Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Population
2.3. Study Protocol and Sample Collections
2.4. DNA Extraction, Amplification for Pyrosequencing, Statistical Analysis
2.5. Statistical Analysis for Nutritional and GI Data
3. Results
3.1. Study Population
3.2. Dietary Habits
3.3. Association between Dietary Habits and Symptoms in IBS Patients
3.4. Faecal Sample Collections
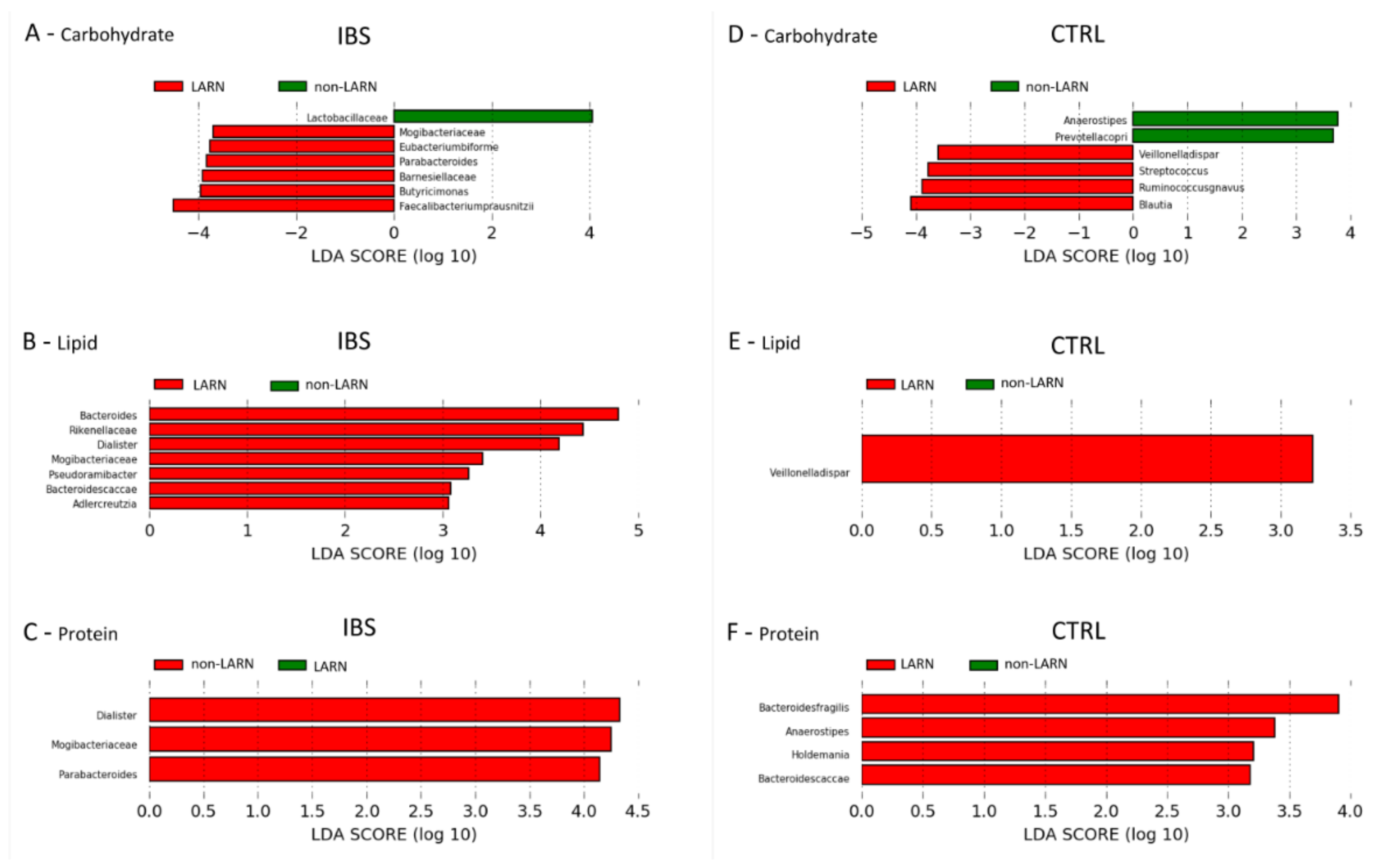
3.5. Microbiota Features Associated to Dietary Habits
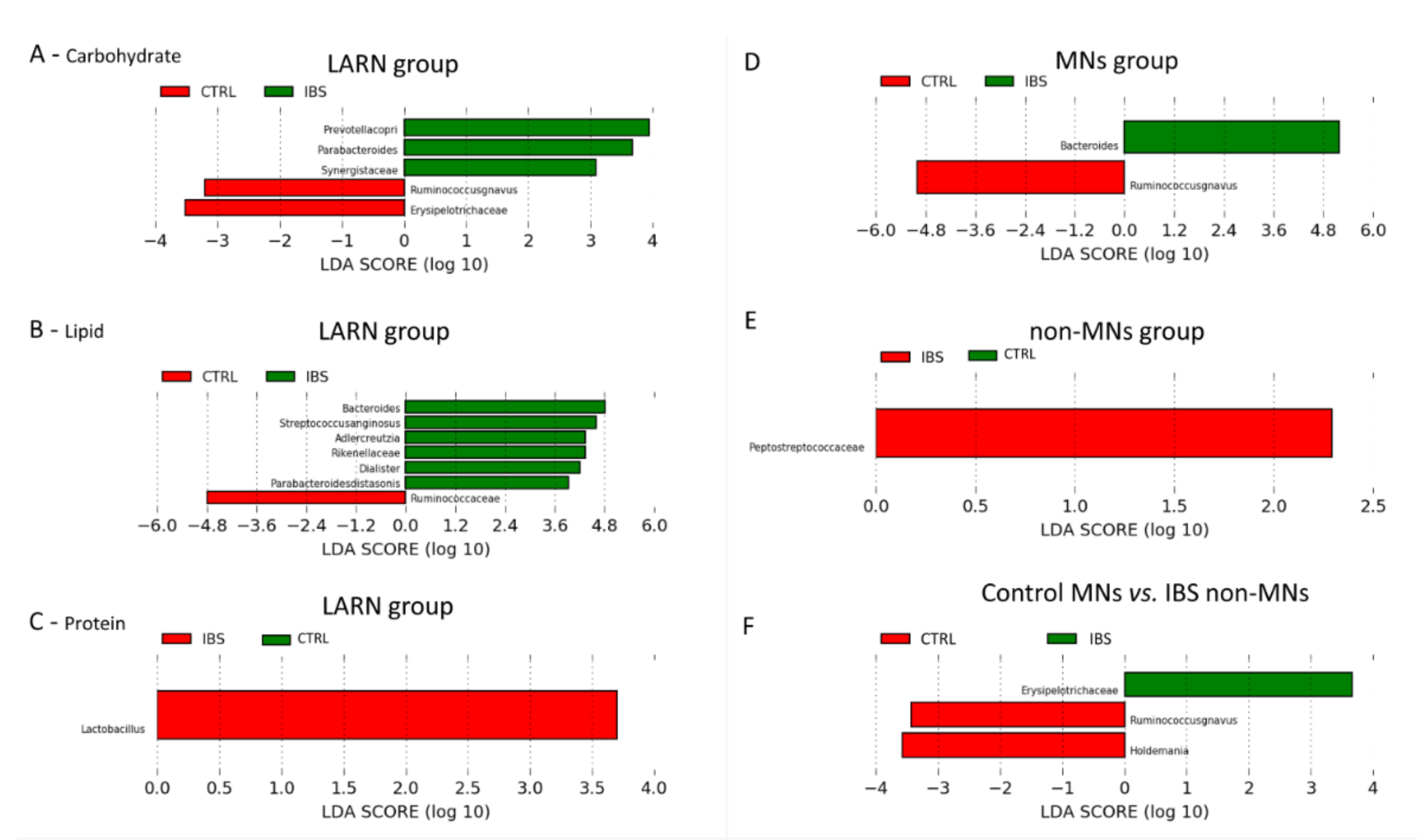
3.6. Comparison between the Microbiota Profiles of IBS vs. Control LARN Group for Carbohydrate, Fat and Protein Intake
3.7. Comparison between the Microbiota Profiles of IBS vs. Control MNs Group and Non-MNs Group
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W. Rome IV—Functional GI Disorders: Disorders of gut-brain interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar]
- Scalera, A.; Loguercio, C. Focus on irritable bowel syndrome. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1155–1171. [Google Scholar] [PubMed]
- Soares, R.L. Irritable bowel syndrome: A clinical review. World J. Gastroenterol. 2014, 20, 12144–12160. [Google Scholar] [CrossRef]
- Van der Veek, P.P.; Dusseldorp, E.; Van Rood, Y.R.; Masclee, A.A. Testing a biobehavioral model of irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2010, 22, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Putignani, L.; Del Chierico, F.; Cocca, S.; Angeletti, S.; Ciccozzi, M.; Tripiciano, C.; Dalla Piccola, B.; Cicala, M.; Guarin, M.P. Gut mucosal-associated microbiota better discloses Inflammatory Bowel Disease differential patterns than faecal microbiota. Dig. Liver Dis. 2019, 51, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable bowel syndrome: A gut microbiota-related disorder? Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef]
- Carroll, I.M.; Chang, Y.H.; Park, J.; Sartor, R.B.; Ringel, Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog. 2010, 2, 19. [Google Scholar] [CrossRef]
- Kerckhoffs, A.P.; Samsom, M.; Van der Rest, M.E.; De Vogel, J.; Knol, J.; Ben-Amor, K.; Akkermans, L.M. Lower Bifidobacteria counts in both duodenal mucosaassociated and fecal microbiota in irritable bowel syndrome patients. World J. Gastroenterol. 2009, 15, 2887–2892. [Google Scholar] [CrossRef]
- Parkes, G.T.; Rayment, N.T.; Hudspith, B.T.; Petrovska, L.; Lomer, M.T.; Brostoff, J.; Whelan, K.; Sanderson, J.T. Distinct microbial populations exist in the mucosaassociated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; O’toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Manichanh, C.; Eck, A.; Varela, E.; Roca, J.; Clemente, J.C.; González, A.; Knights, D.; Knight, R.; Estrella, S.; Hernandez, C.; et al. Anal gas evacuation and colonic microbiota in patients with flatulence: Effect of diet. Gut 2014, 63, 401–408. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 75, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Silvester, K.R.; Cummings, J.H. Does digestibility of meat protein help explain large-bowel cancer risk? Nutr. Cancer 1995, 24, 279–288. [Google Scholar] [CrossRef]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Ostgaard, H.; Gundersen, D.; Hatlebakk, J.G.; Hausken, T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review). Int. J. Mol. Med. 2012, 29, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, M.; Heitkemper, M.M.; Bond, E.F.; Georges, J. Comparison of diet composition in women with and without functional bowel disorder. Gastroenterol. Nurs. 1994, 16, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A.; Locke, G.R.; Weaver, A.L.; Zinsmeister, A.R.; Talley, N.J. Diet and functional gastrointestinal disorders: A population-based case-control study. Am. J. Gastroenterol. 2005, 100, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Ostgaard, H.; Hausken, T.; Gundersen, D.; El-Salhy, M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol. Med. Rep. 2012, 5, 1382–1390. [Google Scholar] [PubMed]
- Staudacher, H.M.; Whelan, K.; Irving, P.M.; Lomer, M.C. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J. Hum. Nutr. Diet. 2011, 24, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.; Storsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Tornblom, H.; Simren, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zito, F.P.; Polese, B.; Vozzella, L.; Gala, A.; Genovese, D.; Verlezza, V.; Medugno, F.; Santini, A.; Barrea, L.; Cargiolli, M.; et al. Good adherence to mediterranean diet can prevent gastrointestinal symptoms: A survey from Southern Italy. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 564–571. [Google Scholar] [CrossRef]
- Paduano, D.; Cingolani, A.; Tanda, E.; Usai, P. Effect of Three Diets (Low-FODMAP, Gluten-free and Balanced) on Irritable Bowel Syndrome Symptoms and Health-Related Quality of Life. Nutrients 2019, 11, 1566. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Patel, N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Vork, L.; Wilms, E.; Penders, J.; Jonkers, D.M.A.E. Stool Consistency: Looking Beyond the Bristol Stool Form Scale. J. Neurogastroenterol. Motil. 2019, 25, 625. [Google Scholar] [CrossRef] [PubMed]
- Berrington de Gonzalez, A.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-Mass Index and Mortality—Prospective Analysis of 1.46 Million White Adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef]
- Consiglio per la Ricerca in Agricoltura e L’analisi Dell’economia Agraria (CREA), Tabella di Composizione Degli Alimenti. Available online: https://www.crea.gov.it/-/tabella-di-composizione-degli-alimenti (accessed on 3 March 2019).
- Greenfield, H.; Southgate, D.A.T. Food Composition Data; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Società Italiana di Nutrizione Umana (SINU), IV Revisione dei Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana (LARN). 2014. Available online: https://sinu.it/tabelle-larn-2014/ (accessed on 3 March 2019).
- Monteagudo, C.; Mariscal-Arcas, M.; Rivas, A.; Lorenzo-Tovar, M.L.; Tur, J.A.; Olea-Serrano, F. Proposal of a Mediterranean Diet Serving Score. PLoS ONE 2015, 10, e0128594. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Ercolini, D.; De Filippis, F.; La Storia, A.; Iacono, M. “Remake” by high-throughput sequencing of the microbiota involved in the production of water buffalo mozzarella cheese. Appl. Environ. Microbiol. 2012, 78, 8142–8145. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Reeder, J.; Knight, R. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 2010, 7, 668–669. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary Reference Values for the EU. 2019. Available online: http://www.efsa.europa.eu/en/interactive-pages/drvs (accessed on 3 March 2019).
- Barrett, J.S.; Irving, P.M.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment. Pharmacol. Ther. 2009, 30, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.; Simren, M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated with More Severe Symptoms and Reduced Quality of Life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Williams, E.; Nai, X.; Corfe, B. Dietary intakes in people with irritable bowel syndrome. BMC Gastroenterol. 2011, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Simren, M.; Mansson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Bjornsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Altomare, A.; Ma, J.; Guarino, M.P.; Cheng, L.; Rieder, F.; Ribolsi, M.; Fiocchi, C.; Biancani, P.; Harnett, K.; Cicala, M. Platelet-activating factor and distinct chemokines are elevated in mucosal biopsies of erosive compared with non-erosive reflux disease patients and controls. Neurogastroenterol. Motil. 2012, 24, 943-e463. [Google Scholar] [CrossRef] [PubMed]
- Carotti, S.; Guarino, M.P.; Cicala, M.; Perrone, G.; Alloni, R.; Segreto, F.; Rabitti, C.; Morini, S. Effect of ursodeoxycholic acid on inflammatory infiltrate in gallbladder muscle of cholesterol gallstone patients. Neurogastroenterol. Motil. 2010, 22, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Bourriaud, C.; Robins, R.J.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol 2005, 99, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, C.I.; Štšepetova, J.; Sepp, E.; Songisepp, E.; Sandrine, P.; Mikelsaar, M. New insights into the impact of Lactobacillus population on host-bacteria metabolic interplay. Oncotarget 2015, 6, 30545–30556. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.; Mierau, I.; Hagting, A.; Poolman, B.; Konings, W.N. The proteolytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 187–221. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Foster, M.L.; Sohail, M.U.; Leutenegger, C.; Queen, E.V.; Steiner, J.M.; Marks, S.L. The fecal microbiome in cats with diarrhea. PLoS ONE 2015, 10, e0127378. [Google Scholar] [CrossRef]
- Martínez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Toyama, M.; Banno, T.; Chonan, O.; Benno, Y.; Watanabe, K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Varela, E.; Antolin, M.; Manichanh, C.; Gallart, M.; Casellas, M.; Torrejón, A.; Borruel, N.; Casellas, F.; Guarner, F. Colonization by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 38, 151–161. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-Medina, M.; Suris-Valls, R.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Flint, H.J.; Garcia-Gil, L.J. Changes in the Abundance of Faecalibacterium prausnitzii Phylogroups I and II in the Intestinal Mucosa of Inflammatory Bowel Disease and Patients with Colorectal Cancer. Inflamm. Bowel Dis. 2016, 22, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Boler, B.M.; Serao, M.C.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C., Jr.; Swanson, K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef]
- Dabard, J.; Bridonneau, C.; Phillipe, C.; Anglade, P.; Molle, D.; Nardi, M.; Ladiré, M.; Girardin, H.; Marcille, F.; Gomez, A.; et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl. Environ. Microbiol. 2001, 67, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680. [Google Scholar] [CrossRef]
- Shen, Y.; Giardino Torchia, M.L.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012, 12, 509–520. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Suutarinen, M.; Heini, T.; Bowers, J.R.; Jasso-Selles, D.; Lemmer, D.; Valentine, M.; Barnes, R.; Engelthaler, D.M.; et al. Isolation of Anti-Inflammatory and Epithelium Reinforcing Bacteroides and Parabacteroides spp. from A Healthy Fecal Donor. Nutrients 2020, 12, 935. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl. Microbiol. Biotechnol. 2019, 103, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Orberg, E.T.; Geis, A.L.; Chan, J.L.; Fu, K.; Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 203–214.e5. [Google Scholar] [CrossRef] [PubMed]
- Lucke, K.; Miehlke, S.; Jacobs, E.; Schuppler, M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J. Med. Microbiol. 2006, 55, 617–624. [Google Scholar] [CrossRef] [PubMed]
- James, S.C.; Fraser, K.; Young, W.; McNabb, W.C.; Roy, N.C. Gut Microbial Metabolites and Biochemical Pathways Involved in Irritable Bowel Syndrome: Effects of Diet and Nutrition on the Microbiome. J. Nutr. 2020, 150, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 21) | IBS (n = 28) | p-Value | |
|---|---|---|---|
| Age years, median (range) | 56 (65–26) | 55 (69–29) | 0.92 |
| Sex, Males, n (%) | 9 (43) | 9 (32) | 0.44 |
| BMI (Kg/m2), mean ± sd | 24.99 ± 3.2 | 27.08 ± 5.2 | 0.16 |
| Underweight, mean ± sd, (n) | - | - | |
| Normal weight, mean ± sd, (n) | 22.14 ± 1.59 (8) | 22.39 ± 2.02 (11) | 0.49 |
| Overweight, mean ± sd, (n) | 25.79 ± 1.22 (11) | 27.63 ± 1.53 (11) | 1.0 |
| Obese, mean ± sd, (n) | 31.95 ± 2.76 (2) | 34.67 ± 4.04 (6) | 0.15 |
| Predominant Bowel Habits, n (%) | |||
| Constipation (IBS-C) | - | 9 (32) | NA |
| Diarrhoea (IBS-D) | - | 11 (39) | NA |
| Mixed (IBS-M) | - | 8 (29) | NA |
| Stool frequency (n/day), mean ± sd | 1.19 ± 0.68 | 1.64 ± 1.37 | 0.17 |
| Stool consistency (BSS), mean ± sd | 3.67 ± 0.80 | 3.79 ± 1.97 | 0.44 |
| Abdominal pain, n (%) | 3 (14) | 28 (100) | <0.001 |
| Frequency (n/day), mean ± sd | 0.24 ± 0.62 | 2.96 ± 2.03 | <0.001 |
| Intensity, mean ± sd | 0.48 ± 1.21 | 6.25 ± 1.24 | <0.001 |
| Flatulence, n (%) | 5 (23) | 28 (100) | <0.001 |
| Frequency (n/day), mean ± sd | 0.62 ± 1.2 | 4.89 ± 2.08 | <0.001 |
| Intensity mean ± sd | 1.05 ± 1.94 | 7.21 ± 1.17 | <0.001 |
| Control (n = 21) | IBS (n = 28) | p-Value a | |
|---|---|---|---|
| Energy, kcal/day | 1425 ± 519.5 | 1484 ± 532.2 | 0.56 |
| Carbohydrates, g/day (E%) | 199 ± 77.6 (49) | 174 ± 75.1 (47) | 0.29 |
| Lipids, g/day (E%) | 60 ± 18.6 (34) | 56 ± 19.5 (37) | 0.46 |
| Proteins, g/day (E%) | 62 ± 21.5 (16) | 61 ± 20.1 (16) | 0.38 |
| Total fibers, g/day | 14 ± 5.1 | 12 ± 5.8 | 0.24 |
| MD score b | 17 ± 4.9 | 11 ± 3.7 | <0.01 |
| Groups | Control (n = 21) | IBS (n = 28) | p-Value a |
|---|---|---|---|
| Carbohydrate intake | |||
| LARN group, (45–60 E%), n (%) | 14 (67) | 12 (43) | 0.15 |
| Non-LARN, (<45–>60 E%), n (%) | 7 (33) | 16 (57) | |
| Lipid intake | |||
| LARN group, (20–35 E%), n (%) | 16 (76) | 9 (32) | 0.003 |
| Non-LARN group, (<20–>35 E%), n (%) | 5 (24) | 19 (68) | |
| Protein intake | |||
| LARN group, (>15 E%), n (%) | 12 (57) | 18 (64) | 0.79 |
| Non-LARN group, (<15 E%), n (%) | 9 (43) | 10 (36) | |
| All Macronutrients Intake | |||
| MNs group, n (%) | 7 (33) | 3 (11) | 0.07 |
| non-MNs group, n (%) | 14 (67) | 25 (89) | |
| Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|
| Mediterranean Diet (MD) | 1.75 | 0.73–41.86 | 0.73 |
| Carbohydrate intake | 0.73 | 0.06–8.43 | 0.80 |
| Lipid intake | 0.67 | 0.07–6.77 | 0.73 |
| Protein intake | 0.81 | 0.004–1.81 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altomare, A.; Del Chierico, F.; Rocchi, G.; Emerenziani, S.; Nuglio, C.; Putignani, L.; Angeletti, S.; Lo Presti, A.; Ciccozzi, M.; Russo, A.; et al. Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study. Nutrients 2021, 13, 1479. https://doi.org/10.3390/nu13051479
Altomare A, Del Chierico F, Rocchi G, Emerenziani S, Nuglio C, Putignani L, Angeletti S, Lo Presti A, Ciccozzi M, Russo A, et al. Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study. Nutrients. 2021; 13(5):1479. https://doi.org/10.3390/nu13051479
Chicago/Turabian StyleAltomare, Annamaria, Federica Del Chierico, Giulia Rocchi, Sara Emerenziani, Chiara Nuglio, Lorenza Putignani, Silvia Angeletti, Alessandra Lo Presti, Massimo Ciccozzi, Alessandra Russo, and et al. 2021. "Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study" Nutrients 13, no. 5: 1479. https://doi.org/10.3390/nu13051479
APA StyleAltomare, A., Del Chierico, F., Rocchi, G., Emerenziani, S., Nuglio, C., Putignani, L., Angeletti, S., Lo Presti, A., Ciccozzi, M., Russo, A., Cocca, S., Ribolsi, M., Muscaritoli, M., Cicala, M., & Guarino, M. P. L. (2021). Association between Dietary Habits and Fecal Microbiota Composition in Irritable Bowel Syndrome Patients: A Pilot Study. Nutrients, 13(5), 1479. https://doi.org/10.3390/nu13051479









