atherosclerosis: The Study of Women’s Health Across the Nation. PLoS ONE 2019, 14, e0219301. [Google Scholar] [CrossRef] [PubMed]
- Berglund, G.; Elmstähl, S.; Janzon, L.; Larsson, S.A. The Malmo Diet and Cancer Study. Design and feasibility. J. Intern. Med. 1993, 233, 45–51. [Google Scholar] [CrossRef]
- Manjer, J.; Carlsson, S.; Elmståhl, S.; Gullberg, B.; Janzon, L.; Lindström, M.; Mattisson, I.; Berglund, G. The Malmö Diet and Cancer Study: Representativity, cancer incidence and mortality in participants and non-participants. Eur. J. Cancer Prev. 2001, 10, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Hedblad, B.; Nilsson, P.; Janzon, L.; Berglund, G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet. Med. 2000, 17, 299–307. [Google Scholar] [CrossRef]
- Sonestedt, E.; Hellstrand, S.; Drake, I.; Schulz, C.A.; Ericson, U.; Hlebowicz, J.; Persson, M.M.; Gullberg, B.; Hedblad, B.; Engström, G.; et al. Diet Quality and Change in Blood Lipids during 16 Years of Follow-up and Their Interaction with Genetic Risk for Dyslipidemia. Nutrients 2016, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Elmståhl, S.; Riboli, E.; Lindgärde, F.; Gullberg, B.; Saracci, R. The Malmö Food Study: The relative validity of a modified diet history method and an extensive food frequency questionnaire for measuring food intake. Eur. J. Clin. Nutr. 1996, 50, 143–151. [Google Scholar]
- Riboli, E.; Elmståhl, S.; Saracci, R.; Gullberg, B.; Lindgärde, F. The Malmö Food Study: Validity of two dietary assessment methods for measuring nutrient intake. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S161–S173. [Google Scholar] [CrossRef]
- Callmer, E.; Riboli, E.; Saracci, R.; Akesson, B.; Lindgärde, F. Dietary assessment methods evaluated in the Malmö food study. J. Intern. Med. 1993, 233, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Wirfält, E.; Mattisson, I.; Johansson, U.; Gullberg, B.; Wallström, P.; Berglund, G. A methodological report from the Malmö Diet and Cancer study: Development and evaluation of altered routines in dietary data processing. Nutr. J. 2002, 1, 3. [Google Scholar] [CrossRef]
- US Department of Agriculture; US Department of Health and Human Services. Nutrition and Your Health: Dietary Guidelines for Americans; USDA: Washington, DC, USA, 2000. [Google Scholar]
- Ramne, S.; Alves Dias, J.; González-Padilla, E.; Olsson, K.; Lindahl, B.; Engström, G.; Ericson, U.; Johansson, I.; Sonestedt, E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population-based prospective cohorts. Am. J. Clin. Nutr. 2019, 109, 411–423. [Google Scholar] [CrossRef]
- World Health Organisation. Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Joint WHO/FAO Expert Consultation. Diet, Nutrition and the Prevention of Chronic Diseases; Technical Report for World Health Organization; WHO: Geneva, Switzerland, April 2003. [Google Scholar]
- González-Padilla, E.; Dias, J.A.; Ramne, S.; Olsson, K.; Nälsén, C.; Sonestedt, E. Association between added sugar intake and micronutrient dilution: A cross-sectional study in two adult Swedish populations. Nutr. Metab. (Lond.) 2020, 17, 15. [Google Scholar] [CrossRef]
- Wendelhag, I.; Gustavsson, T.; Suurküla, M.; Berglund, G.; Wikstrand, J. Ultrasound measurement of wall thickness in the carotid artery: Fundamental principles and description of a computerized analysing system. Clin. Physiol. 1991, 11, 565–577. [Google Scholar] [CrossRef]
- Wendelhag, I.; Liang, Q.; Gustavsson, T.; Wikstrand, J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke 1997, 28, 2195–2200. [Google Scholar] [CrossRef]
- Rosvall, M.; Persson, M.; Östling, G.; Nilsson, P.M.; Melander, O.; Hedblad, B.; Engström, G. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: The Malmö Diet and Cancer Study. Atherosclerosis 2015, 239, 615–621. [Google Scholar] [CrossRef]
- Mutie, P.M.; Drake, I.; Ericson, U.; Teleka, S.; Schulz, C.A.; Stocks, T.; Sonestedt, E. Different domains of self-reported physical activity and risk of type 2 diabetes in a population-based Swedish cohort: The Malmö diet and Cancer study. BMC Public Health 2020, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Frondelius, K.; Borg, M.; Ericson, U.; Borné, Y.; Melander, O.; Sonestedt, E. Lifestyle and Dietary Determinants of Serum Apolipoprotein A1 and Apolipoprotein B Concentrations: Cross-Sectional Analyses within a Swedish Cohort of 24,984 Individuals. Nutrients 2017, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Manjer, J.; Elmståhl, S.; Janzon, L.; Berglund, G. Invitation to a population-based cohort study: Differences between subjects recruited using various strategies. Scand. J. Public Health 2002, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Sallata, P.; Tepper, S.A. Experimental atherosclerosis in rabbits fed cholesterol-free diets. 2. Influence of various carbohydrates. J. Atheroscler. Res. 1968, 8, 697–703. [Google Scholar] [CrossRef]
- Plazas Guerrero, C.G.; Acosta Cota, S.J.; Castro Sánchez, F.H.; Vergara Jiménez, M.J.; Ríos Burgueño, E.R.; Sarmiento Sánchez, J.I.; Picos Corrales, L.A.; Osuna Martínez, U. Evaluation of sucrose-enriched diet consumption in the development of risk factors associated to type 2 diabetes, atherosclerosis and non-alcoholic fatty liver disease in a murine model. Int. J. Environ. Health Res. 2019, 1–19. [Google Scholar] [CrossRef]
- Story, J.A. Dietary carbohydrate and atherosclerosis. Fed. Proc. 1982, 41, 2797–2800. [Google Scholar] [PubMed]
- Mozaffarian, D.; Rimm, E.B.; Herrington, D.M. Dietary fats, carbohydrate, and progression of coronary atherosclerosis in postmenopausal women. Am. J. Clin. Nutr. 2004, 80, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Goñi Ruiz, N.; Martínez González, M.; Salas Salvadó, J.; Buil Cosiales, P.; Diez Espino, J.; Martinez Vila, E.; Irimia Sierra, P.; Ros Rahola, E.; Toledo Atucha, E. Association between Dietary Glycemic Index and Glycemic Load and Intima Media Thickness in a Population at High Cardiovascular Risk: A Subgroup Analysis in the Predimed Trial. Nutr. Hosp. 2015, 32, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Ericson, U.; Brunkwall, L.; Alves Dias, J.; Drake, I.; Hellstrand, S.; Gullberg, B.; Sonestedt, E.; Nilsson, P.M.; Wirfält, E.; Orho-Melander, M. Food patterns in relation to weight change and incidence of type 2 diabetes, coronary events and stroke in the Malmö Diet and Cancer cohort. Eur. J. Nutr. 2019, 58, 1801–1814. [Google Scholar] [CrossRef]
- Akbari-Sedigh, A.; Asghari, G.; Yuzbashian, E.; Dehghan, P.; Imani, H.; Mirmiran, P. Association of dietary pattern with carotid intima media thickness among children with overweight or obesity. Diabetol. Metab. Syndr. 2019, 11, 77. [Google Scholar] [CrossRef]
- Alissa, E.M.; Helmi, S.R.; Al-Salmi, M.M. Relationship between diet quality and carotid intima-media thickness in people with and without carotid atherosclerosis. J. Fam. Med. Prim. Care 2018, 7, 531–537. [Google Scholar] [CrossRef]
- Egusa, G.; Watanabe, H.; Ohshita, K.; Fujikawa, R.; Yamane, K.; Okubo, M.; Kohno, N. Influence of the extent of westernization of lifestyle on the progression of preclinical atherosclerosis in Japanese subjects. J. Atheroscler. Thromb. 2002, 9, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Simova, I. Intima-media thickness: Appropriate evaluation and proper measurement. e-J. Cardiol. Pract. 2015, 13. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-13/Intima-media-thickness-Appropriate-evaluation-and-proper-measurement-described. (accessed on 4 May 2021).
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; Pencina, M.J.; Pencina, K.M.; O’Donnell, C.J.; Wolf, P.A.; D’Agostino, R.B., Sr. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med. 2011, 365, 213–221. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef]
- Lorenz, M.W.; von Kegler, S.; Steinmetz, H.; Markus, H.S.; Sitzer, M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: Prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke 2006, 37, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tosetto, A.; Prati, P.; Baracchini, C.; Manara, R.; Rodeghiero, F. Age-adjusted reference limits for carotid intima-media thickness as better indicator of vascular risk: Population-based estimates from the VITA project. J. Thromb. Haemost. 2005, 3, 1224–1230. [Google Scholar] [CrossRef]
- De Weerd, M.; Greving, J.P.; Hedblad, B.; Lorenz, M.W.; Mathiesen, E.B.; O’Leary, D.H.; Rosvall, M.; Sitzer, M.; de Borst, G.J.; Buskens, E.; et al. Prediction of asymptomatic carotid artery stenosis in the general population: Identification of high-risk groups. Stroke 2014, 45, 2366–2371. [Google Scholar] [CrossRef]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012; Nordic Council of Ministers: Copenhagen, Denmark, 2014. [Google Scholar]
- World Health Organisation (Regional Office for Europe). Incentives and Disincentives for Reducing Sugar in Manufactured Foods; WHO: Copenhagen, Denmark, 2017. [Google Scholar]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
| Added Sugar Intake (%E) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 | 5–7.5 | 7.5–10 | 10–15 | 15–20 | >20 | Total | Ptrends | ||
| N (men/women) | 198/277 | 389/621 | 557/810 | 731/1111 | 190/276 | 49/60 | 2114/3155 | ||
| Age (years) * | Men | 56.0 (5.79) | 56.6 (5.77) | 57.5 (5.96) | 57.5 (6.12) | 58.1 (5.93) | 57.8 (6.13) | 57.3 (5.99) | <0.001 |
| Women | 56.1 (5.79) | 56.4 (5.79) | 57.7 (5.98) | 57.8 (5.98) | 57.8 (5.60) | 57.3 (5.87) | 57.3 (5.92) | <0.001 | |
| BMI (kg/m2) * | Men | 26.9 (4.07) | 26.1 (3.11) | 25.9 (3.24) | 25.8 (3.40) | 25.8 (3.12) | 26.1 (4.10) | 26.0 (3.38) | 0.002 |
| Women | 25.9 (4.57) | 25.5 (4.37) | 25.2 (3.80) | 25.2 (4.04) | 24.5 (3.87) | 24.4 (4.01) | 25.2 (4.09) | <0.001 | |
| Energy intake (kcal/day) *1 | Men | 2385 (722) | 2528 (651) | 2598 (632) | 2700 (690) | 2842 (739) | 2766 (787) | 2626 (688) | <0.001 |
| Women | 1748 (512) | 1903 (455) | 1996 (478) | 2084 (492) | 2231 (554) | 2304 (633) | 2013 (508) | <0.001 | |
| Carbohydrates (%E) * | Men | 40.6 (7.34) | 41.6 (6.15) | 44.2 (5.31) | 46.3 (5.10) | 49.7 (5.65) | 53.0 (4.81) | 44.8 (6.31) | <0.001 |
| Women | 42.7 (7.01) | 43.8 (5.85) | 45.0 (5.40) | 47.2 (5.42) | 49.6 (5.21) | 53.5 (5.30) | 45.9 (6.05) | <0.001 | |
| Protein (%E) * | Men | 17.6 (3.02) | 16.5 (2.36) | 15.7 (1.96) | 14.7 (1.94) | 13.5 (1.74) | 12.3 (2.33) | 15.4 (2.45) | <0.001 |
| Women | 18.7 (3.02) | 17.0 (2.40) | 16.2 (2.25) | 15.1 (2.09) | 13.8 (1.84) | 12.6 (2.25) | 15.9 (2.63) | <0.001 | |
| Fat (%E) * | Men | 41.7 (8.10) | 41.8 (6.67) | 40.2 (5.73) | 39.0 (5.56) | 36.7 (6.03) | 34.7 (5.33) | 39.8 (6.35) | <0.001 |
| Women | 38.6 (7.50) | 39.2 (6.58) | 38.7 (5.85) | 37.7 (5.78) | 36.5 (5.58) | 33.8 (4.99) | 38.1 (6.17) | <0.001 | |
| Saturated fats (%E) * | Men | 17.6 (5.26) | 17.5 (4.25) | 16.9 (3.66) | 16.6 (3.67) | 15.8 (3.96) | 15.2 (3.18) | 16.8 (4.00) | <0.001 |
| Women | 16.3 (4.52) | 16.7 (4.28) | 16.6 (3.67) | 16.2 (3.66) | 16.1 (3.70) | 14.9 (3.25) | 16.4 (3.88) | 0.001 | |
| Fibre (g/1000 kcal) * | Men | 8.58 (3.07) | 8.68 (2.72) | 8.72 (2.47) | 8.33 (2.25) | 8.10 (2.59) | 6.89 (1.98) | 8.47 (2.53) | <0.001 |
| Women | 11.3 (3.65) | 10.5 (2.98) | 10.0 (2.73) | 9.64 (2.66) | 8.74 (2.45) | 7.95 (3.22) | 9.94 (2.92) | <0.001 | |
| Fruits and vegetables (g/day) * | Men | 390 (217) | 356 (182) | 382 (192) | 351 (170) | 343 (180) | 272 (151) | 361 (184) | <0.001 |
| Women | 475 (205) | 454 (203) | 407 (167) | 401 (174) | 371 (169) | 340 (224) | 416 (184) | <0.001 | |
| Meat (g/day) * | Men | 191 (91.1) | 179 (75.6) | 173 (75.2) | 167 (68.7) | 158 (65.3) | 138 (71.5) | 171 (74.4) | <0.001 |
| Women | 118 (55.0) | 118 (48.6) | 116 (46.9) | 112 (45.8) | 107 (44.2) | 104 (53.7) | 114 (47.6) | <0.001 | |
| Fish (g/day) * | Men | 46.0 (49.2) | 48.2 (43.1) | 46.9 (40.1) | 41.0 (30.5) | 38.6 (33.3) | 30.9 (24.9) | 43.9 (37.9) | <0.001 |
| Women | 42.9 (31.5) | 40.1 (29.2) | 40.3 (29.8) | 37.8 (27.2) | 35.1 (28.7) | 25.8 (23.5) | 38.9 (28.9) | <0.001 | |
| Coffee (g/day) * | Men | 612 (525) | 569 (452) | 533 (450) | 539 (401) | 553 (420) | 626 (534) | 553 (441) | 0.233 |
| Women | 556 (473) | 529 (362) | 494 (337) | 504 (380) | 474 (316) | 636 (598) | 511 (376) | 0.094 | |
| SSBs (svg/wk) * | Men | 0.43 (0.96) | 0.65 (1.35) | 1.30 (2.04) | 2.74 (3.57) | 6.01 (5.70) | 10.8 (12.0) | 2.24 (4.02) | <0.001 |
| Women | 0.20 (0.47) | 0.47 (0.93) | 0.85 (1.35) | 1.83 (2.55) | 3.88 (4.18) | 9.93 (9.31) | 1.50 (2.90) | <0.001 | |
| Toppings (svg/wk) * | Men | 2.20 (2.87) | 4.91 (5.15) | 8.95 (7.23) | 15.6 (11.5) | 26.8 (20.2) | 32.7 (26.6) | 12.0 (13.1) | <0.001 |
| Women | 1.32 (1.56) | 2.88 (2.68) | 5.16 (4.05) | 8.48 (7.18) | 14.8 (12.8) | 23.2 (21.1) | 6.73 (8.04) | <0.001 | |
| Treats (svg/wk) * | Men | 2.80 (2.46) | 5.53 (3.81) | 7.13 (4.42) | 9.26 (5.89) | 10.9 (8.00) | 13.4 (12.9) | 7.65 (5.96) | <0.001 |
| Women | 3.00 (2.30) | 4.88 (2.64) | 6.63 (3.71) | 8.65 (4.94) | 11.2 (7.24) | 13.1 (11.2) | 7.20 (5.13) | <0.001 | |
| Time (years) *2 | Men | 0.76 (0.38) | 0.80 (0.37) | 0.79 (0.37) | 0.77 (0.35) | 0.75 (0.40) | 0.74 (0.35) | 0.78 (0.37) | 0.231 |
| Women | 0.84 (0.38) | 0.81 (0.37) | 0.82 (0.37) | 0.81 (0.37) | 0.78 (0.36) | 0.78 (0.34) | 0.81 (0.37) | 0.093 | |
| HbA1c (%) *3 | Men | 4.82 (0.44) | 4.82 (0.61) | 4.76 (0.50) | 4.78 (0.51) | 4.82 (0.45) | 4.86 (0.58) | 4.79 (0.52) | 0.818 |
| Women | 4.82 (0.45) | 4.80 (0.48) | 4.82 (0.42) | 4.83 (0.46) | 4.78 (0.43) | 4.78 (0.51) | 4.81 (0.45) | 0.910 | |
| High plasma glucose **4 | Men | 109 (63.4) | 222 (61.0) | 282 (55.7) | 349 (51.2) | 102 (59.0) | 21 (46.7) | 1085 (55.9) | 0.007 |
| Women | 96 (37.8) | 202 (35.2) | 262 (34.2) | 381 (36.4) | 81 (31.3) | 21 (38.2) | 1043 (35.3) | 0.596 | |
| Alcohol, non-consumers ** | Men | 5 (2.5) | 8 (2.1) | 17 (3.1) | 35 (4.8) | 15 (7.9) | 7 (14.3) | 87 (4.1) | <0.001 |
| Women | 16 (5.8) | 35 (5.6) | 46 (5.7) | 84 (7.6) | 24 (8.7) | 5 (8.3) | 210 (6.7) | <0.001 | |
| Alcohol, high consumers **5 | Men | 65 (32.8) | 92 (23.7) | 112 (20.1) | 114 (15.6) | 28 (14.7) | 8 (16.3) | 419 (19.8) | <0.001 |
| Women | 50 (18.1) | 110 (17.7) | 127 (15.7) | 145 (13.1) | 27 (9.8) | 6 (10.0) | 465 (14.7) | <0.001 | |
| Sedentary **6 | Men | 15 (7.6) | 37 (9.5) | 42 (7.5) | 65 (8.9) | 15 (7.9) | 8 (16.3) | 182 (8.6) | 0.060 |
| Women | 35 (12.6) | 51 (8.2) | 70 (8.6) | 99 (8.9) | 22 (8.0) | 6 (10.0) | 283 (9.0) | 0.617 | |
| University education **7 | Men | 24 (12.1) | 45 (11.6) | 83 (14.9) | 80 (10.9) | 16 (8.4) | 6 (12.2) | 254 (12.0) | 0.185 |
| Women | 46 (16.6) | 103 (16.6) | 104 (12.8) | 110 (9.9) | 16 (5.8) | 3 (5.0) | 382 (12.1) | <0.001 | |
| Current smokers ** | Men | 72 (36.4) | 118 (30.3) | 154 (27.6) | 209 (28.6) | 59 (31.1) | 19 (38.8) | 631 (29.8) | 0.257 |
| Women | 95 (34.3) | 164 (26.4) | 208 (25.7) | 279 (25.1) | 79 (28.6) | 29 (48.3) | 854 (27.1) | <0.001 | |
| High TG **8 | Men | 53 (30.6) | 108 (29.7) | 129 (25.5) | 179 (26.3) | 62 (35.6) | 15 (34.1) | 546 (28.1) | 0.092 |
| Women | 42 (16.6) | 81 (14.1) | 124 (16.1) | 159 (15.1) | 52 (20.2) | 12 (21.8) | 470 (15.9) | 0.351 | |
| Low HDLc **9 | Men | 26 (15.3) | 98 (27.2) | 130 (25.9) | 206 (30.4) | 60 (34.9) | 16 (35.6) | 536 (27.8) | <0.001 |
| Women | 57 (22.7) | 175 (30.8) | 215 (25.3) | 605 (29.5) | 99 (38.1) | 21 (38.2) | 872 (29.8) | 0.004 | |
| High LDLc **10 | Men | 77 (45.8) | 180 (51.3) | 251 (50.9) | 296 (44.3) | 93 (55.4) | 23 (52.3) | 920 (48.6) | 0.055 |
| Women | 110 (44.0) | 256 (45.3) | 388 (51.5) | 506 (49.4) | 124 (48.6) | 26 (47.3) | 1410 (48.6) | 0.189 | |
| Hypertension **11 | Men | 175 (88.4) | 338(86.9) | 4657 (83.8) | 603 (82.5) | 159 (83.7) | 47 (95.9) | 1789 (84.6) | 0.043 |
| Women | 200 (72.2) | 452 (72.8) | 620 (76.5) | 859 (77.3) | 209 (75.7) | 45 (75.0) | 2385 (75.6) | 0.254 | |
| Season, winter **12 | Men | 55 (27.8) | 124 (31.9) | 175 (31.4) | 233 (31.9) | 54 (28.4) | 13 (26.5) | 654 (30.9) | 0.123 |
| Women | 88 (31.8) | 203 (32.7) | 234 (28.9) | 352 (31.7) | 75 (27.2) | 15 (25.0) | 967 (30.6) | 0.351 | |
| Season, spring **12 | Men | 54 (27.3) | 95 (24.4) | 118 (21.2) | 163 (22.3) | 36 (18.9) | 15 (30.6) | 481 (22.8) | 0.123 |
| Women | 70 (25.3) | 142 (22.9) | 198 (24.4) | 240 (21.6) | 72 (26.1) | 18 (30.0) | 740 (23.5) | 0.351 | |
| Season, summer **12 | Men | 29 (14.6) | 29 (10.0) | 64 (11.5) | 88 (12.0) | 21 (11.1) | 11 (22.4) | 252 (11.9) | 0.123 |
| Women | 43 (15.5) | 91 (14.7) | 103 (12.7) | 137 (12.3) | 37 (13.4) | 8 (13.3) | 419 (13.3) | 0.351 | |
| Season, autumn **12 | Men | 60 (30.3) | 131 (33.7) | 200 (35.9) | 247 (33.8) | 79 (41.6) | 10 (20.4) | 727 (34.4) | 0.123 |
| Women | 76 (27.4) | 185 (28.9) | 275 (34.0) | 382 (34.4) | 92 (33.3) | 19 (31.7) | 1029 (32.6) | 0.351 | |
| Added Sugar Intake (%E) | ||||||||
|---|---|---|---|---|---|---|---|---|
| <5 | 5–7.5 | 7.5–10 | 10–15 | 15–20 | >20 | Ptrends | ||
| IMTcca | ||||||||
| N * (men/women) | 173/254 | 365/576 | 507/770 | 685/1052 | 175/261 | 45/55 | ||
| Model 1 | Men | 0.763 (0.738–0.787) | 0.759 (0 742–0.777) | 0.764 (0.749–0.779) | 0.753 (0.740–0.765) | 0.763 (0.738–0.787) | 0.783 (0.736–0.831) | 0.711 |
| Women | 0.715 (0.700–0.731) | 0.727 (0.716–0.737) | 0.719 (0.710–0.728) | 0.717 (0.710–0.728) | 0.731 (0.716–0.746) | 0.747 (0.715–0.780) | 0.621 | |
| Model 2 | Men | 0.755 (0.729–0.782) | 0.756 (0.737–0.776) | 0.767 (0.750–0.783) | 0.754 (0.740–7.769) | 0.764 (0.738–0.790) | 0.784 (0.735–0.832) | 0.811 |
| Women | 0.715 (0.698–0.732) | 0.727 (0.715–0.739) | 0.718 (0.708–0.729) | 0.716 (0.707–0.726) | 0.728 (0.711–0.745) | 0.742 (0.707–0.776) | 0.985 | |
| Model 3 | Men | 0.748 (0.719–0.777) | 0.749 (0.728–0.771) | 0.757 (0.738–0.776) | 0.747 (0.730–0.764) | 0.752 (0.724–0.779) | 0.770 (0.720–0.820) | 0.960 |
| Women | 0.717 (0.699–0.734) | 0.728 (0.715–0.741) | 0.717 (0.705–0.729) | 0.715 (0.704–0.726) | 0.725 (0.707–0.743) | 0.738 (0.704–0.773) | 0.702 | |
| IMTbif | ||||||||
| N * (men/women) | 108/159 | 270/376 | 345/514 | 498/712 | 120/191 | 35/42 | ||
| Model 1 | Men | 1.591 (1.475–1.708) | 1.547 (1.472–1.621) | 1.498 (1.431–1.565) | 1.490 (1.435–1.545) | 1.511 (1.400–1.622) | 1.419 (1.216–1.622) | 0.083 |
| Women | 1.397 (1.312–1.482) | 1.401 (1.346–1.457) | 1.365 (1.317–1.413) | 1.345 (1.305–1.386) | 1.441 (1.364–1.519) | 1.382 (1.217–1.548) | 0.435 | |
| Model 2 | Men | 1.563 (1.435–1.691) | 1.522 (1.438–1.605) | 1.475 (1.399–1.550) | 1.473 (1.408–1.538) | 1.493 (1.373–1.612) | 1.407 (1.198–1.616) | 0.203 |
| Women | 1.400 (1.308–1.492) | 1.416 (1.353–1.479) | 1.388 (1.333–1.443) | 1.370 (1.320–1.420) | 1.450 (1.364–1.537) | 1.373 (1.200–1.546) | 0.402 | |
| Model 3 | Men | 1.555 (1.416–1.693) | 1.501(1.407–1.596) | 1.459 (1.372–1.546) | 1.455 (1.379–1.531) | 1.482 (1.355–1.610) | 1.375 (1.159–1.590) | 0.207 |
| Women | 1.399 (1.302–1.495) | 1.423 (1.353–1.493) | 1.395 (1.333–1.457) | 1.376 (1.318–1.434) | 1.442 (1.351–1.533) | 1.369 (1.197–1.542) | 0.300 | |
| Sugar-Sweetened Beverage Intake (svg/wk) | |||||||
|---|---|---|---|---|---|---|---|
| ≤1 | >1–3 | >3–5 | >5–8 | >8 | Ptrends | ||
| IMTcca | |||||||
| N * (men/women) | 1097/1862 | 369/632 | 195/239 | 133/137 | 156/98 | ||
| Model 1 | Men | 0.755 (0.744–0.765) | 0.758 (0.741–0.775) | 0.771 (0.748–0.794) | 0.781 (0.753–0.809) | 0.765 (0.739–0.790) | 0.129 |
| Women | 0.723 (0.717–0.729) | 0.715 (0.705–0.724) | 0.716 (0.701–0.732) | 0.725(0.704–0.746) | 0.742 (0.717–0.767) | 0.643 | |
| Model 2 | Men | 0.755 (0.742–0.768) | 0.761 (0.742–0.780) | 0.768 (0.744–0.793) | 0.779 (0.750–0.808) | 0.760 (0.733–0.787) | 0.311 |
| Women | 0.723 (0.715–0.731) | 0.712 (0.701–0.724) | 0.714 (0.697–0.731) | 0.720 (0.698–0.742) | 0.736 (0.711–0.762) | 0.914 | |
| Model 3 | Men | 0.745 (0.729–0.761) | 0.750 (0.729–0.771) | 0.764 (0.737–0.790) | 0.774 (0.744–0.804) | 0.748 (0.719–0.777) | 0.257 |
| Women | 0.722 (0.713–0.732) | 0.714 (0.701–0.726) | 0.710 (0.692–0.727) | 0.717 (0.694–0.740) | 0.733 (0.707–0.759) | 0.622 | |
| IMTbif | |||||||
| N * (men/women) | 776/1234 | 264/432 | 135/169 | 91/88 | 110/71 | ||
| Model 1 | Men | 1.497 (1.452–1.543) | 1.560 (1.485–1.635) | 1.499 (1.395–1.604) | 1.468 (1.341–1.595) | 1.535 (1.420–1.650) | 0.776 |
| Women | 1.383 (1.352–1.414) | 1.369 (1.317–1.420) | 1.321 (1.239–1.404) | 1.451 (1.337–1.565) | 1.323 (1.196–1.450) | 0.637 | |
| Model 2 | Men | 1.470 (1.414–1.527) | 1.536 (1.452–1.619) | 1.473 (1.363–1.583) | 1.466 (1.334–1.599) | 1.533 (1.411–1.655) | 0.464 |
| Women | 1.403 (1.361–1.445) | 1.382 (1.323–1.441) | 1.340 (1.253–1.428) | 1.466 (1.347–1.585) | 1.339 (1.207–1.472) | 0.526 | |
| Model 3 | Men | 1.453 (1.382–1.524) | 1.516 (1.423–1.610) | 1.466 (1.346–1.585) | 1.444 (1.304–1.584) | 1.507 (1.375–1.639) | 0.561 |
| Women | 1.408 (1.357–1.460) | 1.390 (1.325–1.454) | 1.338 (1.246–1.431) | 1.463 (1.342–1.584) | 1.322 (1.188–1.456) | 0.307 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Padilla, E.; Janzi, S.; Ramne, S.; Thuneland, C.; Borné, Y.; Sonestedt, E. Association between Sugar Intake and Intima Media Thickness as a Marker for Atherosclerosis: A Cross-Sectional Study in the Malmö Diet and Cancer Study (Sweden). Nutrients 2021, 13, 1555. https://doi.org/10.3390/nu13051555
González-Padilla E, Janzi S, Ramne S, Thuneland C, Borné Y, Sonestedt E. Association between Sugar Intake and Intima Media Thickness as a Marker for Atherosclerosis: A Cross-Sectional Study in the Malmö Diet and Cancer Study (Sweden). Nutrients. 2021; 13(5):1555. https://doi.org/10.3390/nu13051555
Chicago/Turabian StyleGonzález-Padilla, Esther, Suzanne Janzi, Stina Ramne, Camilla Thuneland, Yan Borné, and Emily Sonestedt. 2021. "Association between Sugar Intake and Intima Media Thickness as a Marker for Atherosclerosis: A Cross-Sectional Study in the Malmö Diet and Cancer Study (Sweden)" Nutrients 13, no. 5: 1555. https://doi.org/10.3390/nu13051555
APA StyleGonzález-Padilla, E., Janzi, S., Ramne, S., Thuneland, C., Borné, Y., & Sonestedt, E. (2021). Association between Sugar Intake and Intima Media Thickness as a Marker for Atherosclerosis: A Cross-Sectional Study in the Malmö Diet and Cancer Study (Sweden). Nutrients, 13(5), 1555. https://doi.org/10.3390/nu13051555







