Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
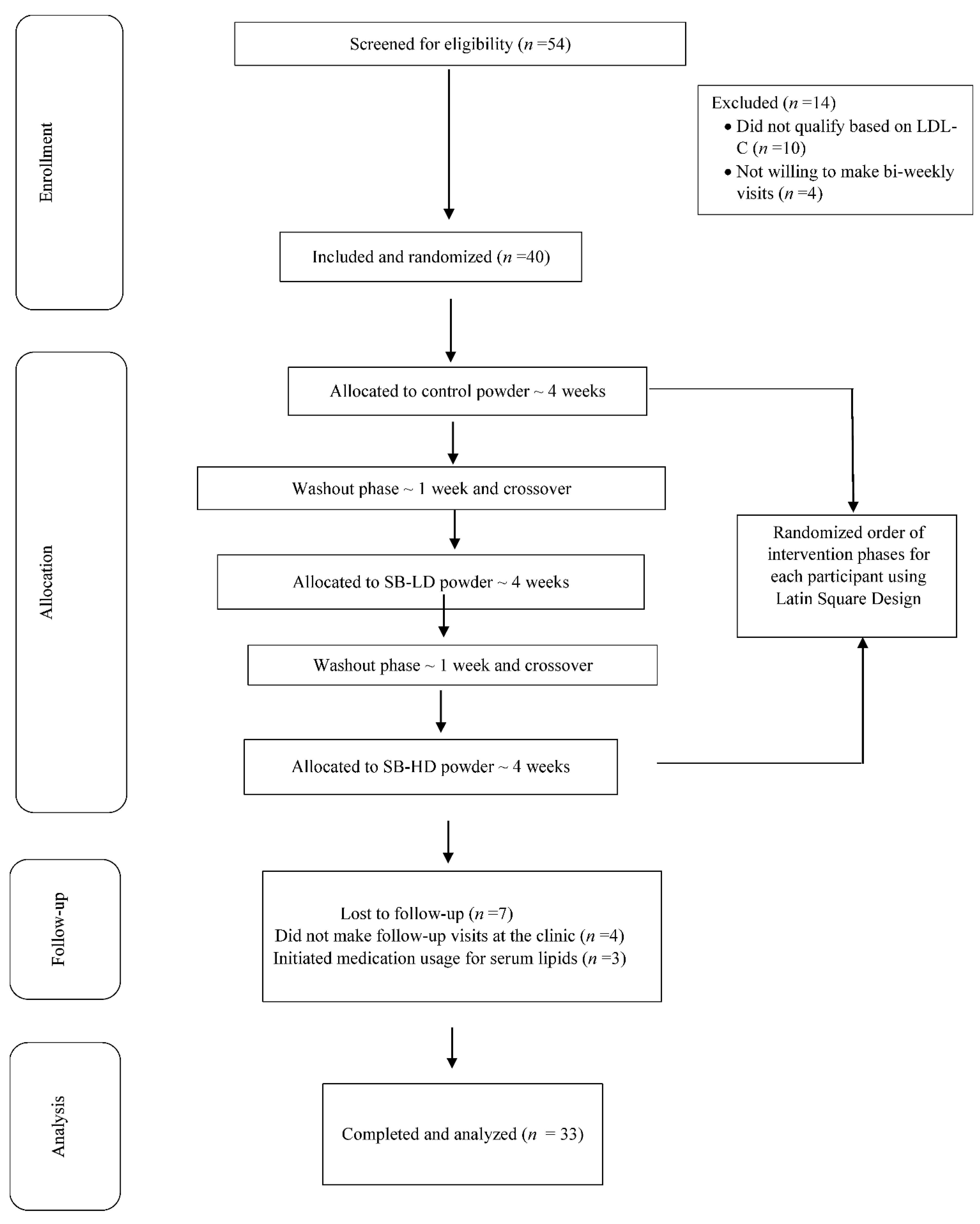
2.1. Study Criteria and Protocol
2.2. Intervention and Control Powders
2.3. Habitual Dietary Intake and Physical Activity Assessment
2.4. Compliance
2.5. Anthropometric Measures and Blood Pressure
2.6. Biochemical Analyses
2.7. Human Diabetes Panel Assays
2.8. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Compliance
3.2. Anthropometrics, Blood Pressure, Glucose, Insulin, and Conventional Lipids
3.3. NMR-Derived Lipid Particle Profiles
3.4. Adipokines, CRP, and Hormonal Biomarkers
3.5. Habitual Dietary Intakes and Physical Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Clinical Trial Registry
References
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Worldwide burden of LDL cholesterol: Implications in cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. Nmcd 2020, 30, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Miao, Y.; Meng, Z.; Zhong, Y. Effects of Vaccinium Berries on Serum Lipids: A Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. Ecam 2015, 2015, 790329. [Google Scholar] [CrossRef]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2012, 60, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Betts, N.M.; Nguyen, A.; Newman, E.D.; Fu, D.; Lyons, T.J. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J. Nutr. 2014, 144, 830–837. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Johnson, N.A.; Barwick, A.L.; Searle, A.; Spink, M.J.; Twigg, S.M.; Chuter, V.H. Self-reported physical activity in community-dwelling adults with diabetes and its association with diabetes complications. J. Diabetes Complicat. 2019, 33, 33–38. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Lyons, T.J.; Jenkins, A.J.; Zheng, D.; Klein, R.L.; Otvos, J.D.; Yu, Y.; Lackland, D.T.; McGee, D.; McHenry, M.B.; Lopes-Virella, M.; et al. Nuclear magnetic resonance-determined lipoprotein subclass profile in the DCCT/EDIC cohort: Associations with carotid intima-media thickness. Diabetes Med. A J. Br. Diabetes Assoc. 2006, 23, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Medina Larque, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonne, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Novotny, J.A.; Baer, D.J.; Khoo, C.; Gebauer, S.K.; Charron, C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015, 145, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Hsia, D.S.; Zhang, D.J.; Beyl, R.S.; Greenway, F.L.; Khoo, C. Effect of daily consumption of cranberry beverage on insulin sensitivity and modification of cardiovascular risk factors in adults with obesity: A pilot, randomised, placebo-controlled study. Br. J. Nutr. 2020, 124, 577–585. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef]
- Aranaz, P.; Romo-Hualde, A.; Zabala, M.; Navarro-Herrera, D.; Ruiz de Galarreta, M.; Gil, A.G.; Martinez, J.A.; Milagro, F.I.; Gonzalez-Navarro, C.J. Freeze-dried strawberry and blueberry attenuates diet-induced obesity and insulin resistance in rats by inhibiting adipogenesis and lipogenesis. Food Funct. 2017, 8, 3999–4013. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. The healthy effects of strawberry bioactive compounds on molecular pathways related to chronic diseases. Ann. N. Y. Acad. Sci. 2017, 1398, 62–71. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and Insulin-Resistance: From Molecular Evidences to Clinical Trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef]
- Garvey, W.T.; Kwon, S.; Zheng, D.; Shaughnessy, S.; Wallace, P.; Hutto, A.; Pugh, K.; Jenkins, A.J.; Klein, R.L.; Liao, Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003, 52, 453–462. [Google Scholar] [CrossRef]
- Basu, A.; Jenkins, A.J.; Zhang, Y.; Stoner, J.A.; Klein, R.L.; Lopes-Virella, M.F.; Garvey, W.T.; Lyons, T.J. Nuclear magnetic resonance-determined lipoprotein subclasses and carotid intima-media thickness in type 1 diabetes. Atherosclerosis 2016, 244, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 2005, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.L.; Edirisinghe, I.; Kappagoda, T.; Burton-Freeman, B. Attenuation of meal-induced inflammatory and thrombotic responses in overweight men and women after 6-week daily strawberry (Fragaria) intake. A randomized placebo-controlled trial. J. Atheroscler. Thromb. 2011, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Juhan-Vague, I.; Alessi, M.C. PAI-1, obesity, insulin resistance and risk of cardiovascular events. Thromb. Haemost. 1997, 78, 656–660. [Google Scholar] [CrossRef]
- Raiko, J.R.; Oikonen, M.; Wendelin-Saarenhovi, M.; Siitonen, N.; Kähönen, M.; Lehtimäki, T.; Viikari, J.; Jula, A.; Loo, B.M.; Huupponen, R.; et al. Plasminogen activator inhitor-1 associates with cardiovascular risk factors in healthy young adults in the Cardiovascular Risk in Young Finns Study. Atherosclerosis 2012, 224, 208–212. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Stewart, P.W.; Ginsberg, H.N.; Tracy, R.P.; Lefevre, M.; Elmer, P.J.; Berglund, L.; Ershow, A.G.; Pearson, T.A.; Ramakrishnan, R.; et al. The Type and Amount of Dietary Fat Affect Plasma Factor VIIc, Fibrinogen, and PAI-1 in Healthy Individuals and Individuals at High Cardiovascular Disease Risk: 2 Randomized Controlled Trials. J. Nutr. 2020, 150, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Pasten, C.; Olave, N.C.; Zhou, L.; Tabengwa, E.M.; Wolkowicz, P.E.; Grenett, H.E. Polyphenols downregulate PAI-1 gene expression in cultured human coronary artery endothelial cells: Molecular contributor to cardiovascular protection. Thromb. Res. 2007, 121, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zagotta, I.; Dimova, E.Y.; Funcke, J.B.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Resveratrol suppresses PAI-1 gene expression in a human in vitro model of inflamed adipose tissue. Oxidative Med. Cell. Longev. 2013, 2013, 793525. [Google Scholar] [CrossRef] [PubMed]
| Variable | Control | Strawberry-LD | Strawberry-HD |
|---|---|---|---|
| Weight, g | 32 | 32 | 32 |
| Calories, Kcal | 122 | 123 | 124 |
| Total Carbohydrates, g | 29 | 28 | 27 |
| Ash, g | 1.1 | 0.7 | 2.0 |
| Dietary fiber, g | 3.2 | 2.1 | 5.0 |
| Vitamin C, mg | ND | 26 | 65 |
| Total phenolics, mg 1 | ND | 400 | 960 |
| Total anthocyanins, mg 2 | ND | 38 | 92 |
| Total ellagic acid, mg | ND | 9 | 25 |
| Total flavan-3-ols, mg | ND | 24 | 60 |
| N | 33 |
| Age, (y) | 53 ± 13 |
| Sex, (M/F) | 2/31 |
| BMI, (kg/m2) | 33 ± 3 |
| Blood pressure medication use, n (%) | 6 (18) |
| Antidepressant use, n (%) | 8 (24) |
| Multivitamin use, n (%) | 5 (15) |
| Meeting exercise recommendations (%) 1 | 11 (33) |
| Variable | Baseline | Control (4-Week) | Strawberry (LD) (4-Week) | Strawberry (HD) (4-Week) | p-Value 1 (Treatment) |
|---|---|---|---|---|---|
| Body weight, lb | 189 ± 23 | 189 ± 23 | 190 ± 23 | 190 ± 23 | 0.99 |
| BMI, kg/m2 | 32.0 ± 2.5 | 32.0 ± 2.4 | 32.0 ± 2.5 | 32.0 ± 2.4 | 0.89 |
| Waist circumference, inches | 40.0 ± 2.8 | 41.0 ± 3.0 | 41.0 ± 3.0 | 41.0 ± 3.0 | 0.92 |
| Systolic blood pressure, mm Hg | 127 ± 8 | 126 ± 10 | 127 ± 9 | 124 ± 8 | 0.78 |
| Diastolic blood pressure, mm Hg | 82 ± 7 | 82 ± 8 | 80 ± 7 | 80 ± 8 | 0.84 |
| Serum total cholesterol, mg/dL | 221 ± 28 | 217 ± 28 | 209 ± 34 | 208 ± 32 | 0.24 |
| Serum LDL cholesterol, mg/dL | 144 ± 25 | 139 ± 23 | 133 ± 29 | 127 ± 24 | 0.05 |
| Serum HDL cholesterol, mg/dL | 54 ± 10 | 53 ± 11 | 53 ± 11 | 53 ± 9 | 0.96 |
| Serum LDL:HDL | 2.8 ± 0.9 | 2.8 ± 0.9 | 2.7 ± 0.9 | 2.6 ± 0.8 | 0.77 |
| Serum Triglycerides, mg/dL | 124 ± 66 | 126 ± 60 | 128 ± 67 | 133 ± 76 | 0.95 |
| Serum Fasting glucose, mg/dL | 93 ± 13 | 93 ± 12 | 94 ± 11 | 93 ± 15 | 0.97 |
| Serum HbA1c, % | 5.5 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.2 | 0.95 |
| Serum Insulin, µIU/mL * | 15.4 ± 6.6 a | 15.2 ± 6.4 a | 14.0 ± 8.2 a | 9.1 ± 3.1 b | 0.0002 |
| Serum HOMA-IR | 3.6 ± 1.5 a | 3.5 ± 1.4 a | 3.3 ± 2.0 a | 2.1 ± 0.5 b | 0.0003 |
| Serum hs-CRP, mg/L | 4.3 ± 3.2 | 4.4 ± 3.5 | 4.3 ± 3.1 | 3.8 ± 2.9 | 0.85 |
| Serum adiponectin, µg/mL | 9.3 ± 5.7 | 10.5 ± 6.2 | 11.4 ± 5.2 | 11.7 ± 7.2 | 0.84 |
| Variable | Baseline | Control (4-Week) | Strawberry (LD) (4-Week) | Strawberry (HD) (4-Week) | p-Value 1 (Treatment) |
|---|---|---|---|---|---|
| VLDL and chylomicron particles (total), nmol/L | 39.2 ± 16.3 a | 41.7 ± 15.9 a | 42.6 ± 16.0 a | 32.3 ± 13.0 b | <0.0001 |
| Large VLDL and chylomicron particles, nmol/L | 6.1 ± 3.9 | 6.7 ± 3.7 | 6.8 ± 3.7 | 6.3 ± 3.2 | 0.06 |
| Medium VLDL particles, nmol/L | 12.9 ± 9.5 | 14.3 ± 9.3 | 14.3 ± 9.3 | 12.7 ± 8.8 | 0.07 |
| Small VLDL particles, nmol/L | 21.8 ± 10.2 | 22.3 ± 10.8 a | 23.1 ± 10.0 a | 13.7 ± 5.4 b | <0.0001 |
| LDL particles (total), nmol/L * | 1126 ± 328 | 1196 ± 326 a | 1204 ± 310 a | 1042 ± 297 b | <0.0001 |
| IDL particles, nmol/L | 341 ± 175 | 359 ± 173 | 362 ± 169 | 357 ± 174 | 0.12 |
| Large LDL particles, nmol/L | 153 ± 99 | 152 ± 93 | 152 ± 92 | 150 ± 93 | 0.76 |
| Small LDL particles (total), nmol/L * | 638 ± 188 a | 695 ± 242 a,b | 681 ± 219 a,b | 535 ± 140 b | <0.0001 |
| HDL particles (total), µmol/L | 29.1 ± 5.6 | 29.5 ± 5.8 | 30.0 ± 5.7 | 29.5 ± 5.8 | 0.18 |
| Large HDL particles, µmol/L | 4.2 ± 1.9 | 4.4 ± 1.8 | 4.5 ± 1.8 | 4.2 ± 1.7 | 0.11 |
| Medium HDL particles, µmol/L | 5.5 ± 4.7 | 5.6 ± 4.8 | 6.0 ± 4.9 | 5.6 ± 4.7 | 0.24 |
| Small HDL particles, µmol/L | 19.5 ± 5.1 | 20.1 ± 4.6 | 20.7 ± 4.1 | 20.0 ± 4.7 | 0.22 |
| VLDL size, nm | 55.4 ± 7.1 | 55.5 ± 7.7 | 55.9 ± 8.0 | 54.1 ± 7.8 | 0.35 |
| LDL size, nm | 20.1 ± 0.6 | 20.8 ± 1.8 | 21.3 ± 1.9 | 20.2 ± 1.9 | 0.45 |
| HDL size, nm | 9.2 ± 0.5 a | 9.2 ± 0.7 a | 11.3 ± 2.4 b | 9.3 ± 0.7 a | <0.0001 |
| Variable | Baseline | Control (4-Week) | Strawberry (LD) (4-Week) | Strawberry (HD) (4-Week) | p-Value 1 (Treatment) |
|---|---|---|---|---|---|
| Serum C-peptide, pg/mL | 1232 ± 929 | 1273 ± 531 | 1254 ± 752 | 1021 ± 680 | 0.68 |
| Serum ghrelin, pg/mL | 486 ± 371 | 371 ± 361 | 380 ± 319 | 482 ± 392 | 0.62 |
| Serum GIP, pg/mL | 478 ± 357 | 354 ± 274 | 378 ± 219 | 564 ± 426 | 0.23 |
| Serum GLP-1, pg/mL | 314 ± 59 | 265 ± 47 | 288 ± 69 | 307 ± 88 | 0.23 |
| Serum glucagon, pg/mL | 654 ± 147 | 609 ± 93 | 635 ± 113 | 725 ± 117 | 0.02 |
| Serum leptin, pg/mL | 13736 ± 9204 | 12795 ± 4841 | 14884 ± 7433 | 9547 ± 3635 | 0.09 |
| Serum PAI-1, pg/Ml * | 5621 ± 1022 | 5812 ± 1240 | 5245 ± 1278 | 4315 ± 1434 | 0.002 |
| Serum resistin, pg/mL | 10487 ± 6799 | 11787 ± 6516 | 11711 ± 6238 | 10199 ± 4811 | 0.82 |
| Serum visfatin, pg/mL | 3240 ± 1310 | 2733 ± 1033 | 2797 ± 954 | 3176 ± 1352 | 0.45 |
| Variable | Baseline | Control (4-Week) | Strawberry (LD) (4-Week) | Strawberry (HD) (4-Week) | p-Value 1 (Treatment) |
|---|---|---|---|---|---|
| Total calories, kcal | 2012 ± 152 | 1988 ± 183 | 2123 ± 113 | 2067 ± 193 | 0.74 |
| Carbohydrates, % kcal | 45 ± 6 | 48 ± 8 | 44 ± 5 | 46 ± 8 | 0.66 |
| Fats, % kcal | 34 ± 4 | 36 ± 7 | 35 ± 5 | 37 ± 5 | 0.45 |
| Proteins, % kcal | 20 ± 5 | 16 ± 6 | 21 ± 7 | 17 ± 6 | 0.42 |
| Total sugars, g * | 85 ± 11 | 78 ± 12 | 72 ± 9 | 75 ± 10 | 0.08 |
| Fiber, g | 15 ± 11 | 18 ± 12 | 22 ± 15 | 21 ± 10 | 0.18 |
| Vitamin E, mg | 9 ± 2 | 11 ± 4 | 11 ± 2 | 10 ± 4 | 0.22 |
| Vitamin C, mg | 45 ± 13 | 46 ± 12 | 49 ± 12 | 49 ± 12 | 0.26 |
| Fruits, cups | 1.0 ± 0.4 | 1.0 ± 0.3 | 0.8 ± 0.2 | 1.0 ± 0.4 | 0.45 |
| Vegetables, cups | 1.3 ± 0.2 | 1.1 ± 0.3 | 0.9 ± 0.2 | 1.1 ± 0.4 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, A.; Izuora, K.; Betts, N.M.; Kinney, J.W.; Salazar, A.M.; Ebersole, J.L.; Scofield, R.H. Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial. Nutrients 2021, 13, 1421. https://doi.org/10.3390/nu13051421
Basu A, Izuora K, Betts NM, Kinney JW, Salazar AM, Ebersole JL, Scofield RH. Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial. Nutrients. 2021; 13(5):1421. https://doi.org/10.3390/nu13051421
Chicago/Turabian StyleBasu, Arpita, Kenneth Izuora, Nancy M. Betts, Jefferson W. Kinney, Arnold M. Salazar, Jeffrey L. Ebersole, and R. Hal Scofield. 2021. "Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial" Nutrients 13, no. 5: 1421. https://doi.org/10.3390/nu13051421
APA StyleBasu, A., Izuora, K., Betts, N. M., Kinney, J. W., Salazar, A. M., Ebersole, J. L., & Scofield, R. H. (2021). Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial. Nutrients, 13(5), 1421. https://doi.org/10.3390/nu13051421







