Hydration Status, Fluid Intake, Sweat Rate, and Sweat Sodium Concentration in Recreational Tropical Native Runners
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Experimental Protocol
2.3. Measurement of Environmental Conditions
2.4. Urine Specific Gravity (USG)
2.5. Whole-Body Sweat Loss (WBSL) and Whole-Body Sweat Rate (WBSR)
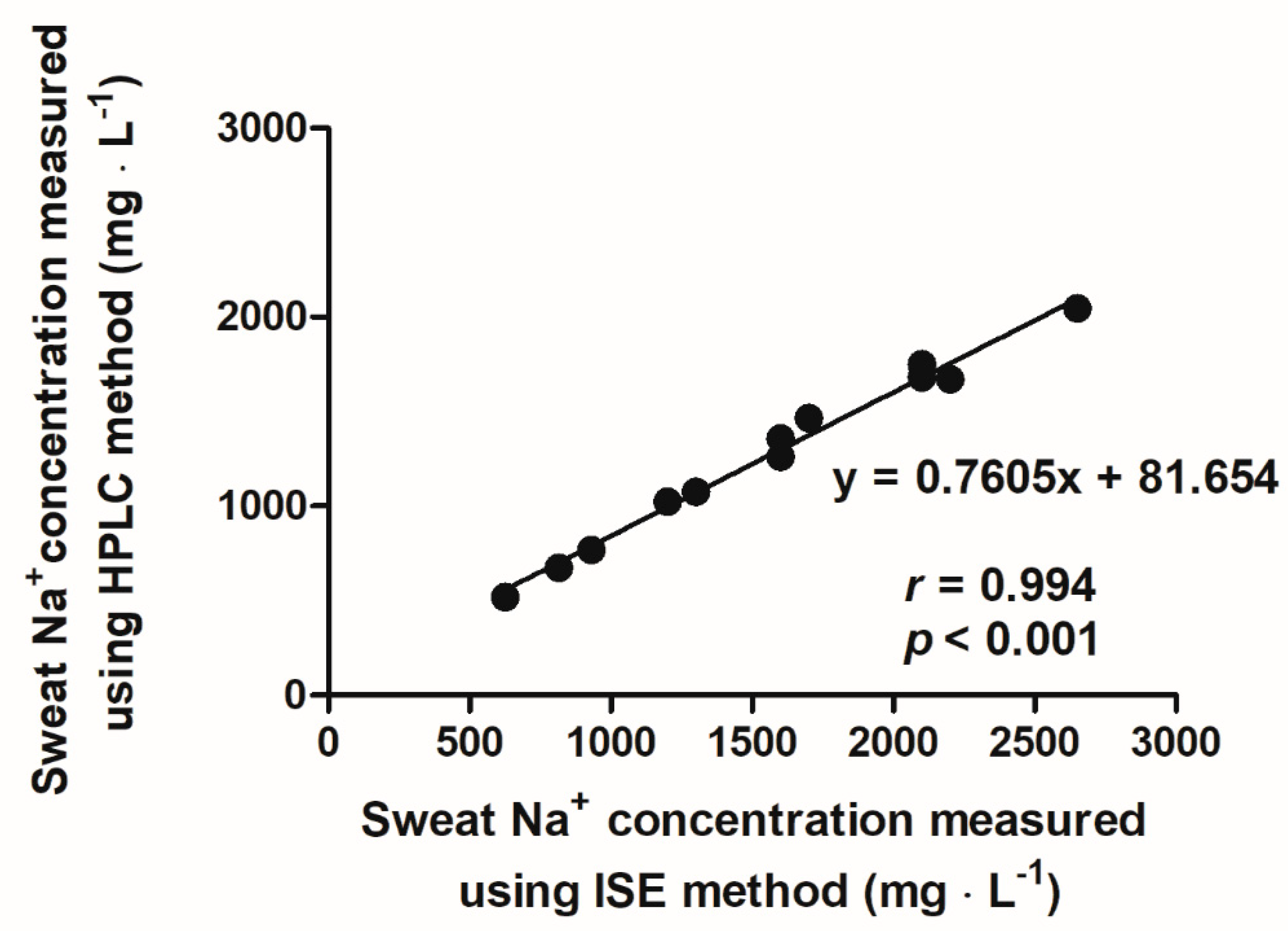
2.6. Whole-Body Sweat Sodium Concentration
2.7. Statistical Analysis
3. Results
3.1. Number of Subjects and Environmental Conditions for Each Running Session
3.2. Participants’ Age, Running Profile, and Body Mass Change across Running
3.3. Urine Specific Gravity
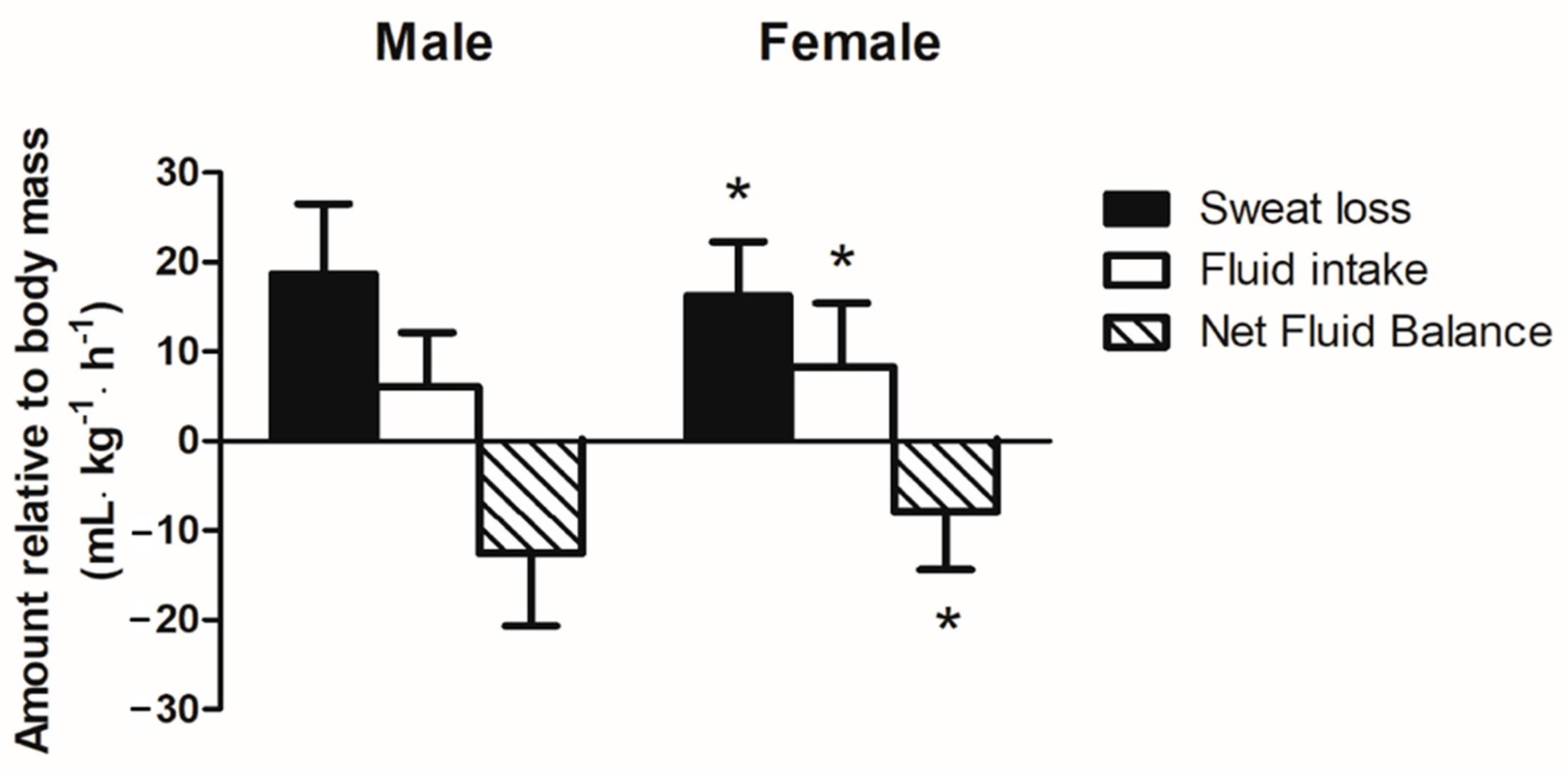
3.4. Relative Sweat Loss and Fluid Intake during Running
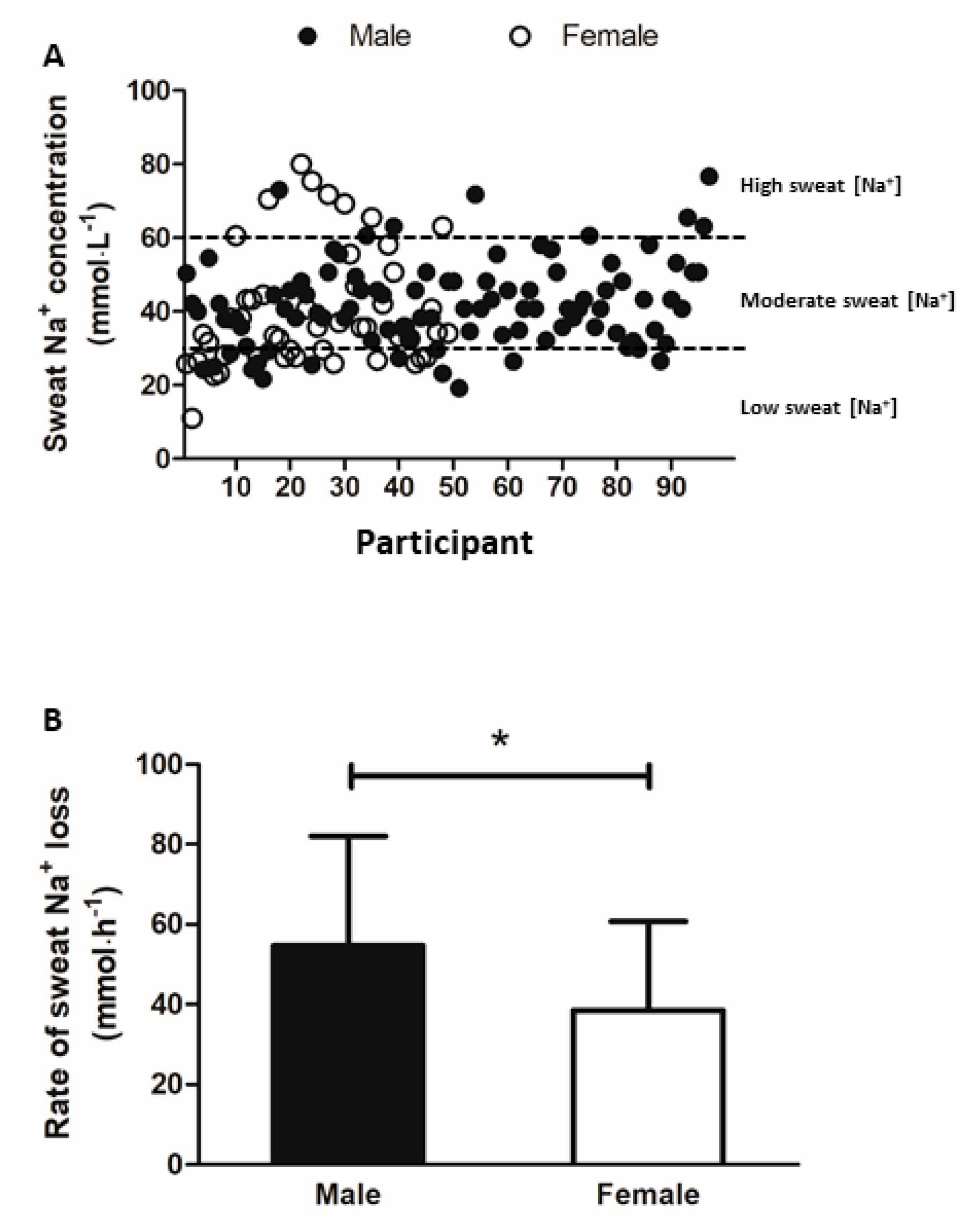
3.5. Sweat Sodium and Potassium Concentration
4. Discussion
Practical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunha, F.; Midgley, A.; Monteiro, W.; Farinatti, P. Influence of cardiopulmonary exercise testing protocol and resting VO2 assessment on %HRmax, %HRR, %VO2max and %VO2R relationships. Int. J. Sports Med. 2010, 31, 319–326. [Google Scholar] [CrossRef]
- Binkley, H.M.; Beckett, J.; Casa, D.J.; Kleiner, D.M.; Plummer, P.E. National Athletic Trainers’ Association position statement: Exertional heat illnesses. J. Athl. Train. 2002, 37, 329. [Google Scholar]
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National Athletic Trainers’ Association position statement: Fluid replacement for the physically active. J. Athl. Train. 2017, 52, 877–895. [Google Scholar] [CrossRef]
- Adams, W.; Fox, R.; Fry, A.; MacDonald, I. Thermoregulation during marathon running in cool, moderate, and hot environments. J. Appl. Physiol. 1975, 38, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, D.; Jay, O.; Kenny, G.P. The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation. J. Physiol. 2013, 591, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.W.; Nio, A.Q.; Lim, C.L.; Teo, E.Y.; Byrne, C. Thermoregulation, pacing and fluid balance during mass participation distance running in a warm and humid environment. Eur. J. Appl. Physiol. 2010, 109, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Reeve, T.; Gordon, R.; Laursen, P.B.; Lee, J.K.W.; Tyler, C.J. Impairment of cycling capacity in the heat in well-trained endurance athletes after high-intensity short-term heat acclimation. Int. J. Sports Physiol. Perform. 2019, 14, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Murray, B. The role of salt and glucose replacement drinks in the marathon. Sports Med. 2007, 37, 358–360. [Google Scholar] [CrossRef]
- Noakes, T. Fluid replacement during marathon running. Clin. J. Sport Med. 2003, 13, 309–318. [Google Scholar] [CrossRef]
- Andersen, J.J. The State of Running 2019. Available online: https://runrepeat.com/state-of-running (accessed on 24 October 2020).
- Gleeson, M. Temperature regulation during exercise. Int. J. Sports Med. 1998, 19, S96–S99. [Google Scholar] [CrossRef]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [PubMed]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26 (Suppl. 5), 575S–584S. [Google Scholar] [CrossRef]
- Amano, T.; Ichinose, M.; Koga, S.; Inoue, Y.; Nishiyasu, T.; Kondo, N. Sweating responses and the muscle metaboreflex under mildly hyperthermic conditions in sprinters and distance runners. J. Appl. Physiol. 2011, 111, 524–529. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating rate and sweat sodium concentration in athletes: A review of methodology and intra/interindividual variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Barnes, K.A.; Anderson, M.L.; Passe, D.H.; Stofan, J.R. Normative data for regional sweat sodium concentration and whole-body sweating rate in athletes. J. Sports Sci. 2016, 34, 358–368. [Google Scholar] [CrossRef]
- Baker, L.B.; Ungaro, C.T.; Sopeña, B.C.; Nuccio, R.P.; Reimel, A.J.; Carter, J.M.; Stofan, J.R.; Barnes, K.A. Body map of regional vs. whole body sweating rate and sweat electrolyte concentrations in men and women during moderate exercise-heat stress. J. Appl. Physiol. 2018, 124, 1304–1318. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Cheuvront, S.N.; Ely, B.R.; Moran, D.S.; Hadid, A.; Endrusick, T.L.; Sawka, M.N. Sweat rate prediction equations for outdoor exercise with transient solar radiation. J. Appl. Physiol. 2012, 112, 1300–1310. [Google Scholar] [CrossRef]
- Kilding, A.; Tunstall, H.; Wraith, E.; Good, M.; Gammon, C.; Smith, C. Sweat rate and sweat electrolyte composition in international female soccer players during game specific training. Int. J. Sports Med. 2009, 30, 443–447. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.; Nimmo, M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M. Development of individual hydration strategies for athletes. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Goulet, E.D. Dehydration and endurance performance in competitive athletes. Nutr. Rev. 2012, 70 (Suppl. 2), S132–S136. [Google Scholar] [CrossRef]
- Almond, C.S.; Shin, A.Y.; Fortescue, E.B.; Mannix, R.C.; Wypij, D.; Binstadt, B.A.; Duncan, C.N.; Olson, D.P.; Salerno, A.E.; Newburger, J.W.; et al. Hyponatremia among runners in the Boston Marathon. N. Engl. J. Med. 2005, 352, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Carter, R., III. Exertional heat illness and hyponatremia: An epidemiological prospective. Curr. Sports Med. Rep. 2008, 7, S20–S27. [Google Scholar] [CrossRef]
- Chorley, J.; Cianca, J.; Divine, J. Risk factors for exercise-associated hyponatremia in non-elite marathon runners. Clin. J. Sport Med. 2007, 17, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Loi, V.; Pani, A.; Rosner, M.H. Exercise-associated hyponatremia: 2017 update. Front. Med. 2017, 4, 21. [Google Scholar] [CrossRef]
- Rosner, M.H. (Ed.) Exercise-associated hyponatremia. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Speedy, D.B.; Noakes, T.D.; Schneider, C. Exercise-associated hyponatremia: A review. Emerg. Med. 2001, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Nadel, E.; Pandolf, K.; Roberts, M.; Stolwijk, J. Mechanisms of thermal acclimation to exercise and heat. J. Appl. Physiol. 1974, 37, 515–520. [Google Scholar] [CrossRef]
- Roberts, M.F.; Wenger, C.B.; Stolwijk, J.A.; Nadel, E.R. Skin blood flow and sweating changes following exercise training and heat acclimation. J. Appl. Physiol. 1977, 43, 133–137. [Google Scholar] [CrossRef]
- Shapiro, Y.; Hubbard, R.W.; Kimbrough, C.M.; Pandolf, K.B. Physiological and hematologic responses to summer and winter dry-heat acclimation. J. Appl. Physiol. 1981, 50, 792–798. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Baker, L.B.; Stofan, J.R.; Hamilton, A.A.; Horswill, C.A. Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise. J. Appl. Physiol. 2009, 107, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.P.; Miller, V.S.; Joubert, D.M. Hydration status of expatriate manual workers during summer in the Middle East. Ann. Occup. Hyg. 2010, 54, 137–143. [Google Scholar]
- Lara, B.; Salinero, J.; Areces, F.; Ruiz-Vicente, D.; Gallo-Salazar, C.; Abián-Vicén, J.; Del Coso, J. Sweat sodium loss influences serum sodium concentration in a marathon. Scand. J. Med. Sci. Sports 2017, 27, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Gallo-Salazar, C.; Puente, C.; Areces, F.; Salinero, J.J.; Del Coso, J. Interindividual variability in sweat electrolyte concentration in marathoners. J. Int. Soc. Sports Nutr. 2016, 13, 31. [Google Scholar] [CrossRef]
- Taylor, R. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Passe, D.; Horn, M.; Stofan, J.; Horswill, C.; Murray, R. Voluntary dehydration in runners despite favorable conditions for fluid intake. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.; Racinais, S.; Sawka, M.N. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand. J. Med. Sci. Sports 2015, 25, 20–38. [Google Scholar] [CrossRef]
- Byrne, C.; Lee, J.K.W.; Chew, S.A.N.; Lim, C.L.; Tan, E.Y.M. Continuous thermoregulatory responses to mass-participation distance running in heat. Med. Sci. Sports Exerc. 2006, 38, 803–810. [Google Scholar] [CrossRef]
- Hargreaves, M.; Dillo, P.; Angus, D.; Febbraio, M. Effect of fluid ingestion on muscle metabolism during prolonged exercise. J. Appl. Physiol. 1996, 80, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N. Physiological consequences of hypohydration: Exercise performance and thermoregulation. Med. Sci. Sports Exerc. 1992, 24, 657–670. [Google Scholar] [CrossRef]
- Bar-Or, O. Effects of age and gender on sweating pattern during exercise. Int. J. Sports Med. 1998, 19, S106–S107. [Google Scholar] [CrossRef]
- Ichinose-Kuwahara, T.; Inoue, Y.; Iseki, Y.; Hara, S.; Ogura, Y.; Kondo, N. Sex differences in the effects of physical training on sweat gland responses during a graded exercise. Exp. Physiol. 2010, 95, 1026–1032. [Google Scholar] [CrossRef]
- Morimoto, T.; Slabochova, Z.; Naman, R.; Sargent, F., 2nd. Sex differences in physiological reactions to thermal stress. J. Appl. Physiol. 1967, 22, 526–532. [Google Scholar] [CrossRef]
- Inoue, Y.; Tanaka, Y.; Omori, K.; Kuwahara, T.; Ogura, Y.; Ueda, H. Sex-and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur. J. Appl. Physiol. 2005, 94, 323–332. [Google Scholar] [CrossRef]
- Hew, T.D. Women hydrate more than men during a marathon race: Hyponatremia in the Houston marathon: A report on 60 cases. Clin. J. Sport Med. 2005, 15, 148–153. [Google Scholar] [CrossRef]
- Tan, X.R.; Low, I.C.C.; Byrne, C.; Wang, R.; Lee, J.K.W. Assessment of dehydration using body mass changes of elite marathoners in the tropics. J. Sci. Med. Sport. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pugh, L.; Corbett, J.; Johnson, R. Rectal temperatures, weight losses, and sweat rates in marathon running. J. Appl. Physiol. 1967, 23, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R. Fluid and electrolyte loss and replacement in exercise. J. Sports Sci. 1991, 9, 117–142. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Brown, I. Sodium Intakes around the World; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Batcagan-Abueg, A.P.; Lee, J.J.; Chan, P.; Rebello, S.A.; Amarra, M.S.V. Salt intakes and salt reduction initiatives in Southeast Asia: A review. Asia Pac. J. Clin. Nutr. 2013, 22, 683. [Google Scholar]
- Braconnier, P.; Milani, B.; Loncle, N.; Lourenco, J.M.; Brito, W.; Delacoste, J.; Maillard, M.; Stuber, M.; Burnier, M.; Pruijm, M. Short-term changes in dietary sodium intake influence sweat sodium concentration and muscle sodium content in healthy individuals. J. Hypertens. 2020, 38, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Ball, K.D.; Kolkhorst, F.W. Sodium ion concentration vs. sweat rate relationship in humans. J. Appl. Physiol. 2007, 103, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Kolding, M.; Leslie, E.; Moreno, D.; Norwood, S.; Ordille, A.; Weller, R. Heat acclimation causes a linear decrease in sweat sodium ion concentration. J. Therm. Biol. 2018, 71, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W.; Cheuvront, S.N. Physiological adjustments to hypohydration: Impact on thermoregulation. Auton. Neurosci. 2016, 196, 47–51. [Google Scholar] [CrossRef] [PubMed]
| Session | Time of Day | Time | Mean Ambient Temperature (°C) | Mean Relative Humidity (%) | Participants | |
|---|---|---|---|---|---|---|
| Male | Female | |||||
| 1 | Morning | 7.00 a.m. to 8.00 a.m. | 28.5 | 75 | 9 | 6 |
| 2 | Morning | 6.00 a.m. to 8.00 a.m. | 30 | 86 | 10 | 4 |
| 3 | Evening | 7.00 p.m. to 8.00 p.m. | 29.5 | 63 | 16 | 8 |
| 4 | Evening | 7.00 p.m. to 8.00 p.m. | 28 | 87 | 31 | 13 |
| 5 | Evening | 7.00 p.m. to 8.00 p.m. | 29.8 | 56 | 12 | 12 |
| 6 | Evening | 5.00 p.m. to 6.00 p.m. | 31.5 | 55 | 24 | 21 |
| Male (n = 102) | Female (n = 64) | |
|---|---|---|
| Age (years) | 36 ± 9 (21–68) | 34 ± 9 (22–62) |
| Running duration (min) | 43.7 ± 14.8 (33–97) | 43.6 ± 13.6 (43–100) |
| Running distance (km) | 6.4 ± 1.1 (2.5–12.5) | 5.3 ± 1.1 * (2.5–10) |
| Running pace (min·km−1) | 6.8 ± 3.7 (3.5–10.0) | 8.2 ± 3.9 * (4.3–11.0) |
| Pre-running body mass (kg) | 70.8 ± 10.6 (46.9–99.7) | 56.7 ± 9.7 * (42.3–93.2) |
| Post-running body mass (kg) | 70.2 ± 10.6 (46.6–99.0) | 56.4 ± 9.6 * (42.3–92.8) |
| Percentage body mass loss (%) | 1.3 ± 0.5 (0.2–3.6) | 1.2 ± 0.5 (0.1–3.7) |
| Whole-body sweat loss (WBSL) (L) | 0.9 ± 0.3 (0.2–2.6) | 0.6 ± 0.3 * (0.1–1.9) |
| Whole-body sweat rate (WBSR) (L·h−1) | 1.3 ± 0.5 (0.2–3.8) | 0.9 ± 0.3 * (0.1–2.2) |
| Fluid intake (L) | 0.3 ± 0.3 (0–1.1) | 0.3 ± 0.2 (0–1.1) |
| Time of Day of Session | Sex | Urine Specific Gravity (USG) | ||
|---|---|---|---|---|
| ≤1.020 | >1.020 | >1.030 | ||
| Morning | Male | 11 (69%) | 4 (31%) | 1 (6%) |
| Female | 7 (78%) | 2 (22%) | 0 (0%) | |
| Total | 18 (72%) | 6 (28%) | 1 (4%) | |
| Evening | Male | 59 (85%) | 10 (15%) | 1 (1%) |
| Female | 43 (84%) | 8 (16%) | 0 (0%) | |
| Total | 102 (85%) | 18 (15%) | 1 (1%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surapongchai, J.; Saengsirisuwan, V.; Rollo, I.; Randell, R.K.; Nithitsuttibuta, K.; Sainiyom, P.; Leow, C.H.W.; Lee, J.K.W. Hydration Status, Fluid Intake, Sweat Rate, and Sweat Sodium Concentration in Recreational Tropical Native Runners. Nutrients 2021, 13, 1374. https://doi.org/10.3390/nu13041374
Surapongchai J, Saengsirisuwan V, Rollo I, Randell RK, Nithitsuttibuta K, Sainiyom P, Leow CHW, Lee JKW. Hydration Status, Fluid Intake, Sweat Rate, and Sweat Sodium Concentration in Recreational Tropical Native Runners. Nutrients. 2021; 13(4):1374. https://doi.org/10.3390/nu13041374
Chicago/Turabian StyleSurapongchai, Juthamard, Vitoon Saengsirisuwan, Ian Rollo, Rebecca K. Randell, Kanpiraya Nithitsuttibuta, Patarawadee Sainiyom, Clarence Hong Wei Leow, and Jason Kai Wei Lee. 2021. "Hydration Status, Fluid Intake, Sweat Rate, and Sweat Sodium Concentration in Recreational Tropical Native Runners" Nutrients 13, no. 4: 1374. https://doi.org/10.3390/nu13041374
APA StyleSurapongchai, J., Saengsirisuwan, V., Rollo, I., Randell, R. K., Nithitsuttibuta, K., Sainiyom, P., Leow, C. H. W., & Lee, J. K. W. (2021). Hydration Status, Fluid Intake, Sweat Rate, and Sweat Sodium Concentration in Recreational Tropical Native Runners. Nutrients, 13(4), 1374. https://doi.org/10.3390/nu13041374






