Individuals Diagnosed with Binge-Eating Disorder Have DNA Hypomethylated Sites in Genes of the Metabolic System: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Population
2.3. Anthropometric and Clinical Characteristics
2.4. DNA Extraction and Microarray DNA Methylation
2.5. Differential Methylated Sites Analysis
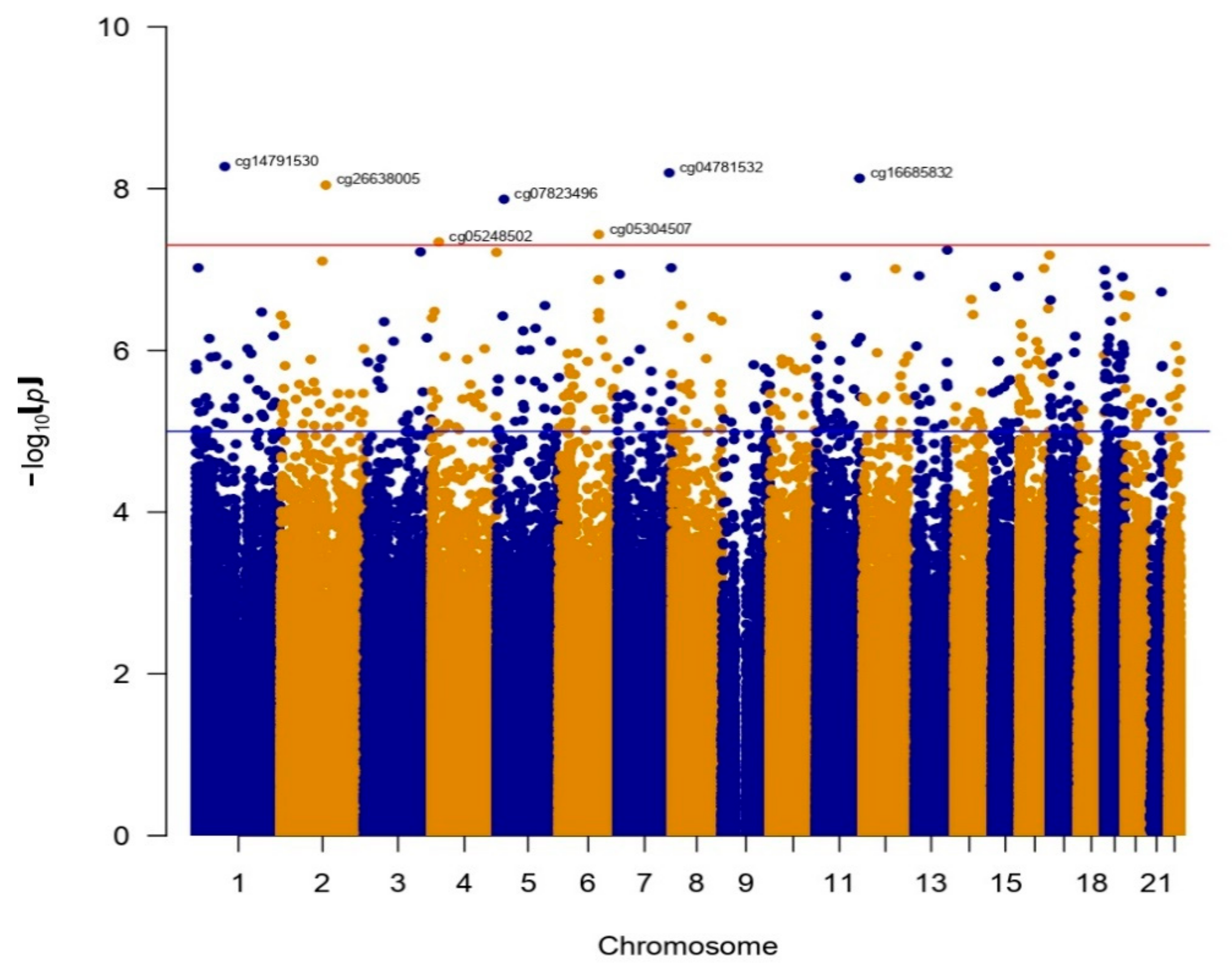
3. Results
Differentially Methylated Sites in BN and BED
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornstein, S.G.; Kunovac, J.L.; Herman, B.K.; Culpepper, L. Recognizing Binge-Eating Disorder in the Clinical Setting: A Review of the Literature. Prim. Care Companion CNS Disord. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Mathes, W.F.; Brownley, K.A.; Mo, X.; Bulik, C.M. The biology of binge eating. Appetite 2009, 52, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Hiripi, E.; Pope, H.G.J.; Kessler, R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 2007, 61, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.A.; Chiu, W.T.; Deitz, A.C.; Hudson, J.I.; Shahly, V.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry 2013, 73, 904–914. [Google Scholar] [CrossRef]
- Mitchison, D.; Hay, P.J. The epidemiology of eating disorders: Genetic, environmental, and societal factors. Clin. Epidemiol. 2014, 6, 89–97. [Google Scholar] [CrossRef]
- Ruiz-Lázaro, P.M.; Comet, M.P.; Calvo, A.I.; Zapata, M.; Cebollada, M.; Trébol, L.; Lobo, A. Prevalence of eating disorders in early adolescent students. Actas Esp. Psiquiatr. 2010, 38, 204–211. [Google Scholar]
- Fairburn, C.G.; Harrison, P.J. Eating Disorders. Lancet 2003, 361, 407–416. [Google Scholar] [CrossRef]
- Mehler, P.S.; Rylander, M. Bulimia Nervosa—Medical complications. J. Eat. Disord. 2015, 3, 12. [Google Scholar] [CrossRef]
- Amianto, F.; Ottone, L.; Abbate Daga, G.; Fassino, S. Binge-eating disorder diagnosis and treatment: A recap in front of DSM-5. BMC Psychiatry 2015, 15, 70. [Google Scholar] [CrossRef]
- Jordan, J.; McIntosh, V.V.W.; Carter, J.D.; Rowe, S.; Taylor, K.; Frampton, C.M.A.; McKenzie, J.M.; Latner, J.; Joyce, P.R. Bulimia nervosa-nonpurging subtype: Closer to the bulimia nervosa-purging subtype or to binge eating disorder? Int. J. Eat. Disord. 2014, 47, 231–238. [Google Scholar] [CrossRef]
- Grilo, C.M.; White, M.A.; Gueorguieva, R.; Wilson, G.T.; Masheb, R.M. Predictive significance of the overvaluation of shape/weight in obese patients with binge eating disorder: Findings from a randomized controlled trial with 12-month follow-up. Psychol. Med. 2013, 43, 1335–1344. [Google Scholar] [CrossRef]
- Fassino, S.; Leombruni, P.; Pierò, A.; Abbate-Daga, G.; Giacomo Rovera, G. Mood, eating attitudes, and anger in obese women with and without Binge Eating Disorder. J. Psychosom. Res. 2003, 54, 559–566. [Google Scholar] [CrossRef]
- Robinson, A.H.; Safer, D.L. Moderators of dialectical behavior therapy for binge eating disorder: Results from a randomized controlled trial. Int. J. Eat. Disord. 2012, 45, 597–602. [Google Scholar] [CrossRef]
- Peterson, C.B.; Swanson, S.A.; Crow, S.J.; Mitchell, J.E.; Agras, W.S.; Halmi, K.A.; Crosby, R.D.; Wonderlich, S.A.; Berg, K.C. Longitudinal stability of binge-eating type in eating disorders. Int. J. Eat. Disord. 2012, 45, 664–669. [Google Scholar] [CrossRef]
- Castellini, G.; Lo Sauro, C.; Mannucci, E.; Ravaldi, C.; Rotella, C.M.; Faravelli, C.; Ricca, V. Diagnostic crossover and outcome predictors in eating disorders according to DSM-IV and DSM-V proposed criteria: A 6-year follow-up study. Psychosom. Med. 2011, 73, 270–279. [Google Scholar] [CrossRef]
- Striegel-Moore, R.H.; Cachelin, F.M.; Dohm, F.-A.; Pike, K.M.; Wilfley, D.E.; Fairburn, C.G. Comparison of binge eating disorder and bulimia nervosa in a community sample. Int. J. Eat. Disord. 2001, 29, 157–165. [Google Scholar] [CrossRef]
- Ruiz-Ramos, D.; Martínez-Magaña, J.J.; García, A.R.; Juarez-Rojop, I.E.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Sarmiento, E.; López-Narvaez, M.L.; Sanchez, N.; Genis-Mendoza, A.D. Psychiatric Comorbidity in Mexican Adolescents with a Diagnosis of Eating Disorders Its Relationship with the Body Mass Index. Int. J. Environ. Res. Public Health 2021, 18. [Google Scholar] [CrossRef]
- Rikani, A.A.; Choudhry, Z.; Choudhry, A.M.; Ikram, H.; Asghar, M.W.; Kajal, D.; Waheed, A.; Mobassarah, N.J. A critique of the literature on etiology of eating disorders. Ann. Neurosci. 2013, 20, 157–161. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Cooper, Z.; Bohn, K.; O’Connor, M.E.; Doll, H.A.; Palmer, R.L. The severity and status of eating disorder NOS: Implications for DSM-V. Behav. Res. Ther. 2007, 45, 1705–1715. [Google Scholar] [CrossRef]
- Sim, L.A.; McAlpine, D.E.; Grothe, K.B.; Himes, S.M.; Cockerill, R.G.; Clark, M.M. Identification and treatment of eating disorders in the primary care setting. Mayo Clin. Proc. 2010, 85, 746–751. [Google Scholar] [CrossRef]
- Halmi, K.A. Anorexia nervosa: An increasing problem in children and adolescents. Dialogues Clin. Neurosci. 2009, 11, 100–103. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef]
- Bulik, C.M.; Yilmaz, Z.; Hardaway, J.A. Genetics and epigenetics of eating disorders. Adv. Genom. Genet. 2015, 5, 131–150. [Google Scholar] [CrossRef]
- Hübel, C.; Marzi, S.J.; Breen, G.; Bulik, C.M. Epigenetics in eating disorders: A systematic review. Mol. Psychiatry 2019, 24, 901–915. [Google Scholar] [CrossRef]
- Turner, B.M. Epigenetic responses to environmental change and their evolutionary implications. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 3403–3418. [Google Scholar] [CrossRef]
- Alegría-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and lifestyle. Epigenomics 2011, 3, 267–277. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef]
- Moosavi, A.; Motevalizadeh Ardekani, A. Role of Epigenetics in Biology and Human Diseases. Iran. Biomed. J. 2016, 20, 246–258. [Google Scholar] [CrossRef]
- Barbarino, J.M.; Whirl-Carrillo, M.; Altman, R.B.; Klein, T.E. PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1417. [Google Scholar] [CrossRef]
- Razin, A.; Cedar, H. DNA methylation and gene expression. Microbiol. Rev. 1991, 55, 451–458. [Google Scholar] [CrossRef]
- Zhong, H.; Kim, S.; Zhi, D.; Cui, X. Predicting gene expression using DNA methylation in three human populations. PeerJ 2019, 7, e6757. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Disease or not, aging is easily treatable. Aging 2018, 10, 3067–3078. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Li, J.; Ni, Y.; Liang, Q.; Zhang, J.; Yeo, G.S.H.; Lyu, J.; Jin, S.; Ding, C. A complex association between DNA methylation and gene expression in human placenta at first and third trimesters. PLoS ONE 2017, 12, e0181155. [Google Scholar] [CrossRef] [PubMed]
- Zerwas, S.; Bulik, C.M. Genetics and Epigenetics of Eating Disorders. Psychiatr. Ann. 2011, 41, 532–538. [Google Scholar] [CrossRef]
- Steiger, H.; Booij, L. Eating Disorders, Heredity and Environmental Activation: Getting Epigenetic Concepts into Practice. J. Clin. Med. 2020, 9, 1332. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Kim, J.-H.; Kim, M.J.; Treasure, J. Differential methylation of the oxytocin receptor gene in patients with anorexia nervosa: A pilot study. PLoS ONE 2014, 9, e88673. [Google Scholar] [CrossRef]
- Yanovski, S.Z.; Marcus, M.D.; Wadden, T.A.; Walsh, B.T. The Questionnaire on Eating and Weight Patterns-5: An updated screening instrument for binge eating disorder. Int. J. Eat. Disord. 2015, 48, 259–261. [Google Scholar] [CrossRef]
- Rivas, T.; Bersabé, R.; Jiménez, M.; Berrocal, C. The eating attitudes test (EAT-26): Reliability and validity in spanish female samples. Span. J. Psychol. 2010, 13, 1044–1056. [Google Scholar] [CrossRef]
- Tovilla-Zárate, C.; Juárez-Rojop, I.; Peralta Jimenez, Y.; Jiménez, M.A.; Vázquez, S.; Bermúdez-Ocaña, D.; Ramón-Frías, T.; Genis Mendoza, A.D.; García, S.P.; Narváez, L.L. Prevalence of anxiety and depression among outpatients with type 2 diabetes in the Mexican population. PLoS ONE 2012, 7, e36887. [Google Scholar] [CrossRef]
- Hernández-Cordero, S.; Cuevas-Nasu, L.; Morán-Ruán, M.C.; Méndez-Gómez Humarán, I.; Ávila-Arcos, M.A.; Rivera-Dommarco, J.A. Overweight and obesity in Mexican children and adolescents during the last 25 years. Nutr. Diabetes 2017, 7. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Campos-Nonato, I.; Cuevas-Nasu, L.; Hernández-Barrera, L.; Morales-Ruán, M.d.C.; Rivera-Dommarco, J.; Barquera, S. Overweight and obesity in Mexican vulnerable population. Results of Ensanut 100k. Salud Publica Mex. 2019, 61, 852–865. [Google Scholar] [CrossRef]
- Tian, Y.; Morris, T.J.; Webster, A.P.; Yang, Z.; Beck, S.; Feber, A.; Teschendorff, A.E. ChAMP: Updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017, 33, 3982–3984. [Google Scholar] [CrossRef]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Graw, S.; Henn, R.; Thompson, J.A.; Koestler, D.C. pwrEWAS: A user-friendly tool for comprehensive power estimation for epigenome wide association studies (EWAS). BMC Bioinform. 2019, 20, 218. [Google Scholar] [CrossRef]
- Pacanowski, C.R.; Mason, T.B.; Crosby, R.D.; Mitchell, J.E.; Crow, S.J.; Wonderlich, S.A.; Peterson, C.B. Weight Change over the Course of Binge Eating Disorder Treatment: Relationship to Binge Episodes and Psychological Factors. Obesity 2018, 26, 838–844. [Google Scholar] [CrossRef]
- Mirch, M.C.; McDuffie, J.R.; Yanovski, S.Z.; Schollnberger, M.; Tanofsky-Kraff, M.; Theim, K.R.; Krakoff, J.; Yanovski, J.A. Effects of binge eating on satiation, satiety, and energy intake of overweight children. Am. J. Clin. Nutr. 2006, 84, 732–738. [Google Scholar] [CrossRef]
- Raymond, N.C.; Neumeyer, B.; Warren, C.S.; Lee, S.S.; Peterson, C.B. Energy intake patterns in obese women with binge eating disorder. Obes. Res. 2003, 11, 869–879. [Google Scholar] [CrossRef]
- Raymond, N.C.; Bartholome, L.T.; Lee, S.S.; Peterson, R.E.; Raatz, S.K. A comparison of energy intake and food selection during laboratory binge eating episodes in obese women with and without a binge eating disorder diagnosis. Int. J. Eat. Disord. 2007, 40, 67–71. [Google Scholar] [CrossRef]
- Roehrig, M.; Masheb, R.M.; White, M.A.; Grilo, C.M. Dieting frequency in obese patients with binge eating disorder: Behavioral and metabolic correlates. Obesity 2009, 17, 689–697. [Google Scholar] [CrossRef]
- Barnes, R.D.; Boeka, A.G.; McKenzie, K.C.; Genao, I.; Garcia, R.L.; Ellman, M.S.; Ellis, P.J.; Masheb, R.M.; Grilo, C.M. Metabolic syndrome in obese patients with binge-eating disorder in primary care clinics: A cross-sectional study. Prim. Care Companion CNS Disord. 2011, 13. [Google Scholar] [CrossRef]
- McCuen-Wurst, C.; Ruggieri, M.; Allison, K.C. Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann. N. Y. Acad. Sci. 2018, 1411, 96–105. [Google Scholar] [CrossRef]
- Wahl, S.; Drong, A.; Lehne, B.; Loh, M.; Scott, W.R.; Kunze, S.; Tsai, P.-C.; Ried, J.S.; Zhang, W.; Yang, Y.; et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017, 541, 81–86. [Google Scholar] [CrossRef]
- Fradin, D.; Boëlle, P.-Y.; Belot, M.-P.; Lachaux, F.; Tost, J.; Besse, C.; Deleuze, J.-F.; De Filippo, G.; Bougnères, P. Genome-Wide Methylation Analysis Identifies Specific Epigenetic Marks in Severely Obese Children. Sci. Rep. 2017, 7, 46311. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Irizarry, R.A.; Fradin, D.; Aryee, M.J.; Murakami, P.; Aspelund, T.; Eiriksdottir, G.; Harris, T.B.; Launer, L.; Gudnason, V.; et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci. Transl. Med. 2010, 2, 49ra67. [Google Scholar] [CrossRef]
- Xu, Y.; Gray, A.; Hardie, D.G.; Uzun, A.; Shaw, S.; Padbury, J.; Phornphutkul, C.; Tseng, Y.-T. A novel, de novo mutation in the PRKAG2 gene: Infantile-onset phenotype and the signaling pathway involved. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H283–H292. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK—Sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef]
- Andersson, U.; Filipsson, K.; Abbott, C.R.; Woods, A.; Smith, K.; Bloom, S.R.; Carling, D.; Small, C.J. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004, 279, 12005–12008. [Google Scholar] [CrossRef]
- Yang, Y.; Atasoy, D.; Su, H.H.; Sternson, S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 2011, 146, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Lage, R.; Saha, A.K.; Pérez-Tilve, D.; Vázquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodríguez-Cuenca, S.; et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.-B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferré, P.; Birnbaum, M.J.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Ashley, S.E.; Andrews, Z.B. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol. Cell. Endocrinol. 2013, 366, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Stocker, C.J.; Ghaffari, S.; Wargent, E.T.; Steeples, V.; Czibik, G.; Pinter, K.; Bellahcene, M.; Woods, A.; Martínez de Morentin, P.B.; et al. Chronic Activation of γ2 AMPK Induces Obesity and Reduces β Cell Function. Cell Metab. 2016, 23, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Claret, M.; Smith, M.A.; Batterham, R.L.; Selman, C.; Choudhury, A.I.; Fryer, L.G.D.; Clements, M.; Al-Qassab, H.; Heffron, H.; Xu, A.W.; et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Investig. 2007, 117, 2325–2336. [Google Scholar] [CrossRef]
- Rojas, J.; Arraiz, N.; Aguirre, M.; Velasco, M.; Bermúdez, V. AMPK as Target for Intervention in Childhood and Adolescent Obesity. J. Obes. 2011, 2011, 252817. [Google Scholar] [CrossRef][Green Version]
- Ha, E.; Yim, S.V.; Jung, K.H.; Yoon, S.H.; Zheng, L.T.; Kim, M.J.; Hong, S.J.; Choe, B.K.; Baik, H.H.; Chung, J.H.; et al. Topiramate stimulates glucose transport through AMP-activated protein kinase-mediated pathway in L6 skeletal muscle cells. Pharm. J. 2006, 6, 327–332. [Google Scholar] [CrossRef]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Okuno, T.; Oda, M.; Kato, K. Urinary volatilome analysis in a mouse model of anxiety and depression. PLoS ONE 2020, 15, e0229269. [Google Scholar] [CrossRef]
- Srimontri, P.; Endo, S.; Sakamoto, T.; Nakayama, Y.; Kurosaka, A.; Itohara, S.; Hirabayashi, Y.; Kato, K. Sialyltransferase ST3Gal IV deletion protects against temporal lobe epilepsy. J. Neurochem. 2014, 131, 675–687. [Google Scholar] [CrossRef]
- Sugie, K.; Kawakami, T.; Maeda, Y.; Kawabe, T.; Uchida, A.; Yodoi, J. Fyn tyrosine kinase associated with Fc epsilon RII/CD23: Possible multiple roles in lymphocyte activation. Proc. Natl. Acad. Sci. USA 1991, 88, 9132–9135. [Google Scholar] [CrossRef]
- Calautti, E.; Grossi, M.; Mammucari, C.; Aoyama, Y.; Pirro, M.; Ono, Y.; Li, J.; Dotto, G.P. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J. Cell Biol. 2002, 156, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Mkaddem, S.B.; Murua, A.; Flament, H.; Titeca-Beauport, D.; Bounaix, C.; Danelli, L.; Launay, P.; Benhamou, M.; Blank, U.; Daugas, E.; et al. Lyn and Fyn function as molecular switches that control immunoreceptors to direct homeostasis or inflammation. Nat. Commun. 2017, 8, 246. [Google Scholar] [CrossRef]
- Shang, G.; Tang, X.; Gao, P.; Guo, F.; Liu, H.; Zhao, Z.; Chen, Q.; Jiang, T.; Zhang, N.; Li, H. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J. Nutr. Biochem. 2015, 26, 596–606. [Google Scholar] [CrossRef]
- Lim, J.C.; Kim, G.; Levine, R.L. Stereospecific oxidation of calmodulin by methionine sulfoxide reductase A. Free Radic. Biol. Med. 2013, 61, 257–264. [Google Scholar] [CrossRef]
- Annerén, C. Dual role of the tyrosine kinase GTK and the adaptor protein SHB in beta-cell growth: Enhanced beta-cell replication after 60% pancreatectomy and increased sensitivity to streptozotocin. J. Endocrinol. 2002, 172, 145–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Annerén, C.; Welsh, M. Increased cytokine-induced cytotoxicity of pancreatic islet cells from transgenic mice expressing the Src-like tyrosine kinase GTK. Mol. Med. 2001, 7, 301–310. [Google Scholar] [CrossRef]
- Boyd, H.K.; Bodell, L.P.; Jennings, K.M.; Graham, A.K.; Crosby, R.D.; Wildes, J.E. Relationship between desired weight constructs and eating disorder severity following treatment for anorexia nervosa. Int. J. Eat. Disord. 2018, 51, 870–878. [Google Scholar] [CrossRef]
| BN (n = 25) | BED (n = 21) | Total (n = 46) | |
|---|---|---|---|
| Gender | |||
| Female (n, %) | 22 (88.00) | 12 (57.14) | 34 (73.91) |
| Male (n, %) | 3 (12.00) | 9 (42.86) | 12 (26.09) |
| Age (s.d) | 13.76 (1.56) | 14.10 (1.51) | 13.91 (1.53) |
| Body Mass Index | |||
| BMI z-score (s.d) | 0.87 (0.74) | 1.69 (0.65) | 1.25 (0.81) |
| Normal weight (n, %) | 14 (56.00) | 1 (4.76) | 15 (32.61) |
| Overweight (n, %) | 5 (20.00) | 7 (33.33) | 12 (26.09) |
| Obese (n, %) | 6 (24.00) | 13 (61.90) | 19 (41.30) |
| Eating behavior | |||
| Compensatory (n, %) | 25 (100.0) | 0 (0.00) | 25 (47.83) |
| Binge eating (n, %) | 17 (68.00) | 21 (100.00) | 38 (82.61) |
| Position 1 | CpG Site | LogFC 2 | p-Value | BED Avg | BN Avg | Gene | Gene Loc 3 | CGI 4 |
|---|---|---|---|---|---|---|---|---|
| 1:85040857 | cg14791530 | −0.0499 | 5.3305 × 10−9 | 0.8150 | 0.8550 | CTBS | TSS1500 | Shore |
| 2:131090049 | cg26638005 | −0.0509 | 9.0552 × 10−9 | 0.3135 | 0.3644 | IGR | OpenSea | |
| 3:164907027 | cg00059161 | 0.0216 | 6.0427 × 10−8 | 0.7929 | 0.7713 | SLITRK3 | Body | OpenSea |
| 4:20702180 | cg05248502 | 0.0211 | 4.5662 × 10−8 | 0.0737 | 0.0526 | PACRGL | 1st Exon | Island |
| 4:189258348 | cg22740817 | −0.0138 | 6.1212 × 10−8 | 0.9427 | 0.9565 | IGR 5 | OpenSea | |
| 5:20305935 | cg07823496 | −0.0386 | 1.3534 × 10−8 | 0.9039 | 0.9424 | IGR | Island | |
| 6:116381966 | cg05304507 | −0.0765 | 3.6910 × 10−8 | 0.7500 | 0.8265 | FRK | TSS200 | OpenSea |
| 7:151565722 | cg04781532 | −0.0190 | 6.3694 × 10−9 | 0.9376 | 0.9566 | PRKAG2 | Body | OpenSea |
| 11:126284163 | cg16685832 | −0.0282 | 7.4533 × 10−9 | 0.8750 | 0.9032 | ST3GAL4 | 3′-UTR | Shelf |
| 13:114318347 | cg21211187 | −0.0115 | 5.7405 × 10−8 | 0.9501 | 0.9616 | IGR | OpenSea | |
| 16:88110197 | cg10838260 | −0.0245 | 6.6465 × 10−8 | 0.8966 | 0.9211 | BANP | Body | Island |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-López, M.L.; Martínez-Magaña, J.J.; Ruiz-Ramos, D.; García, A.R.; Gonzalez, L.; Tovilla-Zarate, C.A.; Sarmiento, E.; Juárez-Rojop, I.E.; Nicolini, H.; Gonzalez-Castro, T.B.; et al. Individuals Diagnosed with Binge-Eating Disorder Have DNA Hypomethylated Sites in Genes of the Metabolic System: A Pilot Study. Nutrients 2021, 13, 1413. https://doi.org/10.3390/nu13051413
Rodríguez-López ML, Martínez-Magaña JJ, Ruiz-Ramos D, García AR, Gonzalez L, Tovilla-Zarate CA, Sarmiento E, Juárez-Rojop IE, Nicolini H, Gonzalez-Castro TB, et al. Individuals Diagnosed with Binge-Eating Disorder Have DNA Hypomethylated Sites in Genes of the Metabolic System: A Pilot Study. Nutrients. 2021; 13(5):1413. https://doi.org/10.3390/nu13051413
Chicago/Turabian StyleRodríguez-López, Mariana Lizbeth, José Jaime Martínez-Magaña, David Ruiz-Ramos, Ana Rosa García, Laura Gonzalez, Carlos Alfonso Tovilla-Zarate, Emmanuel Sarmiento, Isela Esther Juárez-Rojop, Humberto Nicolini, Thelma Beatriz Gonzalez-Castro, and et al. 2021. "Individuals Diagnosed with Binge-Eating Disorder Have DNA Hypomethylated Sites in Genes of the Metabolic System: A Pilot Study" Nutrients 13, no. 5: 1413. https://doi.org/10.3390/nu13051413
APA StyleRodríguez-López, M. L., Martínez-Magaña, J. J., Ruiz-Ramos, D., García, A. R., Gonzalez, L., Tovilla-Zarate, C. A., Sarmiento, E., Juárez-Rojop, I. E., Nicolini, H., Gonzalez-Castro, T. B., & Genis-Mendoza, A. D. (2021). Individuals Diagnosed with Binge-Eating Disorder Have DNA Hypomethylated Sites in Genes of the Metabolic System: A Pilot Study. Nutrients, 13(5), 1413. https://doi.org/10.3390/nu13051413









