Dietary Intakes of Recipients of Faecal Microbiota Transplantation: An Observational Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Background to FMT Program at Our Centre
2.2. Study Procedures
2.3. Data Analysis
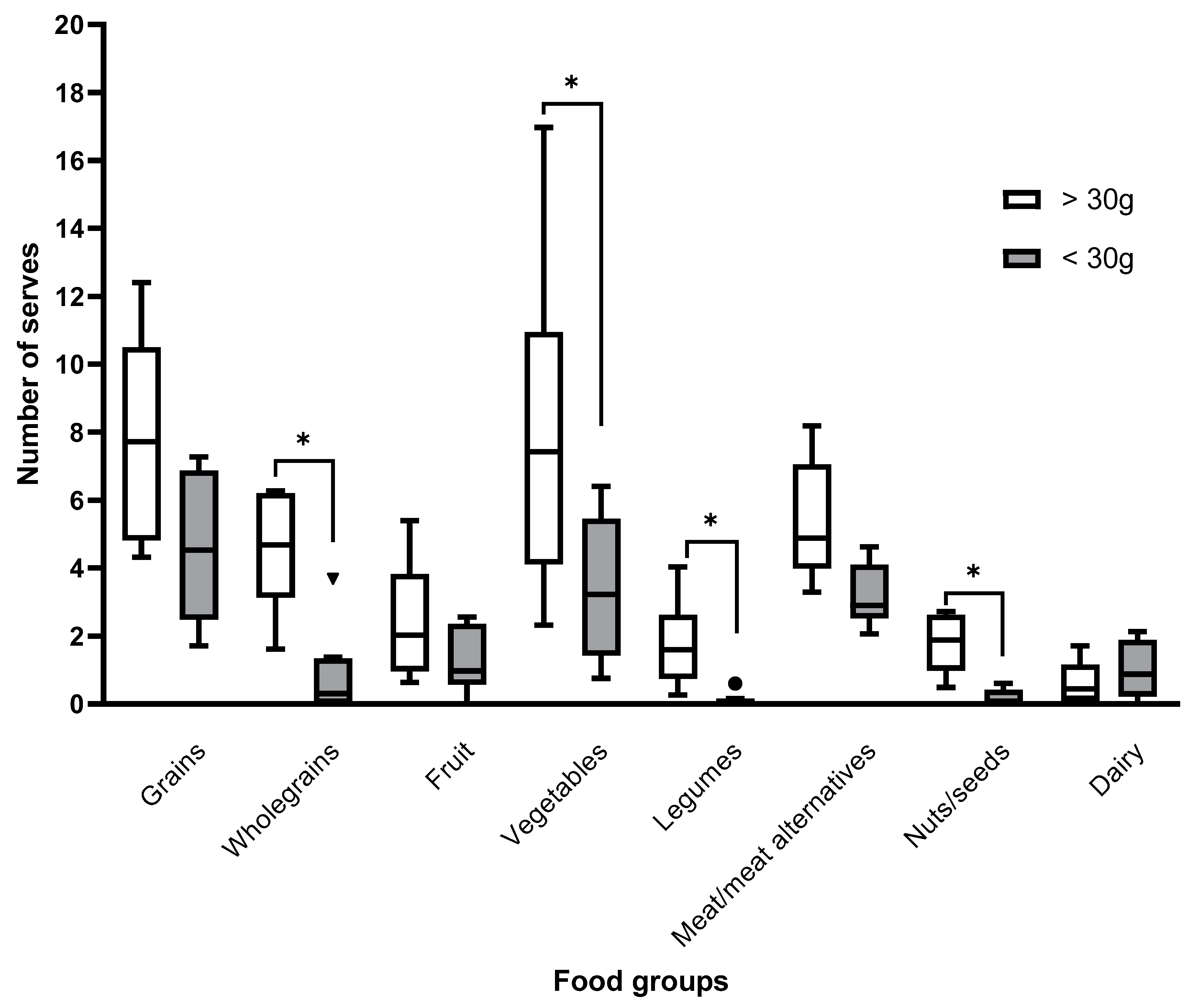
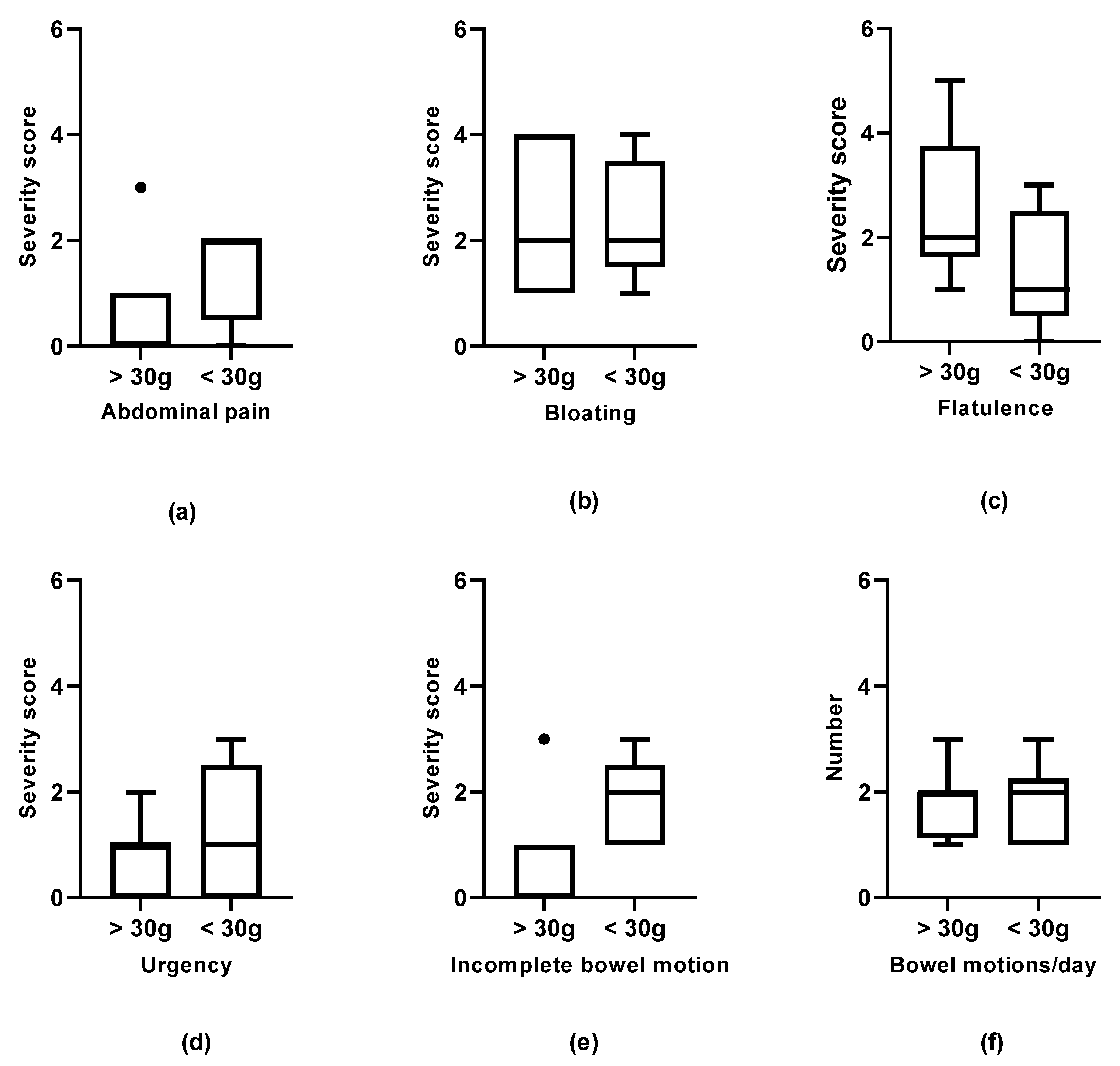
3. Results
3.1. Baseline
3.2. Week Four Follow-Up
3.3. Week 12 Follow-Up
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on FMT in clinical practice. BMJ Open 2017, 66, 569–580. [Google Scholar]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, V.L.; Steiner, C.A.; Berinstein, J.A.; Eswaran, S.; Waljee, A.K.; Higgins, P.D.R.; Owyang, C. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2019, 114, 1043. [Google Scholar] [CrossRef]
- O’Grady, J.; O’Connor, E.M.; Shanahan, F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019, 49, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Fayet-Moore, F.; Cassettari, T.; Tuck, K.; McConnell, A.; Petocz, P. Dietary fibre intake in Australia. Paper I: Associations with demographic, socio-economic, and anthropometric factors. Nutrients 2018, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Day, A.; Barrett, J.; Vanlint, A.; Andrews, J.M.; Costello, S.P.; Bryant, R.V. Habitual dietary fibre and prebiotic intake is inadequate in patients with inflammatory bowel disease: Findings from a multicentre cross-sectional study. J. Hum. Nutr. Diet. 2020, 34, 420–428. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- Staudacher, H.M. Nutritional, microbiological and psychosocial implications of the low FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32, 16–19. [Google Scholar] [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef]
- Wong, C.; Harris, P.J.; Ferguson, L.R. Potential benefits of dietary fibre intervention in inflammatory bowel disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar] [CrossRef]
- Thompson, S.; Guetterman, H.M.; Taylor, A.; Bogner, A.; Martin, D.; Farrell, J.; Swanson, K.; Holshcer, H. Dietary Predictors of Fecal Microbiota Transplantation Success. J. Acad. Nutr. Diet. 2016, 116, A76. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Zhu, W.; Tian, H.; Ding, C.; Gu, L.; Li, N.; Li, J. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Ge, X.; Tian, H.; Ding, C.; Gu, L.; Wei, Y.; Gong, J.; Zhu, W.; Li, N.; Li, J. Fecal Microbiota Transplantation in Combination with Soluble Dietary Fiber for Treatment of Slow Transit Constipation: A Pilot Study. Arch. Med. Res. 2016, 47, 236–242. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Fundamentals of Biostatistics, 4th ed.; Duxbury Press: Belmont, CA, USA, 1995. [Google Scholar]
- Haifer, C.; Kelly, C.R.; Paramsothy, S.; Andresen, D.; Papanicolas, L.E.; McKew, G.L.; Borody, T.J.; Kamm, M.; Costello, S.P.; Andrews, J.M.; et al. Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut 2020, 69, 801–810. [Google Scholar] [CrossRef]
- Bovenschen, H.J.; Janssen, M.J.R.; Van Oijen, M.G.H.; Laheij, R.J.F.; Van Rossum, L.G.M.; Jansen, J.B.M.J. Evaluation of a gastrointestinal symptoms questionnaire. Dig. Dis. Sci. 2006, 51, 1509–1515. [Google Scholar] [CrossRef]
- Russell, A.; Ball, J.; Spallek, M. The SF-36 Calculated. Available online: https://www.alswh.org.au/images/content/pdf/InfoData/Data_Dictionary_Supplement/DDSSection2SF36.pdf (accessed on 4 January 2017).
- Xyris Pty Ltd. Software Foodworks Professional v10.0; Xyris Pty Ltd.: Brisbane, Australia, 2019. [Google Scholar]
- Food Standards Australia New Zealand AUSNUT 2011–2013. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/pages/default.aspx (accessed on 6 March 2020).
- Black, A. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics National Health Survey: SF-36 Population Norms Australia; Australian Bureau of Statistics: Canberra, Australia, 1995; pp. 1–37.
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand including Recommended Dietary Intakes; Commonwealth Department of Health and Ageing: Canberra, Australia, 2006.
- National Health and Medical Research Council. Australian Dietary Guidelines; Commonwealth Department of Health and Ageing: Canberra, Australia, 2013.
- Qasem, A.; Elkamel, E.; Naser, S. Anti-MAP Triple Therapy Supports Immunomodulatory Therapeutic Response in Crohn’s Disease through Downregulation of NF-κB Activation in the Absence of MAP Detection. Biomedicines 2020, 8, 513. [Google Scholar] [CrossRef]
- Opstelten, J.L.; de Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics Australian Health Survey: Consumption of Food Groups from the Australian Dietary Guidelines, 2011–2012. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.012main+features12011-12 (accessed on 6 August 2020).
- Peters, V.; Tigchelaar-Feenstra, E.F.; Imhann, F.; Dekens, J.A.M.; Swertz, M.A.; Franke, L.H.; Wijmenga, C.; Weersma, R.K.; Alizadeh, B.Z.; Dijkstra, G.; et al. Habitual dietary intake of IBD patients differs from population controls: A case–control study. Eur. J. Nutr. 2021, 60, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef]
- Costello, S.P.; Day, A.; Yao, C.K.; Bryant, R.V. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 2020, 13, 2019–2021. [Google Scholar] [CrossRef]
- Brown, J.R.M.; Flemer, B.; Joyce, S.A.; Zulquernain, A.; Sheehan, D.; Shanahan, F.; O’Toole, P.W. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef] [PubMed]
| Total Cohort | Irritable Bowel Syndrome | Ulcerative Colitis | Crohn’s Disease | p-Value * | |
|---|---|---|---|---|---|
| (n = 18) | (n = 7) | (n = 4) | (n = 7) | ||
| Age median (range) | 35 (18–57) | 34 (25–50) | 44 (21–57) | 33 (18–55) | 0.63 |
| Males n (%) | 8 (44) | 3 (43) | 2 (50) | 3 (43) | |
| Bowel motions/day median (range) | 2 (0–6) | 2 (0–3) | 1 (1–5) | 2 (0–6) | 0.73 |
| Abdominal symptoms n (%) | |||||
| Urgency | 10 (55) | 3 (43) | 2 (50) | 5 (71) | |
| Nausea n (%) | 10 (55) | 5 (71) | 2 (50) | 3 (43) | |
| Abdominal pain n (%) | 9 (50) | 4 (57) | 2 (50) | 3 (43) | |
| Bloating n (%) | 14 (78) | 6 (86) | 3 (75) | 3 (43) | |
| Flatulence n (%) | 12 (67) | 4 (57) | 3 (75) | 5 (71) | |
| SF-36 Quality of Life median (range) | |||||
| Physical functioning | 88 (35–100) | 80 (35–95) | 95 (90–100) | 85 (40–100) | 0.11 |
| Role physical | 25 (0–100) | 0 (0–25) | 100 (0–100) | 75 (0–100) | 0.04 |
| Bodily pain | 58 (0–100) | 28 (0–58) | 74 (55–90) | 78 (33–100) | 0.02 |
| General health | 40 (20–70) | 30 (20–60) | 65 (40–65) | 45 (25–70) | 0.05 |
| Vitality | 38 (10–75) | 30 (10–75) | 50 (30–65) | 40 (10–70) | 0.52 |
| Social functioning | 50 (0–100) | 19 (0–50) | 69 (50–88) | 75 (25–100) | 0.01 |
| Role emotional | 33 (0–100) | 0 (0–100) | 83 (0–100) | 67 (0–100) | 0.19 |
| Mental health | 60 (16–88) | 56 (16–68) | 68 (52–76) | 40 (10–70) | 0.33 |
| BMI median (range) | 23 (20–29) | 22 (20–25) | 22 (21–28) | 26 (21–29) | 0.43 |
| Nutrient | EAR/AI | Intake (Including Supplements) Median (Range) | Number of Participants with Intakes (Including Supplements) below the EAR/AI n (%) | Intake (Excluding Supplements) Median (Range) | Number of Participants with Intakes (Excluding Supplements) below the EAR/AI n (%) |
|---|---|---|---|---|---|
| Fibre (g) | 30 g | 34.9 (15.0–90.5) | 6 (43%) | 27.2 (6.0–85.7) | 8 (57%) |
| Vitamin A (µg) | 500–625 | 1105.7 (147.4–4270.6) | 1 (7%) | 1105.7 (126.6–4270.6) | 1 (7%) |
| Thiamine (B1) (mg) | 0.9–1 | 1.8 (1.1–52.2) | 0 (0%) | 1.4 (0.5–38.4) | 2 (14%) |
| Riboflavin (B2) (mg) | 0.9–1.1 | 1.8 (1.0–102.8) | 0 (0%) | 1.5 (0.7–2.7) | 1 (7%) |
| Niacin (B3) (mg) | 11–12 | 24.1 (15.7–521.9) | 0 (0%) | 21.9 (15.7–58.8) | 0 (0%) |
| Vitamin B6 (mg) | 1.1 | 2.2 (1.4–11.5) | 0 (0%) | 1.9 (1.0–4.3) | 1 (7%) |
| Folate (B9) (µg) | 320 | 609.1 (405.2–1024.4) | 0 (0%) | 609.1 (263.7–1024.4) | 2 (14%) |
| Vitamin B12 (µg) | 2.0 | 4.8 (1.1–1000.5) | 1 (7%) | 4.0 (0.5–5.7) | 3 (21%) |
| Vitamin C (mg) | 30 | 157.5 (45.6–358.1) | 0 (0%) | 124.7 (14.1–301.5) | 1 (7%) |
| Calcium (mg) | 840 | 832.1 (356.2–1029.7) | 8 (57%) | 786.9 (212.5–998.2) | 10 (71%) |
| Iron (mg) | 6–8 | 14.4 (7.0–35.1) | 1 (7%) | 12.5 (4.8–28.7) | 2 (14%) |
| Magnesium (mg) | 255–350 | 479.9 (215.8–888.4) | 4 (29%) | 375.2 (141.4–860.8) | 6 (43%) |
| Zinc (mg) | 6.5–12 | 13.2 (9.3–31.5) | 1 (7%) | 12.7 (5.1–17.2) | 3 (21%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clancy, A.K.; Lee, C.; Hamblin, H.; Gunaratne, A.W.; LeBusque, A.; Beck, E.J.; Dawson, M.V.; Borody, T.J. Dietary Intakes of Recipients of Faecal Microbiota Transplantation: An Observational Pilot Study. Nutrients 2021, 13, 1487. https://doi.org/10.3390/nu13051487
Clancy AK, Lee C, Hamblin H, Gunaratne AW, LeBusque A, Beck EJ, Dawson MV, Borody TJ. Dietary Intakes of Recipients of Faecal Microbiota Transplantation: An Observational Pilot Study. Nutrients. 2021; 13(5):1487. https://doi.org/10.3390/nu13051487
Chicago/Turabian StyleClancy, Annabel K., Christina Lee, Harrison Hamblin, Anoja W. Gunaratne, Antoinette LeBusque, Eleanor J. Beck, Marie V. Dawson, and Thomas J. Borody. 2021. "Dietary Intakes of Recipients of Faecal Microbiota Transplantation: An Observational Pilot Study" Nutrients 13, no. 5: 1487. https://doi.org/10.3390/nu13051487
APA StyleClancy, A. K., Lee, C., Hamblin, H., Gunaratne, A. W., LeBusque, A., Beck, E. J., Dawson, M. V., & Borody, T. J. (2021). Dietary Intakes of Recipients of Faecal Microbiota Transplantation: An Observational Pilot Study. Nutrients, 13(5), 1487. https://doi.org/10.3390/nu13051487






