Effect of Oat β-Glucan on Affective and Physical Feeling States in Healthy Adults: Evidence for Reduced Headache, Fatigue, Anxiety and Limb/Joint Pains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Visits
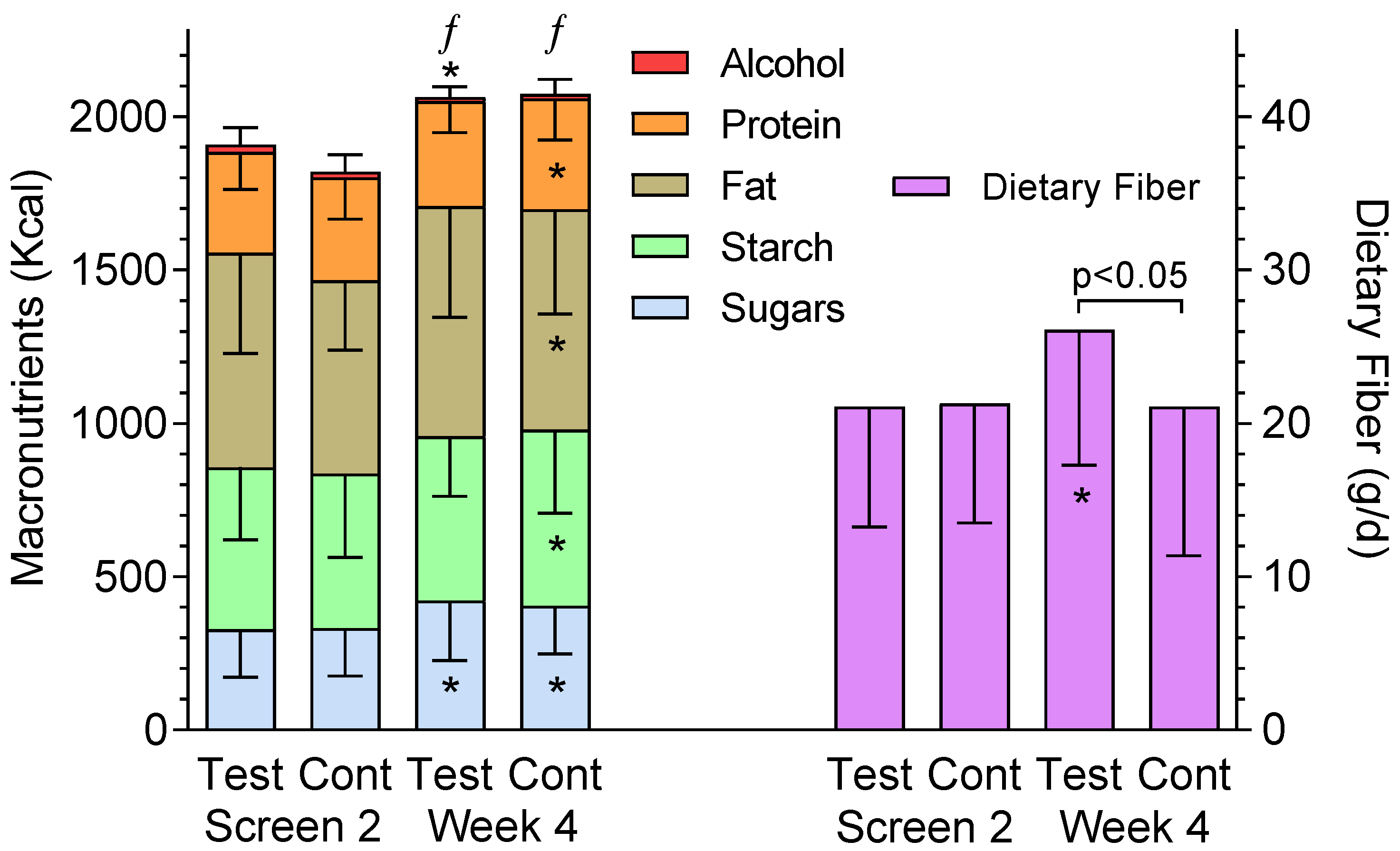
2.2. Interventions
2.3. Symptoms Questionnaire
2.4. Data Analysis, Management and Calculations
2.5. Power Analysis
2.6. Statistical Analysis
3. Results
3.1. Gastrointestinal (GI) Symptoms
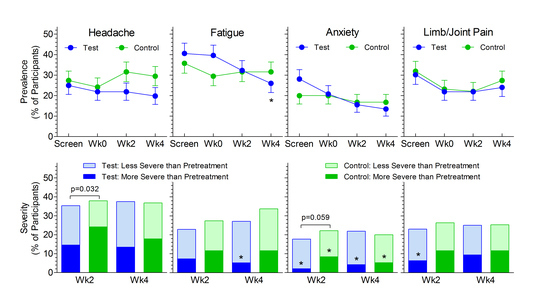
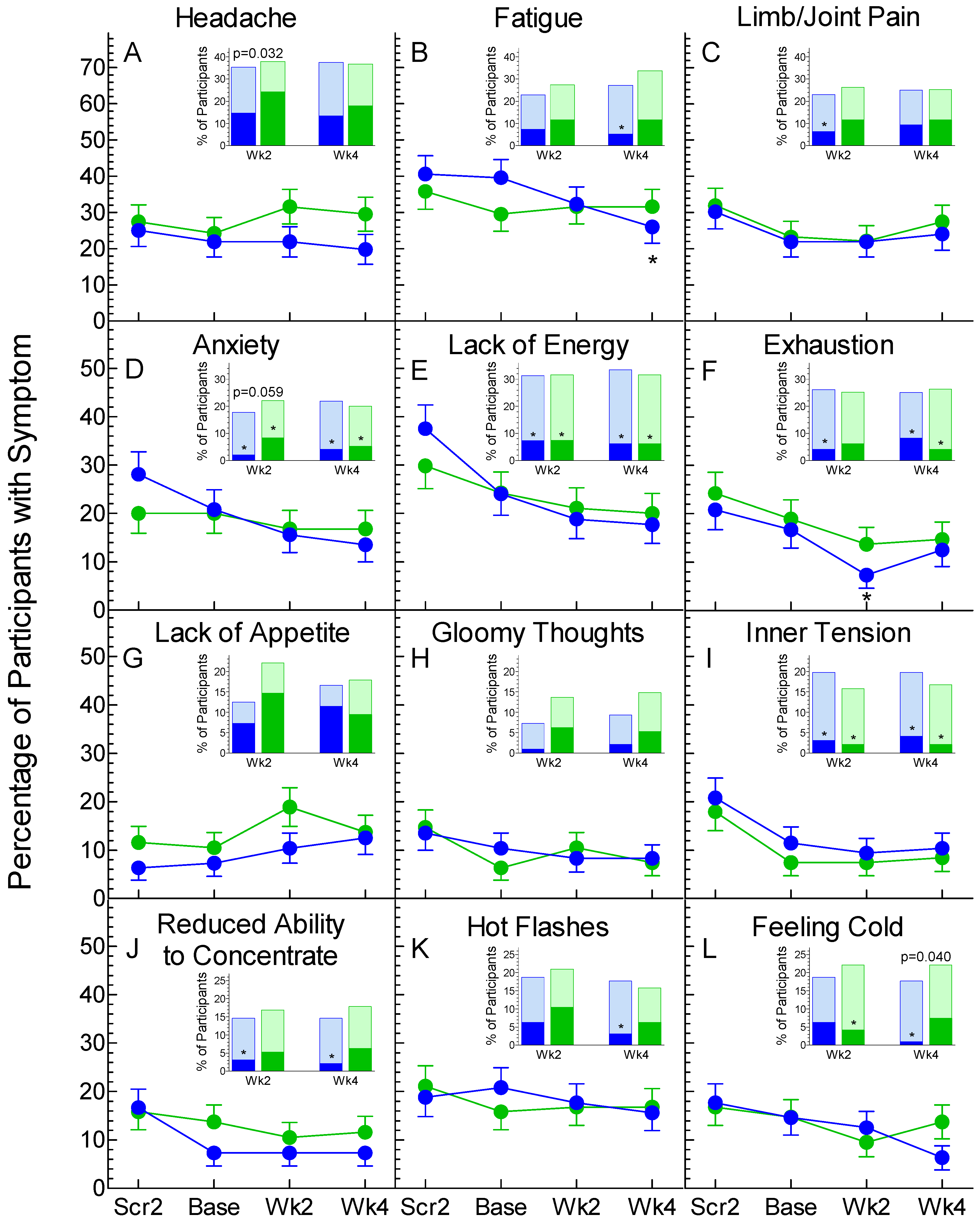
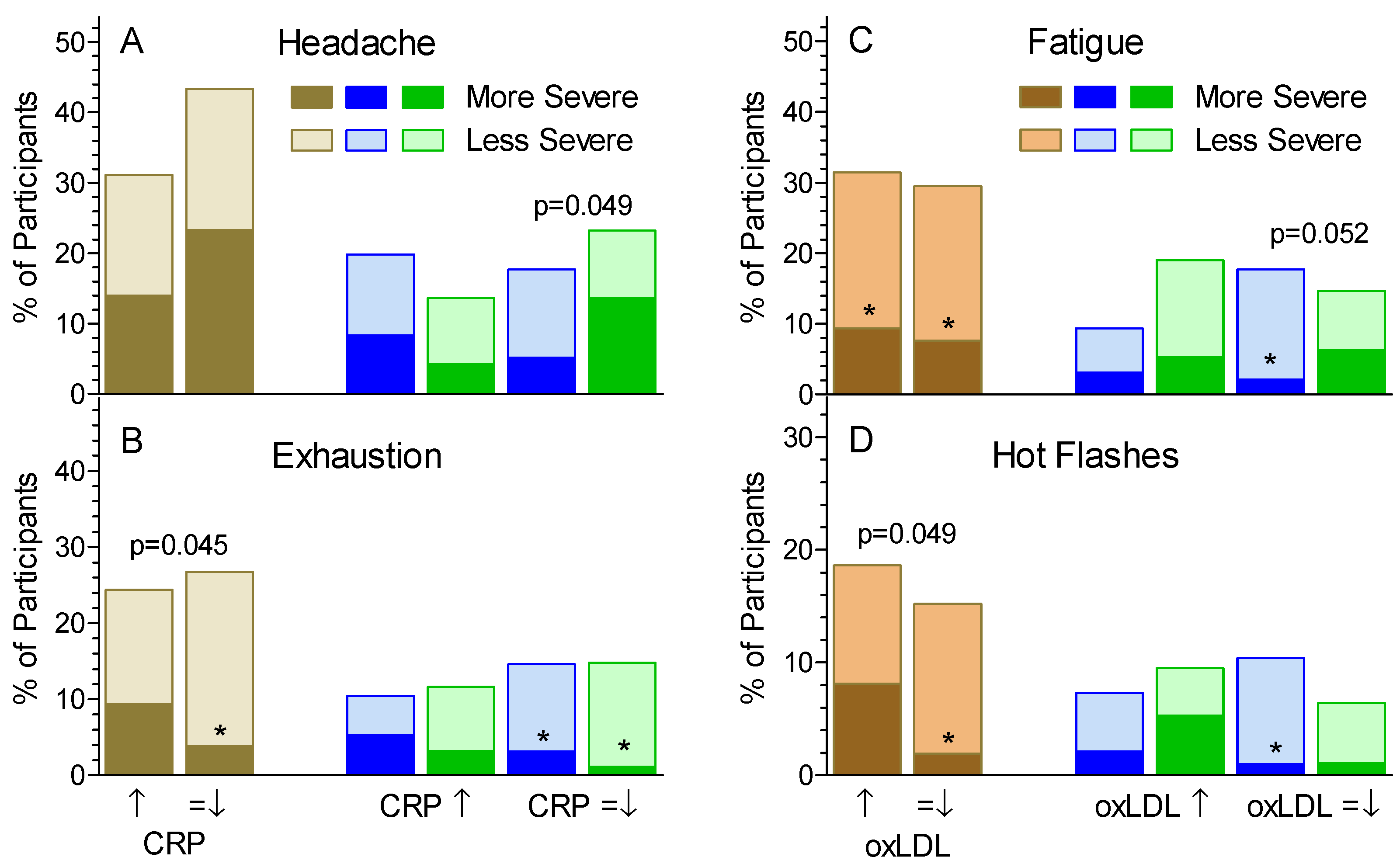
3.2. Non-GI Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Healthy Diet; Fact Sheet No. 394, Updated August 2018. World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs394?en/ (accessed on 20 November 2020).
- Wood, P.J. Oat β-glucan: Structure, location and properties. In Oats: Chemistry and Technology; Webster, F.H., Ed.; AACC Inc.: St. Paul, MN, USA, 1986; pp. 121–152. [Google Scholar]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M.S. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomized-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Zurbau, A.; Noronha, J.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The effect of oat β-glucan on postprandial blood glucose and insulin responses: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2021. published on line before print. [Google Scholar] [CrossRef] [PubMed]
- Delahoy, P.J.; Magliano, D.J.; Webb, K.; Grobler, M.; Liew, D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: An updated metaanalysis. Clin. Ther. 2009, 31, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta-analysis of prospective cohort studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef]
- Grabistske, H.A.; Slavin, J.L. Gastrointestinal effects of low-digestible carbohydrates. Crit. Rev. Food Sci. Nutr. 2009, 49, 327–360. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Rahn, M.; Dioum, E.; Spruill, S.E.; Ezatagha, A.; Campbell, J.E.; Jenkins, A.L.; Chu, Y. Impact of an oat β-glucan beverage on LDL-cholesterol and cardiovascular risk in men and women with borderline high cholesterol: A double-blind, randomized, controlled clinical-trial. J. Nutr. 2021, in press. [Google Scholar]
- Kaukinen, K.; Collin, P.; Huhtala, H.; Mäki, M. Long-term consumption of oats in adult celiac disease patients. Nutrients 2013, 5, 4380–4389. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. 1), S2–S26. [Google Scholar] [CrossRef] [PubMed]
- Humes, D.; Smith, J.K.; Spiller, R.C. Colonic diverticular disease. BMJ Clin. Evid. 2011, 2011, 0405. [Google Scholar]
- Cumming, J.H. The effect of dietary fiber on fecal weight and composition. In CRC Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; Spiller, G.A., Ed.; CRC Press: Roca Raton, FL, USA, 1985; pp. 183–252. [Google Scholar]
- Korczak, R.; Kocher, M.; Swanson, K.S. Effects of oats on gastrointestinal health as assessed by in vitro, animal and human studies. Nutr. Rev. 2020, 78, 343–363. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Luca, M.; Di Mauro, M.; Di Maruo, M.; Luca, A. Gut microbiota in Alzheimer’s disease, depression, and type 2 diabetes mellitus: The role of oxidative stress. Oxid. Med. Cell Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schrieber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- O’Reilly, G.A.; Huh, J.; Schembre, S.M.; Tate, E.B.; Pentz, M.A.; Dunton, G. Association of usual self-reported dietary intake with ecological momentary measures of affective and physical feeling states in children. Appetite 2015, 92, 314–321. [Google Scholar] [CrossRef]
- Smith, A.; Bazzoni, C.; Beale, J.; Elliott-Smith, J.; Tiley, M. High fibre breakfast cereals reduce fatigue. Appetite 2001, 37, 249–250. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Chiasson, J.-L.; Josse, R.G.; Leiter, L.A.; Maheux, P.; Rabasa-Lhoret, R.; Rodger, N.W.; Ryan, E.A. Effects of changing the amount and source of dietary carbohydrates on symptoms and dietary satisfaction over a 1-year period in subjects with type 2 diabetes: Canadian trial of carbohydrates in diabetes (CCD). Can. J. Diab. 2017, 41, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lv, J.; Lo, M.; Cui, S.W.; Hu, X.; Fan, M. Effects of oat β-glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohydr. Polym. 2013, 92, 1159–1165. [Google Scholar] [CrossRef]
- Chiasson, J.-L.; Josse, R.G.; Hunt, J.A.; Palmason, C.; Rodger, N.W.; Ross, S.A.; Ryan, E.A.; Tan, M.H.; Wolever, T.M.S. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann. Int. Med. 1994, 121, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Chiasson, J.-L.; Josse, R.G.; Hunt, J.A.; Palmason, C.; Rodger, N.W.; Ross, S.A.; Ryan, E.A.; Tan, M.H. No relationship between carbohydrate intake and effect of acarbose on HbA1c or gastrointestinal symptoms in type 2 diabetic subjects consuming 30–60% of energy from carbohydrate. Diabetes Care 1998, 21, 1612–1618. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef]
- Hindiyeh, N.; Aurora, S.K. What the gut can teach us about migrane. Curr. Pain Headache Rep. 2015, 19, 33. [Google Scholar] [CrossRef]
- Lasser, R.B.; Bond, J.H.; Levitt, M.D. The role of intestinal gas in functional abdominal pain. N. Eng. J. Med. 1975, 293, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Devroede, G.; Girard, G.; Bouchoucha, M.; Roy, T.; Black, R.; Camerlain, M.; Pinard, G.; Schang, J.-C.; Arhan, P. Idiopathic constipation by colonic dysfunction: Relationship with personality and anxiety. Dig. Dis. Sci. 1989, 34, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.; Johansson, M.; Nilsson, A.; Björk, I.; Nyman, M. Dietary supplementation with β-glucan enriched oat bran increases faecal concentration of carboxylic acids in healthy subjects. Eur. J. Clin. Nutr. 2008, 62, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.L.; Tzounis, X.; Tuohy, K.M.; Lovegrove, J.A. Hypocholesterolemic and probiotic effects of a whole-grain oat-based granola breakfast cereal in a cardio-metabolic “at risk” population. Front. Microbiol. 2016, 7, 1675. [Google Scholar] [CrossRef]
- Valeur, J.; Puaschitz, N.G.; Midtvedt, T.; Berstad, A. Oatmeal porridge: Impact on microflora-associated characteristics in healthy subjects. Brit. J. Nutr. 2016, 115, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Sun, J.; Chen, Y.; Ren, Q.; Li, Z.; Zhao, Y.; Pan, Y.; Xue, H. Oatmeal induced gut microbiota alteration and its relationship with improved lipid profiles: A secondary analysis of a randomized clinical trial. Nutr. Metab. (Lond.) 2020, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Micriobiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; von Wright, A.; Vilpponen-Salmela, T.; Ban-Amor, K.; Akkermans, A.D.L.; de Vos, W.M. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and different from the community recovered from feces. Appl. Environ. Microbiol. 2002, 68, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.A.; Ishii-Schrade, K.B.; Pencharz, P.B.; Wolever, T.M.S. L-Ramnose increases serum propionate after long-term supplementation, but lactulose does not raise serum acetate. Am. J. Clin. Nutr. 2004, 80, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.A.; Wolever, T.M.S. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J. Nutr. 2003, 133, 3145–3148. [Google Scholar] [CrossRef]
- Torelli, P.; Manzoni, G.C. Fasting headache. Curr. Pain Headache Rep. 2010, 14, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Rapoport, A.M. Obesity and chronic daily headache. Curr. Pain Headache Rep. 2010, 16, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, J.; Zhang, Y.; Men, Y.; Zhang, B.; Sun, Y. Prebiotic, immunomodulating and anti-fatigue effects of konjac oligosaccharide. J. Food Sci. 2018, 83, 3110–3117. [Google Scholar] [CrossRef]
- Buigues, C.; Fernández-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martínez, R.; Martínez-Martínez, M.; Verdejo, Y.; Mascarós, M.C.; Peris, C.; Cauli, O. Effect of a prebiotic formulation on frailty syndrome: A randomized, double-blind clinical trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef]
- Thomas, K.S.; Motivala, S.; Olmstead, R.; Irwin, M.R. Sleep depth and fatigue: Role of cellular inflammatory activation. Brain Behav. Immun. 2011, 25, 53–58. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Han, T.S.; Seidell, J.C. Impairment of health and quality of life in people with large waist circumference. Lancet 1998, 351, 853–856. [Google Scholar] [CrossRef]
- Schaible, H.G. Spinal mechanisms contributing to joint pain. Novartis Found. Symp. 2004, 260, 4–22. [Google Scholar]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Raj, S.; Mishra, R.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Elucidation of dietary risk factors in osteoarthritis knee: A case-control study. J. Am. Coll. Nutr. 2015, 34, 15–20. [Google Scholar] [CrossRef]
- Bravo-Blas, A.; Wessel, H.; Milling, S. Microbiota and arthritis: Correlations or cause? Curr. Opin. Rheumatol. 2016, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Najdi, A.; Bisson, J.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Brit. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Cowen, P.J.; Harmer, C.H.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychoparmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.E.; Aasmoe, L.; Aardal, L.; Andorsen, G.S.; Bjørnbakk, A.K.; Egeness, C.; Espejord, I.; Kramvik, E. Feeling cold at work increases the risk of symptoms from muscles, skin and airways in seafood industry workers. Am. J. Ind. Med. 2005, 47, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, E.; Remes, J.; Hassi, J.; Pienimäki, T.; Rintamäki, H. Factors associated with self-estimated work ability and musculoskeletal symptoms among male and female workers in cooled food-processing facilities. Ind. Health 2009, 47, 271–282. [Google Scholar] [CrossRef][Green Version]
- Farbu, E.H.; Skandfer, M.; Nielsen, C.; Brenn, T.; Stubhaug, A.; Höper, A.C. Working in a cold environment, feeling cold at work and chronic pain: A cross-sectional analysis of the Tromsø study. BMJ Open 2019, 9, e031248. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Mendoza-Núñez, V.M. Association between hot flashes severity and oxidative stress among Mexican postmenopausal women: A cross-sectional study. PLoS ONE 2019, 14, e0214264. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bergamini, C.M. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin. Chem. Lab. Med. 2016, 54, 739–753. [Google Scholar] [CrossRef]
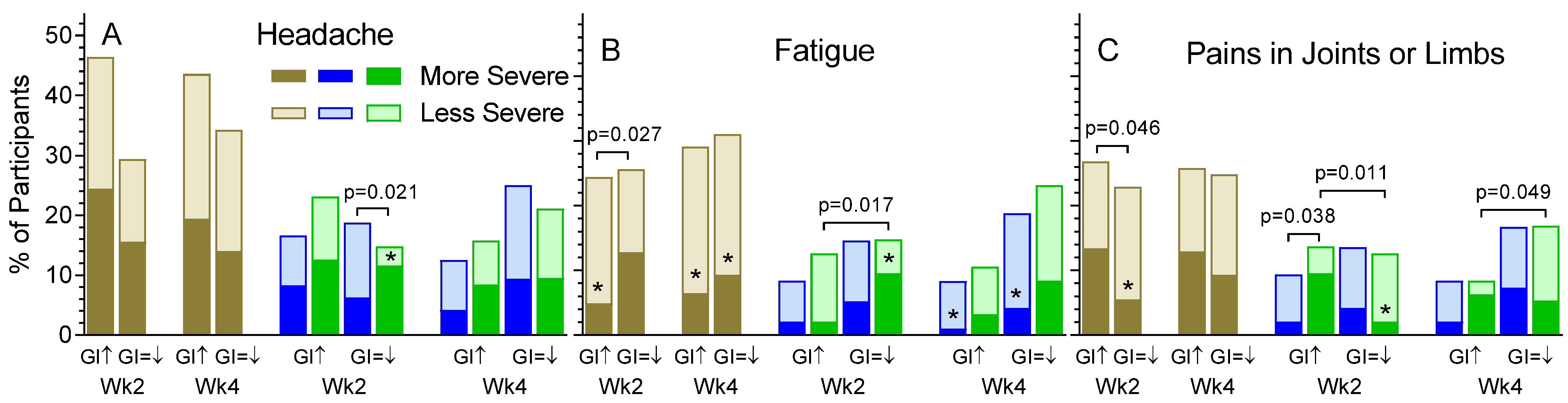
| Non-GI Symptom | Major GI Symptom | p | |
|---|---|---|---|
| Absent (n = 103) | Present (n = 88) | ||
| Headache | 16 (16) | 28 (32) | 0.008 |
| Fatigue | 20 (19) | 46 (52) | <0.001 |
| Pains in joints or limbs | 8 (8) | 35 (40) | <0.001 |
| Feelings of anxiety | 12 (12) | 27 (31) | 0.001 |
| Lack of energy | 7 (7) | 39 (44) | <0.001 |
| Tend to become exhausted | 8 (8) | 26 (30) | <0.001 |
| Lack of appetite | 6 (6) | 11 (13) | 0.11 |
| Gloomy thoughts | 3 (3) | 13 (15) | 0.003 |
| Inner tension | 4 (4) | 14 (16) | 0.005 |
| Diminished ability to concentrate | 2 (2) | 18 (20) | <0.001 |
| Hot flashes | 10 (10) | 25 (28) | <0.001 |
| Feeling cold | 5 (5) | 23 (26) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolever, T.M.S.; Rahn, M.; Dioum, E.H.; Jenkins, A.L.; Ezatagha, A.; Campbell, J.E.; Chu, Y. Effect of Oat β-Glucan on Affective and Physical Feeling States in Healthy Adults: Evidence for Reduced Headache, Fatigue, Anxiety and Limb/Joint Pains. Nutrients 2021, 13, 1534. https://doi.org/10.3390/nu13051534
Wolever TMS, Rahn M, Dioum EH, Jenkins AL, Ezatagha A, Campbell JE, Chu Y. Effect of Oat β-Glucan on Affective and Physical Feeling States in Healthy Adults: Evidence for Reduced Headache, Fatigue, Anxiety and Limb/Joint Pains. Nutrients. 2021; 13(5):1534. https://doi.org/10.3390/nu13051534
Chicago/Turabian StyleWolever, Thomas M. S., Maike Rahn, El Hadji Dioum, Alexandra L. Jenkins, Adish Ezatagha, Janice E. Campbell, and YiFang Chu. 2021. "Effect of Oat β-Glucan on Affective and Physical Feeling States in Healthy Adults: Evidence for Reduced Headache, Fatigue, Anxiety and Limb/Joint Pains" Nutrients 13, no. 5: 1534. https://doi.org/10.3390/nu13051534
APA StyleWolever, T. M. S., Rahn, M., Dioum, E. H., Jenkins, A. L., Ezatagha, A., Campbell, J. E., & Chu, Y. (2021). Effect of Oat β-Glucan on Affective and Physical Feeling States in Healthy Adults: Evidence for Reduced Headache, Fatigue, Anxiety and Limb/Joint Pains. Nutrients, 13(5), 1534. https://doi.org/10.3390/nu13051534