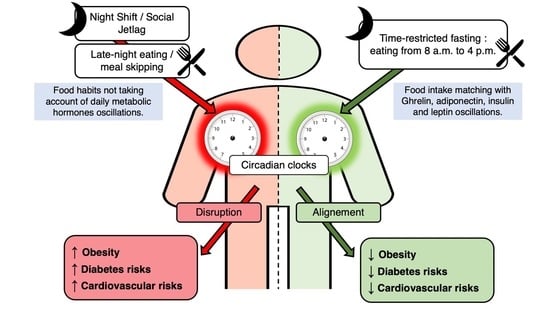
Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock
Abstract
:1. Introduction
2. Obesity and Diabetes Mellitus: Epidemiology and Physiopathology
3. Intermittent Fasting and Time-Restricted Feeding: Definition, History, and Principle
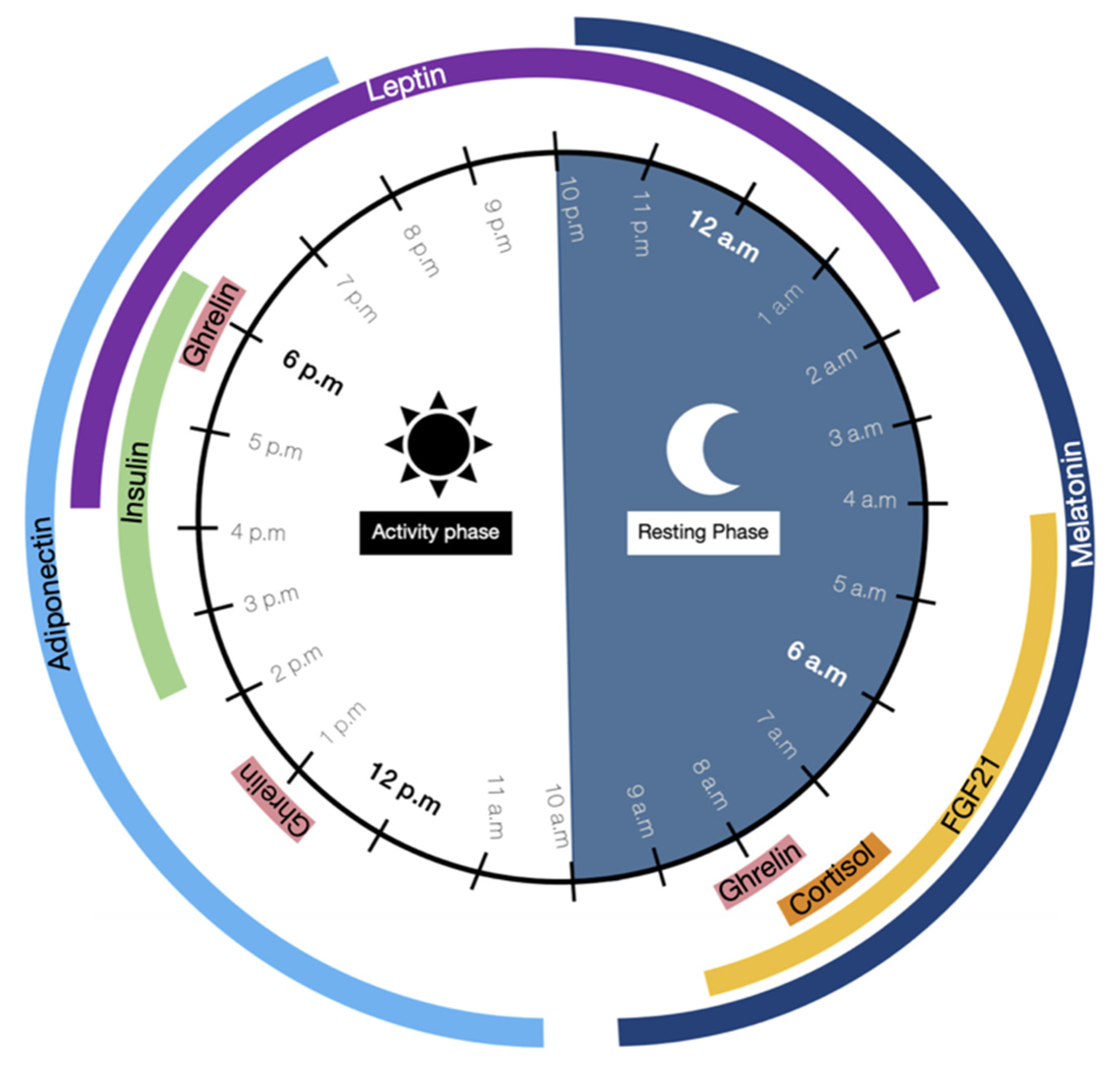
4. Circadian Clock, a Key Regulator of the Physiological Processes
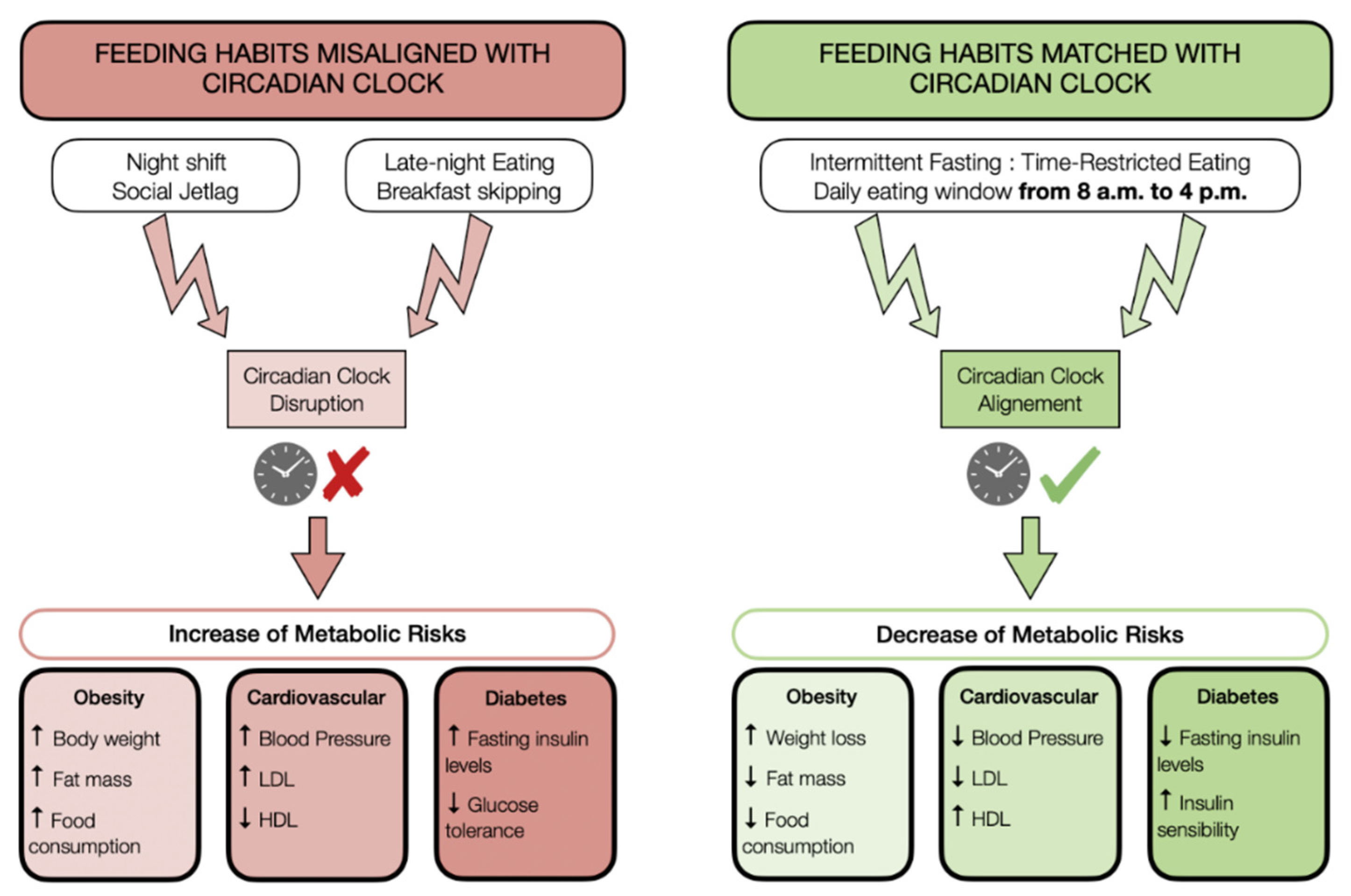
5. Modern Lifestyle and Circadian Disruption: Leaving the Door Open to Metabolic Diseases
6. Association between Time-Restricted Feeding and Normal Circadian Rhythms: A Relevant Approach for the Fight against Metabolic Diseases
7. Warnings and Limits of the Association between Intermittent Fasting and Normal Circadian Rhythms
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 November 2020).
- Kissane, N.A.; Pratt, J.S.A. Medical and Surgical Treatment of Obesity. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.N.; Hocking, S.L.; Markovic, T.P. Pharmacotherapy for the Treatment of Obesity. Mol. Cell. Endocrinol. 2015, 418 Pt 2, 173–183. [Google Scholar] [CrossRef]
- Bal, B.S.; Finelli, F.C.; Shope, T.R.; Koch, T.R. Nutritional Deficiencies after Bariatric Surgery. Nat. Rev. Endocrinol. 2012, 8, 544–556. [Google Scholar] [CrossRef]
- Jammah, A.A. Endocrine and Metabolic Complications After Bariatric Surgery. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2015, 21, 269–277. [Google Scholar] [CrossRef]
- Lyn, R.; Heath, E.; Dubhashi, J. Global Implementation of Obesity Prevention Policies: A Review of Progress, Politics, and the Path Forward. Curr. Obes. Rep. 2019, 8, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Kanter, R.; Vanderlee, L.; Vandevijvere, S. Front-of-Package Nutrition Labelling Policy: Global Progress and Future Directions. Public Health Nutr. 2018, 21, 1399–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, B.; Niven, P.; Dixon, H.; Swanson, M.; Szybiak, M.; Shilton, T.; Pratt, I.S.; Slevin, T.; Hill, D.; Wakefield, M. Population-Based Evaluation of the ‘LiveLighter’ Healthy Weight and Lifestyle Mass Media Campaign. Health Educ. Res. 2016, 31, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Cacan, R. Régulation Métabolique: Gènes, Enzymes, Hormones et Nutriments; Ellipses: Paris, France, 2008; ISBN 978-2-7298-3893-5. [Google Scholar]
- Abdul-Ghani, M.A.; Muller, F.L.; Liu, Y.; Chavez, A.O.; Balas, B.; Zuo, P.; Chang, Z.; Tripathy, D.; Jani, R.; Molina-Carrion, M.; et al. Deleterious Action of FA Metabolites on ATP Synthesis: Possible Link between Lipotoxicity, Mitochondrial Dysfunction, and Insulin Resistance. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E678–E685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferré, P. Signalisation insulinique et résistance à l’insuline. Therapies 2007, 62, 277–284. [Google Scholar] [CrossRef]
- Alessi, M.; Peiretti, F.; Morange, P.; Henry, M.; Nalbone, G.; Juhan-Vague, I. Production of Plasminogen Activator Inhibitor 1 by Human Adipose Tissue: Possible Link between Visceral Fat Accumulation and Vascular Disease. Diabetes 1997, 46, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Albosta, M.; Bakke, J. Intermittent Fasting: Is There a Role in the Treatment of Diabetes? A Review of the Literature and Guide for Primary Care Physicians. Clin. Diabetes Endocrinol. 2021, 7, 3. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even Without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [Green Version]
- Jamshed, H.; Beyl, R.; Della Manna, D.; Yang, E.; Ravussin, E.; Peterson, C. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Hong, N.; Kim, K.; Cho, S.; Lee, M.; Lee, Y.; Lee, Y.; Kang, E.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secor, S.M.; Carey, H.V. Integrative Physiology of Fasting. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 773–825. ISBN 978-0-470-65071-4. [Google Scholar]
- Espi Forcen, F.; Espi Forcen, C. The Practice of Holy Fasting in the Late Middle Ages: A Psychiatric Approach. J. Nerv. Ment. Dis. 2015, 203, 650–653. [Google Scholar] [CrossRef]
- Höhn, S.; Dozières-Puyravel, B.; Auvin, S. History of Dietary Treatment from Wilder’s Hypothesis to the First Open Studies in the 1920s. Epilepsy Behav. 2019, 101, 106588. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent Fasting Interventions for Treatment of Overweight and Obesity in Adults: A Systematic Review and Meta-Analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.; Zhu, L.; Kord-Varkaneh, H.; Santos, H.O.; Tinsley, G.M.; Fu, P. Effects of Intermittent Fasting and Energy-Restricted Diets on Lipid Profile: A Systematic Review and Meta-Analysis. Nutr. Burbank Los Angel. Cty. Calif 2020, 77, 110801. [Google Scholar] [CrossRef]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Harder-Lauridsen, N.M.; Rosenberg, A.; Benatti, F.B.; Damm, J.A.; Thomsen, C.; Mortensen, E.L.; Pedersen, B.K.; Krogh-Madsen, R. Ramadan Model of Intermittent Fasting for 28 d Had No Major Effect on Body Composition, Glucose Metabolism, or Cognitive Functions in Healthy Lean Men. Nutrition 2017, 37, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Al-barha, N.S.; Aljaloud, K.S. The Effect of Ramadan Fasting on Body Composition and Metabolic Syndrome in Apparently Healthy Men. Am. J. Mens Health 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Myers, K.; Dhanji, A.-R.; West, O.; McRobbie, H. Weight Change during and after Ramadan Fasting. J. Public Health Oxf. Engl. 2012, 34, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, R. Scientific Evidence of Diets for Weight Loss: Different Macronutrient Composition, Intermittent Fasting, and Popular Diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of Time-Restricted Feeding on Body Weight and Metabolism. A Systematic Review and Meta-Analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian Clock Control of Endocrine Factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.E.; Laughlin, G.A.; Sears, D.D.; LaCroix, A.Z.; Marinac, C.; Gallo, L.C.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian Disruption and Metabolic Disease: Findings from Animal Models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef] [Green Version]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.E.; Kalsbeek, A.; la Fleur, S.E.; Belsham, D.D. Impact of Nutrients on Circadian Rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzog, E.D.; Hermanstyne, T.; Smyllie, N.J.; Hastings, M.H. Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbe, I.; Brehm, N.; Oster, H. Interplay of Central and Peripheral Circadian Clocks in Energy Metabolism Regulation. J. Neuroendocrinol. 2019, 31, e12659. [Google Scholar] [CrossRef]
- Gnocchi, D.; Bruscalupi, G. Circadian Rhythms and Hormonal Homeostasis: Pathophysiological Implications. Biology 2017, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skene, D.J.; Arendt, J. Human Circadian Rhythms: Physiological and Therapeutic Relevance of Light and Melatonin. Ann. Clin. Biochem. 2006, 43, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Broadway, J. Light and Melatonin as Zeitgebers in Man. Chronobiol. Int. 1987, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Gavrila, A.; Peng, C.-K.; Chan, J.L.; Mietus, J.E.; Goldberger, A.L.; Mantzoros, C.S. Diurnal and Ultradian Dynamics of Serum Adiponectin in Healthy Men: Comparison with Leptin, Circulating Soluble Leptin Receptor, and Cortisol Patterns. J. Clin. Endocrinol. Metab. 2003, 88, 2838–2843. [Google Scholar] [CrossRef] [Green Version]
- Louiset, E. Perturbation Du Rythme Circadien Du Cortisol. Corresp. Métabolismes Horm. Diabètes Nutr. 2009, 8, 115–119. [Google Scholar]
- Chan, J.L.; Bullen, J.; Lee, J.H.; Yiannakouris, N.; Mantzoros, C.S. Ghrelin Levels Are Not Regulated by Recombinant Leptin Administration and/or Three Days of Fasting in Healthy Subjects. J. Clin. Endocrinol. Metab. 2004, 89, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Espelund, U.; Hansen, T.K.; Højlund, K.; Beck-Nielsen, H.; Clausen, J.T.; Hansen, B.S.; Orskov, H.; Jørgensen, J.O.L.; Frystyk, J. Fasting Unmasks a Strong Inverse Association between Ghrelin and Cortisol in Serum: Studies in Obese and Normal-Weight Subjects. J. Clin. Endocrinol. Metab. 2005, 90, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, G.; Ruiz, J.; Urbain, J.L.; Chen, X. Evidence for a Circadian Rhythm of Insulin Secretion. Am. J. Physiol.-Endocrinol. Metab. 1996, 271, E246–E252. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Lee, J.I.; Shin, D.; Kim, H.T.; Jung, H.Y.; Lee, T.G.; Kang, L.-W.; Ahn, Y.-J.; Cho, H.-S.; Heo, Y.-S. Molecular Mechanism for the Regulation of Human ACC2 through Phosphorylation by AMPK. Biochem. Biophys. Res. Commun. 2010, 391, 187–192. [Google Scholar] [CrossRef]
- Yu, H.; Xia, F.; Lam, K.S.L.; Wang, Y.; Bao, Y.; Zhang, J.; Gu, Y.; Zhou, P.; Lu, J.; Jia, W.; et al. Circadian Rhythm of Circulating Fibroblast Growth Factor 21 Is Related to Diurnal Changes in Fatty Acids in Humans. Clin. Chem. 2011, 57, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran-Ramos, S.; Baez-Ruiz, A.; Buijs, R.M.; Escobar, C. When to Eat? The Influence of Circadian Rhythms on Metabolic Health: Are Animal Studies Providing the Evidence? Nutr. Res. Rev. 2016, 29, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Manoogian, E.N.C.; Panda, S. Circadian Rhythms, Time-Restricted Feeding, and Healthy Aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kadowaki, T. Physiological and Pathophysiological Roles of Adiponectin and Adiponectin Receptors in the Integrated Regulation of Metabolic and Cardiovascular Diseases. Int. J. Obes. 2005 2008, 32 (Suppl. 7), S13–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’souza, A.M.; Neumann, U.H.; Glavas, M.M.; Kieffer, T.J. The Glucoregulatory Actions of Leptin. Mol. Metab. 2017, 6, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehr, T.A. Melatonin and Seasonal Rhythms. J. Biol. Rhythms 1997, 12, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.P.; Barrett, P. The Regulation of Seasonal Changes in Food Intake and Body Weight. J. Neuroendocrinol. 2008, 20, 827–833. [Google Scholar] [CrossRef]
- Nelson, R.J. Seasonal Immune Function and Sickness Responses. Trends Immunol. 2004, 25, 187–192. [Google Scholar] [CrossRef]
- O’Hare, C.; O’Sullivan, V.; Flood, S.; Kenny, R.A. Seasonal and Meteorological Associations with Depressive Symptoms in Older Adults: A Geo-Epidemiological Study. J. Affect. Disord. 2016, 191, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Ebling, F.J.P. On the Value of Seasonal Mammals for Identifying Mechanisms Underlying the Control of Food Intake and Body Weight. Horm. Behav. 2014, 66, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Wirz-Justice, A. Seasonality in Affective Disorders. Gen. Comp. Endocrinol. 2018, 258, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The Circadian Regulation of Food Intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.L.; Husse, J.; Bode, B.; Naujokat, N.; Meyer-Kovac, J.; Schmid, S.M.; Lehnert, H.; Oster, H. Circadian Desynchrony Promotes Metabolic Disruption in a Mouse Model of Shiftwork. PLoS ONE 2012, 7, e37150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maury, E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef] [Green Version]
- Crafts, N. Explaining the First Industrial Revolution: Two Views. Eur. Rev. Econ. Hist. 2011, 15, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Daumas, J.-C. Consommation de masse et grande distribution, Une révolution permanente (1957–2005). Vingtième Siècle Rev. Hist. 2006, 93, 57–76. [Google Scholar] [CrossRef]
- Claval, P. Mondialisation et enjeux géo-culturels. Conférence donnée le 9 octobre 2006 à l’École Normale Supérieure de Lyon. Confins Rev. Fr.-Brés. Géographie Rev. Fr.-Bras. Geogr. 2007. [Google Scholar] [CrossRef]
- Marinache, R. Sleep, Work and Globalization: The Evening/Night Shift Employees in Call Centre in Romania. Int. Rev. Soc. Res. 2016, 6, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Boivin, D.B.; Boudreau, P. Impacts of Shift Work on Sleep and Circadian Rhythms. Pathol. Biol. 2014, 62, 292–301. [Google Scholar] [CrossRef]
- Papantoniou, K.; Pozo, O.J.; Espinosa, A.; Marcos, J.; Castaño-Vinyals, G.; Basagaña, X.; Ribas, F.C.; Mirabent, J.; Martín, J.; Carenys, G.; et al. Circadian Variation of Melatonin, Light Exposure, and Diurnal Preference in Day and Night Shift Workers of Both Sexes. Cancer Epidemiol. Prev. Biomark. 2014, 23, 1176–1186. [Google Scholar] [CrossRef] [Green Version]
- Dumont, M.; Paquet, J. Progressive Decrease of Melatonin Production over Consecutive Days of Simulated Night Work. Chronobiol. Int. 2014, 31, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Hung, E.W.M.; Aronson, K.J.; Leung, M.; Day, A.; Tranmer, J. Shift Work Parameters and Disruption of Diurnal Cortisol Production in Female Hospital Employees. Chronobiol. Int. 2016, 33, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Ritonja, J.; Aronson, K.J.; Day, A.G.; Korsiak, J.; Tranmer, J. Investigating Cortisol Production and Pattern as Mediators in the Relationship Between Shift Work and Cardiometabolic Risk. Can. J. Cardiol. 2018, 34, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M.V.; Garg, A.X.; Iansavichus, A.V.; Costella, J.; Donner, A.; Laugsand, L.E.; Janszky, I.; Mrkobrada, M.; Parraga, G.; Hackam, D.G. Shift Work and Vascular Events: Systematic Review and Meta-Analysis. BMJ 2012, 345, e4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726. [Google Scholar] [CrossRef]
- Martino, T.A.; Oudit, G.Y.; Herzenberg, A.M.; Tata, N.; Koletar, M.M.; Kabir, G.M.; Belsham, D.D.; Backx, P.H.; Ralph, M.R.; Sole, M.J. Circadian Rhythm Disorganization Produces Profound Cardiovascular and Renal Disease in Hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1675–R1683. [Google Scholar] [CrossRef] [Green Version]
- Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-Specific Bmal1 Deletion in Mice Triggers Diastolic Dysfunction, Extracellular Matrix Response, and Impaired Resolution of Inflammation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1827–H1836. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Rutters, F.; Lemmens, S.G.; Adam, T.C.; Bremmer, M.A.; Elders, P.J.; Nijpels, G.; Dekker, J.M. Is Social Jetlag Associated with an Adverse Endocrine, Behavioral, and Cardiovascular Risk Profile? J. Biol. Rhythms 2014, 29, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.M.; Hasler, B.P.; Kamarck, T.W.; Muldoon, M.F.; Manuck, S.B. Social Jetlag, Chronotype, and Cardiometabolic Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4612–4620. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Moffitt, T.; Gregory, A.; Goldman-Mellor, S.; Nolan, P.; Poulton, R.; Caspi, A. Social Jetlag, Obesity and Metabolic Disorder: Investigation in a Cohort Study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pot, G.K. Sleep and Dietary Habits in the Urban Environment: The Role of Chrono-Nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Socarrás, V. Hábitos Alimentarios y Conductas Relacionadas con la salud en una. Nutr. Hosp. 2015, 31, 449–457. [Google Scholar] [CrossRef]
- Njike, V.Y.; Smith, T.M.; Shuval, O.; Shuval, K.; Edshteyn, I.; Kalantari, V.; Yaroch, A.L. Snack Food, Satiety, and Weight123. Adv. Nutr. 2016, 7, 866–878. [Google Scholar] [CrossRef] [Green Version]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later Circadian Timing of Food Intake Is Associated with Increased Body Fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of Night Eating Habits with Metabolic Syndrome and Its Components: A Longitudinal Study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef] [PubMed]
- Vera, B.; Dashti, H.S.; Gómez-Abellán, P.; Hernández-Martínez, A.M.; Esteban, A.; Scheer, F.A.J.L.; Saxena, R.; Garaulet, M. Modifiable Lifestyle Behaviors, but Not a Genetic Risk Score, Associate with Metabolic Syndrome in Evening Chronotypes. Sci. Rep. 2018, 8, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roßbach, S.; Diederichs, T.; Nöthlings, U.; Buyken, A.E.; Alexy, U. Relevance of Chronotype for Eating Patterns in Adolescents. Chronobiol. Int. 2018, 35, 336–347. [Google Scholar] [CrossRef]
- Froy, O.; Chapnik, N.; Miskin, R. Effect of Intermittent Fasting on Circadian Rhythms in Mice Depends on Feeding Time. Mech. Ageing Dev. 2009, 130, 154–160. [Google Scholar] [CrossRef]
- Hara, R.; Wan, K.; Wakamatsu, H.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted Feeding Entrains Liver Clock without Participation of the Suprachiasmatic Nucleus. Genes Cells Devoted Mol. Cell. Mech. 2001, 6, 269–278. [Google Scholar] [CrossRef]
- Yoon, J.-A.; Han, D.-H.; Noh, J.-Y.; Kim, M.-H.; Son, G.H.; Kim, K.; Kim, C.-J.; Pak, Y.K.; Cho, S. Meal Time Shift Disturbs Circadian Rhythmicity along with Metabolic and Behavioral Alterations in Mice. PLoS ONE 2012, 7, e44053. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A.J.L. Endogenous Circadian System and Circadian Misalignment Impact Glucose Tolerance via Separate Mechanisms in Humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Scheer, F.A. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. TEM 2016, 27, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of Circadian Rhythmicity and Sleep in Human Glucose Regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, F.S.d.; Dias, M.D.S.; Mintem, G.C.; Oliveira, I.O.d.; Gigante, D.P. Food Processing and Cardiometabolic Risk Factors: A Systematic Review. Rev. Saúde Pública 2020, 54, 70. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The Evidence for Saturated Fat and for Sugar Related to Coronary Heart Disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Smith, M.; Karthikeyan, S.; Jeffers, M.S.; Janik, R.; Thomason, L.A.; Stefanovic, B.; Corbett, D. A Physiological Characterization of the Cafeteria Diet Model of Metabolic Syndrome in the Rat. Physiol. Behav. 2016, 167, 382–391. [Google Scholar] [CrossRef]
- Barrington, W.E.; Beresford, S.A.A. Eating Occasions, Obesity and Related Behaviors in Working Adults: Does It Matter When You Snack? Nutrients 2019, 11, 2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R.; Steffen, L.M.; Van Horn, L.; Ludwig, D.S.; Pereira, M.A. Breakfast Frequency and Development of Metabolic Risk. Diabetes Care 2013, 36, 3100–3106. [Google Scholar] [CrossRef] [Green Version]
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The Relationship Between Breakfast Skipping, Chronotype, and Glycemic Control in Type 2 Diabetes. Chronobiol. Int. 2014, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Witbracht, M.; Keim, N.L.; Forester, S.; Widaman, A.; Laugero, K. Female Breakfast Skippers Display a Disrupted Cortisol Rhythm and Elevated Blood Pressure. Physiol. Behav. 2015, 140, 215–221. [Google Scholar] [CrossRef]
- Sharma, K.; Shah, K.; Brahmbhatt, P.; Kandre, Y. Skipping Breakfast and the Risk of Coronary Artery Disease. QJM Mon. J. Assoc. Physicians 2018, 111, 715–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans Who Are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans That Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A Pilot Feasibility Study Exploring the Effects of a Moderate Time-Restricted Feeding Intervention on Energy Intake, Adiposity and Metabolic Physiology in Free-Living Human Subjects. J. Nutr. Sci. 2018, 7, e22. [Google Scholar] [CrossRef] [Green Version]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-Hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation but Does Not Affect Energy Expenditure in Humans. Obes. Silver Spring Md 2019, 27, 1244–1254. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Cermak, P.; Gulich, M.; Kesztyüs, T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre–Post Design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef] [Green Version]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, J.d.N.; Macedo, R.C.O.; Tinsley, G.M.; Reischak-Oliveira, A. Time-Restricted Eating and Circadian Rhythms: The Biological Clock Is Ticking. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Regmi, P.; Heilbronn, L.K. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef] [PubMed]
- Melkani, G.C.; Panda, S. Time-restricted Feeding for Prevention and Treatment of Cardiometabolic Disorders. J. Physiol. 2017, 595, 3691–3700. [Google Scholar] [CrossRef] [Green Version]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Duncan, M.J.; Smith, J.T.; Narbaiza, J.; Mueez, F.; Bustle, L.B.; Qureshi, S.; Fieseler, C.; Legan, S.J. Restricting Feeding to the Active Phase in Middle-Aged Mice Attenuates Adverse Metabolic Effects of a High-Fat Diet. Physiol. Behav. 2016, 167, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Yan, L. Time-Restricted Feeding Reduces Adiposity in Mice Fed a High-Fat Diet. Nutr. Res. 2016, 36, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.; Chou, W.; Sears, D.D.; Patterson, R.E.; Webster, N.J.G.; Ellies, L.G. Time-Restricted Feeding Improves Insulin Resistance and Hepatic Steatosis in a Mouse Model of Postmenopausal Obesity. Metabolism 2016, 65, 1743–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouichat, S.; Chayah, M.; Bouguerra-Aouichat, S.; Agil, A. Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue. Nutrients 2020, 12, 2185. [Google Scholar] [CrossRef] [PubMed]
- de Goede, P.; Foppen, E.; Ritsema, W.I.G.R.; Korpel, N.L.; Yi, C.-X.; Kalsbeek, A. Time-Restricted Feeding Improves Glucose Tolerance in Rats, but Only When in Line With the Circadian Timing System. Front. Endocrinol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolotkin, R.L.; Andersen, J.R. A Systematic Review of Reviews: Exploring the Relationship between Obesity, Weight Loss and Health-related Quality of Life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and Prevention of Cardiovascular Disease and Diabetes Mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef]
- Potter, G.D.M.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Nutrition and the Circadian System. Br. J. Nutr. 2016, 116, 434–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obes. Silver Spring Md 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Elgar, F.J.; Craig, W.; Trites, S.J. Family Dinners, Communication, and Mental Health in Canadian Adolescents. J. Adolesc. Health 2013, 52, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Vesnaver, E.; Keller, H.H. Social Influences and Eating Behavior in Later Life: A Review. J. Nutr. Gerontol. Geriatr. 2011, 30, 2–23. [Google Scholar] [CrossRef]
- Kimura, Y.; Wada, T.; Okumiya, K.; Ishimoto, Y.; Fukutomi, E.; Kasahara, Y.; Chen, W.; Sakamoto, R.; Fujisawa, M.; Otsuka, K.; et al. Eating Alone among Community-Dwelling Japanese Elderly: Association with Depression and Food Diversity. J. Nutr. Health Aging 2012, 16, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Erzen, E.; Çikrikci, Ö. The Effect of Loneliness on Depression: A Meta-Analysis. Int. J. Soc. Psychiatry 2018, 64, 427–435. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of Reduced Meal Frequency Without Caloric Restriction on Glucose Regulation in Healthy, Normal Weight Middle-Aged Men and Women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.B.; Cornelissen, G.; Mojto, V.; Fatima, G.; Wichansawakun, S.; Singh, M.; Kartikey, K.; Sharma, J.P.; Torshin, V.I.; Chibisov, S.; et al. Effects of Circadian Restricted Feeding on Parameters of Metabolic Syndrome among Healthy Subjects. Chronobiol. Int. 2020, 37, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Berne, C.; Svärdsudd, K.; Garmo, H.; Melhus, H.; Zethelius, B. Seasonal Variations of Insulin Sensitivity from a Euglycemic Insulin Clamp in Elderly Men. Ups. J. Med. Sci. 2012, 117, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.; Tuplin, E.; Holahan, M.R. Circannual Changes in Stress and Feeding Hormones and Their Effect on Food-Seeking Behaviors. Front. Neurosci. 2013, 7, 140. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charlot, A.; Hutt, F.; Sabatier, E.; Zoll, J. Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients 2021, 13, 1405. https://doi.org/10.3390/nu13051405
Charlot A, Hutt F, Sabatier E, Zoll J. Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients. 2021; 13(5):1405. https://doi.org/10.3390/nu13051405
Chicago/Turabian StyleCharlot, Anouk, Fanny Hutt, Eugénie Sabatier, and Joffrey Zoll. 2021. "Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock" Nutrients 13, no. 5: 1405. https://doi.org/10.3390/nu13051405
APA StyleCharlot, A., Hutt, F., Sabatier, E., & Zoll, J. (2021). Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients, 13(5), 1405. https://doi.org/10.3390/nu13051405