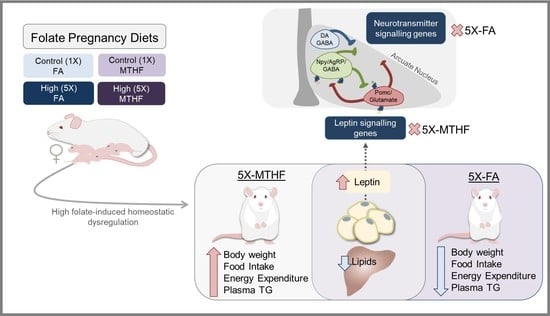
High Intakes of [6S]-5-Methyltetrahydrofolic Acid Compared with Folic Acid during Pregnancy Programs Central and Peripheral Mechanisms Favouring Increased Food Intake and Body Weight of Mature Female Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Phenotypic and Biochemical Analyses
2.3. Folate and Related 1-Carbon Metabolites
2.4. Energy Expenditure and Locomotor Activity
2.5. Brain Dissections and qRT-PCR
2.6. Statistical Analyses
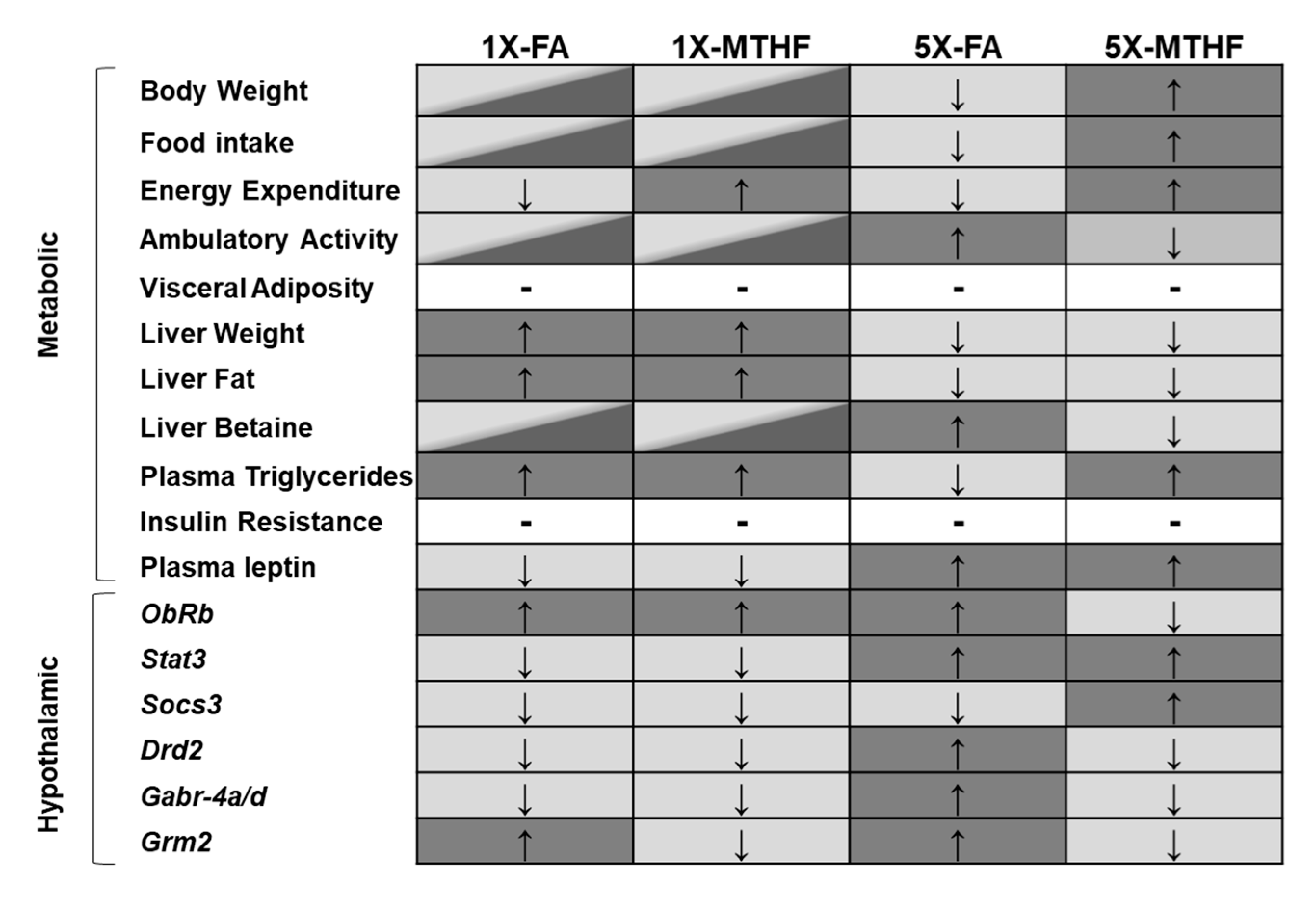
3. Results
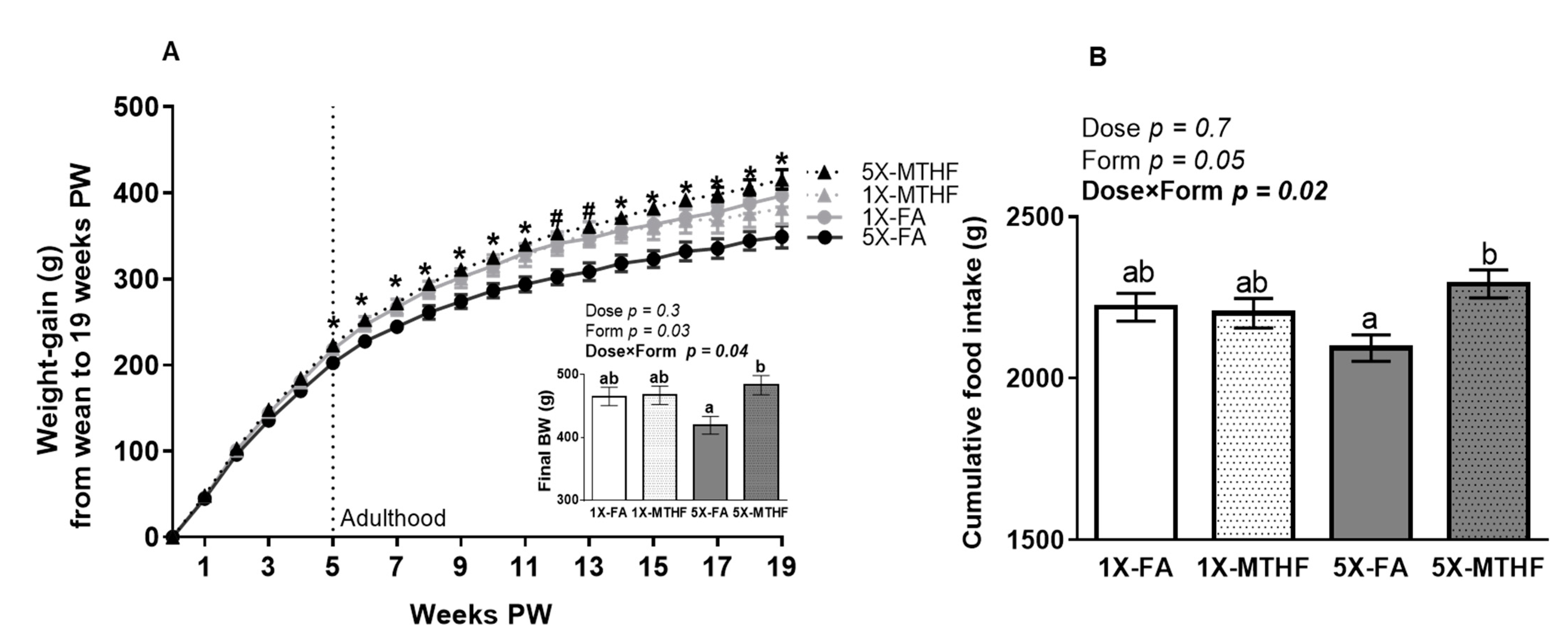
3.1. X-MTHF Offspring Gained more Weight and Ate more Food Than 5X-FA Offspring
3.2. X offspring have Higher Plasma Leptin at Birth and at 19 Weeks Post-Weaning Than 1X Offspring
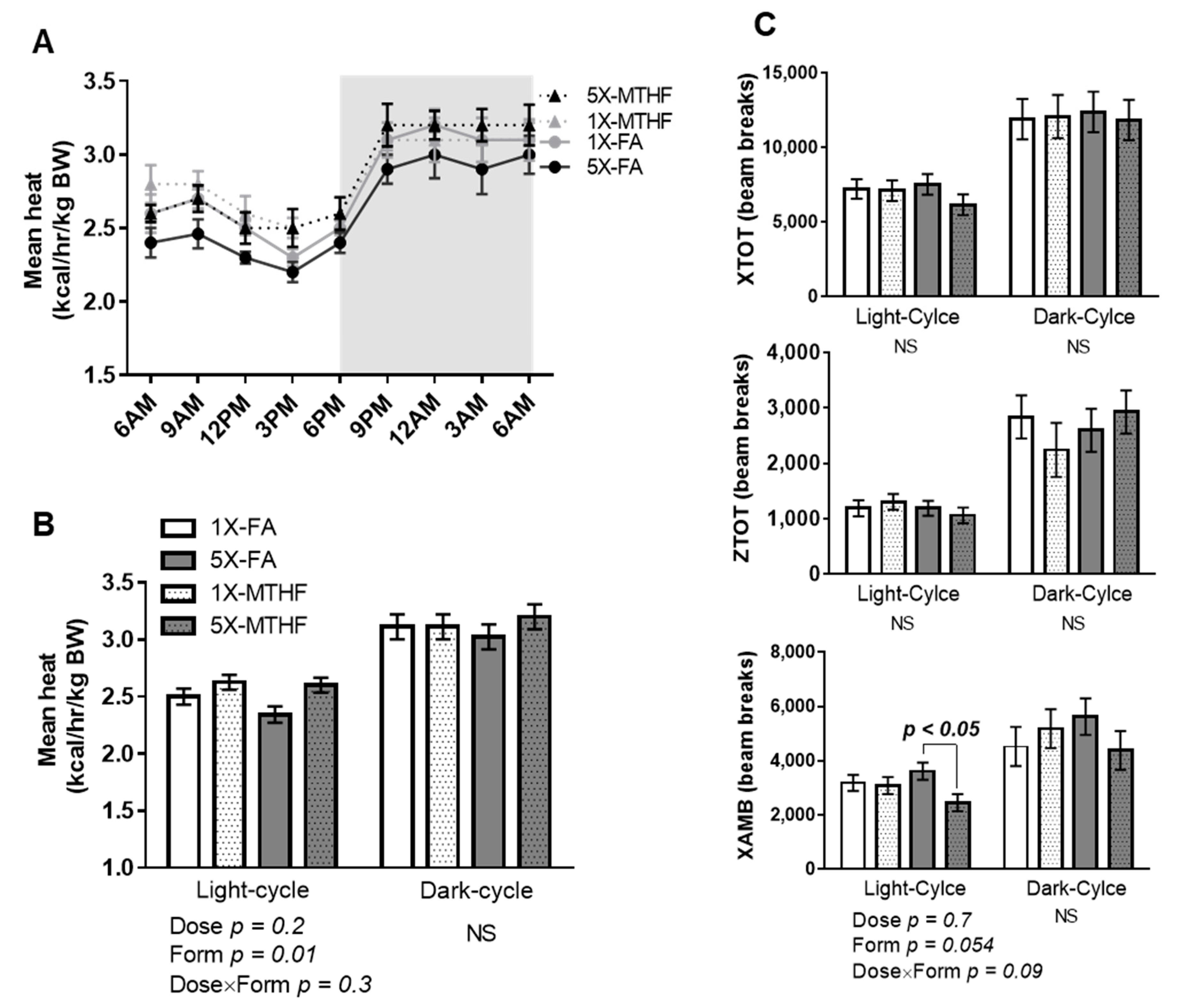
3.3. Post-Weaning Energy Expenditure and Locomotor Activity Are Affected by Gestational Folate Diets
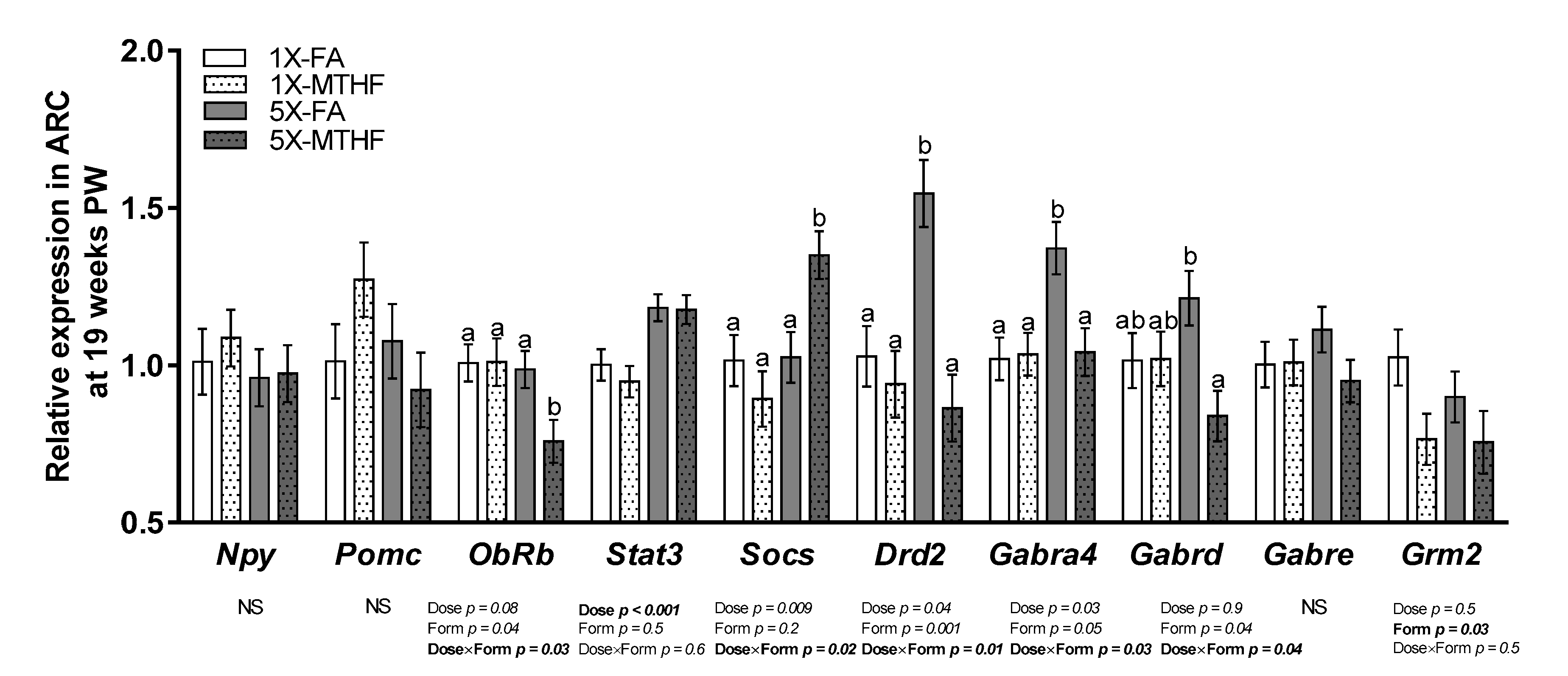
3.4. Post-Weaning Hypothalamic Energy Regulatory Genes Are Affected by Gestational Folate Diets
3.5. Plasma 5-MTHF and Related 1-Carbon Metabolites Are Affected by Folate Gestational Diets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- Pannia, E.; Cho, C.E.; Kubant, R.; Sánchez-Hernández, D.; Huot, P.S.; Anderson, G.H. Role of maternal vitamins in programming health and chronic disease. Nutr. Rev. 2016, 74, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J.; Field, M.S. Trafficking of intracellular folates. Adv. Nutr. 2011, 2, 325–331. [Google Scholar] [CrossRef]
- Christensen, K.E.; Mikael, L.G.; Leung, K.-Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, E.N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Mudryj, A.N.; de Groh, M.; Aukema, H.M.; Yu, N. Folate intakes from diet and supplements may place certain Canadians at risk for folic acid toxicity. Corrigendum. Br. J. Nutr. 2016, 116, 1995. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L.; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.-J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.-I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Maruvada, P.; Stover, P.J.; Mason, J.B.; Bailey, R.L.; Davis, C.D.; Field, M.S.; Finnell, R.H.; Garza, C.; Green, R.; Gueant, J.-L.; et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: A summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020, 112, 1390–1403. [Google Scholar] [CrossRef]
- Naninck, E.F.G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef]
- Keating, E.; Correia-Branco, A.; Araújo, J.R.; Meireles, M.; Fernandes, R.; Guardão, L.; Guimarães, J.T.; Martel, F.; Calhau, C. Excess perigestational folic acid exposure induces metabolic dysfunction in post-natal life. J. Endocrinol. 2015, 224, 245–259. [Google Scholar] [CrossRef]
- Giudicelli, F.; Brabant, A.-L.; Grit, I.; Parnet, P.; Amarger, V. Excess of Methyl Donor in the Perinatal Period Reduces Postnatal Leptin Secretion in Rat and Interacts with the Effect of Protein Content in Diet. PLoS ONE 2013, 8, e68268. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Goh, C.E.; Demmer, R.T.; Whitcomb, B.W.; Du, P.; Liu, Z. Association between Serum Folate and Insulin Resistance among U.S. Nondiabetic Adults. Sci. Rep. 2017, 7, 9187. [Google Scholar] [CrossRef]
- Narin, F.; Atabek, M.E.; Karakukcu, M.; Narin, N.; Kurtoglu, S.; Gumus, H.; Çoksevim, B.; Erez, R. The association of plasma homocysteine levels with serum leptin and apolipoprotein B levels in childhood obesity. Ann. Saudi Med. 2005, 25, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Pannia, E.; Huot, P.S.P.; Sánchez-Hernández, D.; Kubant, R.; Dodington, D.W.; Ward, W.E.; Bazinet, R.P.; Anderson, G.H. Methyl vitamins contribute to obesogenic effects of a high multivitamin gestational diet and epigenetic alterations in hypothalamic feeding pathways in Wistar rat offspring. Mol. Nutr. Food Res. 2014, 59, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Huot, P.S.; Ly, A.; Szeto, I.M.; Reza-López, S.A.; Cho, D.; Kim, Y.-I.; Anderson, G.H. Maternal and postweaning folic acid supplementation interact to influence body weight, insulin resistance, and food intake regulatory gene expression in rat offspring in a sex-specific manner. Appl. Physiol. Nutr. Metab. 2016, 41, 411–420. [Google Scholar] [CrossRef]
- Yang, N.V.; Pannia, E.; Chatterjee, D.; Kubant, R.; Ho, M.; Hammoud, R.; Pausova, Z.; Anderson, G.H. Gestational folic acid content alters the development and function of hypothalamic food intake regulating neurons in Wistar rat offspring post-weaning. Nutr. Neurosci. 2018, 23, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Fu, Z.; Li, H.; Gu, X.; Cai, Z.; Xu, P.; Cui, X.; You, L.; Wang, X.; Zhu, L.; et al. High folate intake contributes to the risk of large for gestational age birth and obesity in male offspring. J. Cell. Physiol. 2018, 233, 9383–9389. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse Offspring Fed a High-Fat Diet. Int. J. Mol. Sci. 2014, 15, 6298–6313. [Google Scholar] [CrossRef]
- Huot, P.S.P.; Dodington, D.W.; Mollard, R.C.; Reza-López, S.A.; Sanchez-Hernandez, D.; Cho, C.E.; Kuk, J.; Ward, W.E.; Anderson, G.H. High Folic Acid Intake during Pregnancy Lowers Body Weight and Reduces Femoral Area and Strength in Female Rat Offspring. J. Osteoporos. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sie, K.K.Y.; Li, J.; Ly, A.; Sohn, K.-J.; Croxford, R.; Kim, Y.-I. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol. Nutr. Food Res. 2013, 57, 677–685. [Google Scholar] [CrossRef]
- Lamers, Y.; Macfarlane, A.J.; O’Connor, D.L.; Fontaine-Bisson, B. Periconceptional intake of folic acid among low-risk women in Canada: Summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am. J. Clin. Nutr. 2018, 108, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.G.; Dwyer, J.T.; Haggans, C.J.; Mills, J.L.; Potischman, N. Perspective: Time to Resolve Confusion on Folate Amounts, Units, and Forms in Prenatal Supplements. Adv. Nutr. 2020, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services; Food and Drug Administration; Center for Food Safety and Applied Nutrition. Guidance for Industry: Converting Units of Measure for Folate, Niacin, And Vitamins A, D, and E on the Nutrition and Supplement Facts Labels. Available online: https://www.fda.gov/media/129863/download (accessed on 19 March 2021).
- Pannia, E.; Hammoud, R.; Simonian, R.; Arning, E.; Ashcraft, P.; Wasek, B.; Bottiglieri, T.; Pausova, Z.; Kubant, R.; Anderson, G.H. [6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth. Nutrition 2020, 13, 48. [Google Scholar] [CrossRef]
- Delgado, T.C. Glutamate and GABA in Appetite Regulation. Front. Endocrinol. 2013, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Savella, G.M.; Church, T.R.; Góez-Mogollón, L.; Sosinsky, A.Z.; Noe, O.B.; Kaimal, A.; Cohen, L.S. A prenatal supplement with methylfolate for the treatment and prevention of depression in women trying to conceive and during pregnancy. Ann. Clin. Psychiatry 2019, 31, 4–16. [Google Scholar]
- Jain, R.; Manning, S.; Cutler, A.J. Good, better, best: Clinical scenarios for the use of L-methylfolate in patients with MDD. CNS Spectr. 2019, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Battista, M.-C.; Hivert, M.-F.; Duval, K.; Baillargeon, J.-P. Intergenerational Cycle of Obesity and Diabetes: How Can We Reduce the Burdens of These Conditions on the Health of Future Generations? Exp. Diabetes Res. 2011, 2011, 596060. [Google Scholar] [CrossRef]
- Lamers, Y.; Prinz-Langenohl, R.; Moser, R.; Pietrzik, K. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am. J. Clin. Nutr. 2004, 79, 473–478. [Google Scholar] [CrossRef]
- Lamers, Y.; Prinz-Langenohl, R.; Brämswig, S.; Pietrzik, K. Red blood cell folate concentrations increase more after supplementation with [6 S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am. J. Clin. Nutr. 2006, 84, 156–161. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient requirements of the laboratory rat. In Nutrient Requirements of Laboratory Animals, 4th ed.; National Academy Press: Washington, DC, USA, 1995; pp. 11–79. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Arning, E.; Bottiglieri, T. Quantitation of S-Adenosylmethionine and S-Adenosylhomocysteine in Plasma Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Adv. Struct. Saf. Stud. 2016, 1378, 255–262. [Google Scholar] [CrossRef]
- Nandania, J.; Kokkonen, M.; Euro, L.; Velagapudi, V. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2018, 1092, 168–178. [Google Scholar] [CrossRef]
- Rooney, M.; Bottiglieri, T.; Wasek-Patterson, B.; McMahon, A.; Hughes, C.F.; McCann, A.; Horigan, G.; Strain, J.; McNulty, H.; Ward, M. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie 2020, 173, 91–99. [Google Scholar] [CrossRef]
- Ducros, V.; Belva-Besnet, H.; Casetta, B.; Favier, A. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin. Chem. Lab. Med. 2006, 44, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.R.; Emson, P.C. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J. Neurosci. Methods 1980, 3, 129–149. [Google Scholar] [CrossRef]
- Herrera, E.; Lasunción, M.; Huerta, L.; Martín-Hidalgo, A. Plasma leptin levels in rat mother and offspring during pregnancy and lactation. Biol. Neonate 2000, 78, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Simonds, S.E.; Cowley, M.A.; Enriori, P.J. Leptin increasing sympathetic nerve outflow in obesity. Adipocyte 2012, 1, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obesity 2018, 26, 1026–1033. [Google Scholar] [CrossRef]
- Bjursell, M.; Gerdin, A.-K.; Lelliott, C.J.; Egecioglu, E.; Elmgren, A.; Törnell, J.; Oscarsson, J.; Bohlooly-Y, M. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am. J. Physiol. Metab. 2008, 294, E251–E260. [Google Scholar] [CrossRef]
- Cosín-Tomás, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess Folic Acid Supplementation Before and During Pregnancy and Lactation Activates Fos Gene Expression and Alters Behaviors in Male Mouse Offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef]
- Shen, W.; Wang, C.; Xia, L.; Fan, C.; Dong, H.; Deckelbaum, R.J.; Qi, K. Epigenetic Modification of the Leptin Promoter in Diet-Induced Obese Mice and the Effects of N-3 Polyunsaturated Fatty Acids. Sci. Rep. 2014, 4, 5282. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, J.; Birkenstock, A.; Buchholz, V.; Müller, A.; Aly, S.A.; Gruner-Labitzke, K.; Koehler, H.; Lichtinghagen, R.; Jahn, K.; Groh, A.; et al. Promoter Methylation of LEP and LEPR before and after Bariatric Surgery: A Cross-Sectional Study. Obes. Facts 2021, 14, 93–99. [Google Scholar] [CrossRef]
- Lecoutre, S.; Oger, F.; Pourpe, C.; Butruille, L.; Marousez, L.; Dickes-Coopman, A.; Laborie, C.; Guinez, C.; Lesage, J.; Vieau, D.; et al. Maternal obesity programs increased leptin gene expression in rat male offspring via epigenetic modifications in a depot-specific manner. Mol. Metab. 2017, 6, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Forhead, A.J.; Fowden, A.L. The hungry fetus? Role of leptin as a nutritional signal before birth. J. Physiol. 2009, 587, 1145–1152. [Google Scholar] [CrossRef]
- Glavas, M.M.; Kirigiti, M.A.; Xiao, X.Q.; Enriori, P.J.; Fisher, S.K.; Evans, A.E.; Grayson, B.E.; Cowley, M.A.; Smith, M.S.; Grove, K.L. Early Overnutrition Results in Early-Onset Arcuate Leptin Resistance and Increased Sensitivity to High-Fat Diet. Endocrinology 2010, 151, 1598–1610. [Google Scholar] [CrossRef]
- Alexe, D.-M.; Syridou, G.; Petridou, E.T. Determinants of Early Life Leptin Levels and Later Life Degenerative Outcomes. Clin. Med. Res. 2006, 4, 326–335. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef]
- Reed, A.S.; Unger, E.K.; Olofsson, L.E.; Piper, M.L.; Myers, M.G.; Xu, A.W. Functional Role of Suppressor of Cytokine Signaling 3 Upregulation in Hypothalamic Leptin Resistance and Long-Term Energy Homeostasis. Diabetes 2010, 59, 894–906. [Google Scholar] [CrossRef]
- Wauman, J. Leptin receptor signaling: Pathways to leptin resistance. Front. Biosci. 2011, 16, 2771–2793. [Google Scholar] [CrossRef]
- Rahmouni, K.; Morgan, D.A.; Morgan, G.M.; Mark, A.L.; Haynes, W.G. Role of Selective Leptin Resistance in Diet-Induced Obesity Hypertension. Diabetes 2005, 54, 2012–2018. [Google Scholar] [CrossRef]
- Lee, A.K.; Mojtahed-Jaberi, M.; Kyriakou, T.; Astarloa, E.A.-O.; Arno, M.; Marshall, N.J.; Brain, S.D.; O’Dell, S.D. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition 2010, 26, 411–422. [Google Scholar] [CrossRef]
- Qiu, J.; Rivera, H.M.; Bosch, M.A.; Padilla, S.L.; Stincic, T.L.; Palmiter, R.D.; Kelly, M.J.; Rønnekleiv, O.K. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 2018, 7. [Google Scholar] [CrossRef]
- Billes, S.K.; Simonds, S.E.; Cowley, M.A. Leptin reduces food intake via a dopamine D2 receptor-dependent mechanism. Mol. Metab. 2012, 1, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vicchi, F.; Ladyman, S.R.; Ornstein, A.M.; Gustafson, P.; Knowles, P.; Luque, G.M.; Grattan, D.R.; Becu-Villalobos, D. Chronic high prolactin levels impact on gene expression at discrete hypothalamic nuclei involved in food intake. FASEB J. 2020, 34, 3902–3914. [Google Scholar] [CrossRef]
- Suyama, S.; Yada, T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Guertin, J.M.; Jacob, T.C. GABA type a receptor trafficking and the architecture of synaptic inhibition. Dev. Neurobiol. 2017, 78, 238–270. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.T.; Yao, X.X.; Zhao, F.L.; Zou, X.H.; Yang, W.; Cui, R.J.; Li, B.J. Role of leptin in the regulation of food intake in fasted mice. J. Cell. Mol. Med. 2020, 24, 4524–4532. [Google Scholar] [CrossRef]
- Bekdash, R.A.; Harrison, N.L. Downregulation of Gabra4 expression during alcohol withdrawal is mediated by specific micro RNA s in cultured mouse cortical neurons. Brain Behav. 2015, 5, e00355. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.M.; Lozano, J.J.; Sumoy, L.; Dierssen, M.; Visa, J.; Gratacòs, M.; Estivill, X. Hypothalamus transcriptome profile suggests an anorexia-cachexia syndrome in the anx/anx mouse model. Physiol. Genom. 2008, 35, 341–350. [Google Scholar] [CrossRef]

| Gene | Forward Sequence (5’-3’) | Reverse Sequence (3’-5’) | Acc. No |
|---|---|---|---|
| B2M | GTGCTTGCCATTCAGAAAACTCC | AACTGAGACACGTAGCAGTTGAG | NM_012512.2 |
| Pomc | GACCTCACCACGGAAAGCAA | TGTTCATCTCCGTTGCCTGG | NM_139326.3 |
| Npy | TGGCCAGATACTACTCCGCT | TTCAAGCCTTGTTCTGGGGG | NM_012614.2 |
| ObRb | CCAGTACCCAGAGCCAAAGT | GGATCGGGCTTCACAACAAGC | NM_012596.1 |
| Stat3 | TGCATTGATAAGGACTCTGGGG | CTGCCGTTGTTGGACTCCTC | NM_012747.2 |
| Socs3 | CCTCCAGCATCTTTGTCGGAAGAC | TACTGGTCCAGGAACTCCCGAATG | NM_053565.1 |
| Drd2 | GTTGTCTACCTGGAGGTGGTG | AGGTTCAGGATGCTTGCTGTG | NM_012547 |
| Gabra4 | ACAGTGATCCTTTCTCAAGTTTCC | CGTGAGGACTGTGGTTATTCCAA | NM_080587.3 |
| Gabrd | TTTATACAGCATCCGCATCACCT | GAAGAGTAGCCATAGCTCTCCAG | NM_017289.2 |
| Gabre | AGGAATTCTAAGAGGACCCAAGA | GCATCAATGGTCATCCTAACTGTG | NM_023091.1 |
| Grm2 | CTCCAGTGATTATCGGGTGCAG | TTCTGTGGCTGGAAAAGGATGAT | NM_001105711.1 |
| 1X | 5X | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X-FA | 1X-MTHF | 5X-FA | 5X-MTHF | Two-way ANOVA p-Value | |||||||||||
| Mean | ± | S.E.M. | Mean | ± | S.E.M. | Mean | ± | S.E.M. | Mean | ± | S.E.M. | Dose | Form | Dose × Form | |
| Birth | |||||||||||||||
| Leptin ng/mL | 0.86 | ± | 0.25 | 1.32 | ± | 0.28 | 1.93 | ± | 0.28 | 1.64 | ± | 0.37 | 0.03 | 0.78 | 0.23 |
| Insulin ng/mL | 0.30 | ± | 0.05 | 0.19 | ± | 0.05 | 0.35 | ± | 0.05 | 0.29 | ± | 0.08 | 0.20 | 0.17 | 0.68 |
| Glucose mg/dL | 74.38 | ± | 6.39 | 70.30 | ± | 7.83 | 77.30 | ± | 7.24 | 72.05 | ± | 9.58 | 0.77 | 0.56 | 0.94 |
| HOMA-IR | 0.22 | ± | 0.05 | 0.15 | ± | 0.07 | 0.29 | ± | 0.06 | 0.21 | ± | 0.07 | 0.34 | 0.27 | 0.97 |
| 19 weeks PW | |||||||||||||||
| Leptin ng/mL | 14.08 | ± | 2.12 | 11.15 | ± | 2.12 | 17.18 | ± | 1.95 | 18.35 | ± | 2.12 | 0.02 | 0.67 | 0.33 |
| Leptin/VAT | 0.29 | ± | 0.05 | 0.27 | ± | 0.04 | 0.40 | ± | 0.04 | 0.37 | ± | 0.04 | 0.02 | 0.53 | 0.85 |
| Ghrelin ng/mL | 153.24 | ± | 18.23 | 110.19 | ± | 19.21 | 110.19 | ± | 17.38 | 102.79 | ± | 19.21 | 0.37 | 0.08 | 0.16 |
| Insulin ng/mL | 2.00 | ± | 0.24 | 1.76 | ± | 0.22 | 2.25 | ± | 0.26 | 1.95 | ± | 0.22 | 0.62 | 0.26 | 0.91 |
| Glucose mg/dL | 120.57 | ± | 7.11 | 115.81 | ± | 6.71 | 129.40 | ± | 7.60 | 127.67 | ± | 6.36 | 0.15 | 0.64 | 0.83 |
| HOMA-IR | 2.53 | ± | 0.38 | 2.54 | ± | 0.36 | 3.05 | ± | 0.41 | 2.62 | ± | 0.34 | 0.43 | 0.58 | 0.56 |
| TG nmol/dL | 70.01 | ± | 7.88 | 83.08 | ± | 7.37 | 45.17 | ± | 7.88 | 70.08 | ± | 7.88 | 0.02 | 0.02 | 0.45 |
| 1X | 5X | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1X-FA | 1X-MTHF | 5X-FA | 5X-MTHF | Two-Way ANOVA p-Value | |||||||||||
| Mean | ± | S.E.M. | Mean | ± | S.E.M. | Mean | ± | S.E.M. | Mean | ± | S.E.M. | Dose | Form | Dose×Form | |
| Birth Plasma | |||||||||||||||
| 5-MTHF (nmol/L) | 217.0 | ± | 25.34 | 174.80 | ± | 25.34 | 275.60 | ± | 25.34 | 331.2 | ± | 25.34 | 0.004 | 0.81 | 0.09 |
| 19 weeks PW Plasma | |||||||||||||||
| 5-MTHF (nmol/L) | 127.14 | ± | 7.83 | 144.38 | ± | 7.33 | 126.34 | ± | 7.33 | 133.38 | ± | 7.33 | 0.44 | 0.12 | 0.55 |
| Met (µmol/L) | 73.77 | ± | 2.87 | 73.41 | ± | 2.68 | 69.39 | ± | 2.87 | 74.26 | ± | 2.68 | 0.53 | 0.42 | 0.35 |
| SAM (nmol/L) | 269.02 | ± | 11.77 | 269.87 | ± | 11.77 | 267.97 | ± | 12.58 | 248.94 | ± | 11.77 | 0.37 | 0.45 | 0.41 |
| SAH (nmol/L) | 74.16 | ± | 8.84 | 76.43 | ± | 8.84 | 79.68 | ± | 8.84 | 72.90 | ± | 8.84 | 0.91 | 0.80 | 0.61 |
| Homocysteine (µmol/L) | 5.81 | ± | 0.88 | 4.20 | ± | 1.01 | 5.66 | ± | 0.88 | 5.84 | ± | 0.94 | 0.43 | 0.45 | 0.34 |
| Cystathionine (nmol/L) | 830.27 | ± | 83.80 | 827.55 | ± | 83.80 | 700.03 | ± | 83.80 | 611.89 | ± | 83.80 | 0.04 | 0.59 | 0.61 |
| Choline (µmol/L) | 10.89 | ± | 0.72 | 11.36 | ± | 0.72 | 10.70 | ± | 0.72 | 10.34 | ± | 0.72 | 0.41 | 0.94 | 0.57 |
| Betaine (µmol/L) | 90.71 | ± | 7.53 | 86.57 | ± | 6.25 | 85.18 | ± | 6.68 | 67.06 | ± | 6.25 | 0.06 | 0.09 | 0.29 |
| 19 weeks PW Liver | |||||||||||||||
| 5-MTHF (nmol/L) | 27.78 | 1.63 | 32.31 | 1.63 | 29.57 | 1.63 | 30.75 | 1.63 | 0.94 | 0.09 | 0.31 | ||||
| Met (µmol/L) | 447.80 | ± | 45.17 | 447.88 | ± | 45.17 | 506.25 | ± | 45.17 | 508.25 | ± | 45.17 | 0.20 | 0.98 | 0.98 |
| SAM (nmol/L) | 8.75 | ± | 3.83 | 15.85 | ± | 3.32 | 17.33 | ± | 3.32 | 17.15 | ± | 3.32 | 0.15 | 0.34 | 0.32 |
| SAH (nmol/L) | 15.55 | ± | 3.36 | 21.78 | ± | 2.91 | 18.41 | ± | 2.91 | 21.56 | ± | 2.91 | 0.67 | 0.13 | 0.61 |
| Cystathionine (nmol/L) | 25.48 | ± | 2.51 | 23.77 | ± | 2.51 | 26.61 | ± | 2.51 | 26.99 | ± | 2.51 | 0.51 | 0.95 | 0.54 |
| Choline (µmol/L) | 318.63 | ± | 64.36 | 384.00 | ± | 64.36 | 317.75 | ± | 64.37 | 346.38 | ± | 64.36 | 0.77 | 0.47 | 0.77 |
| Betaine (µmol/L) | 2458.75 ab | ± | 233.63 | 2585 ab | ± | 233.63 | 3045 a | ± | 249.76 | 2230.75 b | ± | 233.63 | 0.60 | 0.13 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannia, E.; Hammoud, R.; Kubant, R.; Sa, J.Y.; Simonian, R.; Wasek, B.; Ashcraft, P.; Bottiglieri, T.; Pausova, Z.; Anderson, G.H. High Intakes of [6S]-5-Methyltetrahydrofolic Acid Compared with Folic Acid during Pregnancy Programs Central and Peripheral Mechanisms Favouring Increased Food Intake and Body Weight of Mature Female Offspring. Nutrients 2021, 13, 1477. https://doi.org/10.3390/nu13051477
Pannia E, Hammoud R, Kubant R, Sa JY, Simonian R, Wasek B, Ashcraft P, Bottiglieri T, Pausova Z, Anderson GH. High Intakes of [6S]-5-Methyltetrahydrofolic Acid Compared with Folic Acid during Pregnancy Programs Central and Peripheral Mechanisms Favouring Increased Food Intake and Body Weight of Mature Female Offspring. Nutrients. 2021; 13(5):1477. https://doi.org/10.3390/nu13051477
Chicago/Turabian StylePannia, Emanuela, Rola Hammoud, Ruslan Kubant, Jong Yup Sa, Rebecca Simonian, Brandi Wasek, Paula Ashcraft, Teodoro Bottiglieri, Zdenka Pausova, and G. Harvey Anderson. 2021. "High Intakes of [6S]-5-Methyltetrahydrofolic Acid Compared with Folic Acid during Pregnancy Programs Central and Peripheral Mechanisms Favouring Increased Food Intake and Body Weight of Mature Female Offspring" Nutrients 13, no. 5: 1477. https://doi.org/10.3390/nu13051477
APA StylePannia, E., Hammoud, R., Kubant, R., Sa, J. Y., Simonian, R., Wasek, B., Ashcraft, P., Bottiglieri, T., Pausova, Z., & Anderson, G. H. (2021). High Intakes of [6S]-5-Methyltetrahydrofolic Acid Compared with Folic Acid during Pregnancy Programs Central and Peripheral Mechanisms Favouring Increased Food Intake and Body Weight of Mature Female Offspring. Nutrients, 13(5), 1477. https://doi.org/10.3390/nu13051477