Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
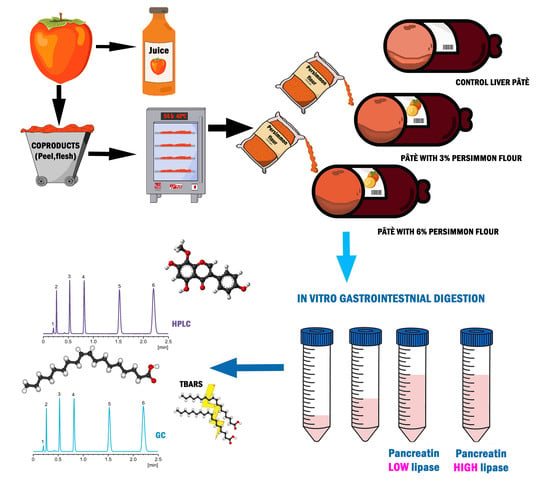
2.2. Sample Preparation
2.3. In Vitro Gastrointestinal Digestion
2.4. Extraction, Identification, and Quantification of Bound and Free Polyphenol Fractions of Both Persimmon Flour and Pork Liver Pâté
2.4.1. Persimmon Flour and Undigested Pork Liver Pâté
2.4.2. Digested Pork Liver Pâté
- PFi: polyphenols (individual or total) in bound fraction after intestinal phase (µg/g d.w).
- BPC: Bound polyphenol (individual or total) in undigested enriched pâté (µg /g d.w).
2.5. Fatty Acid Profile
2.5.1. Fat Extraction
2.5.2. Methylation of Fatty Acids
- FAu: Fatty acid content in undigested pâté samples (mg/100 g fatty acid).
- FAid: Fatty acid content after intestinal digestion (mg/100 g fatty acid).
2.6. Lipid Oxidation (TBARs)
- TBARsu: TBARs values in undigested pâté samples (µmol MDA/Kg).
- TBARsd: TBARs values in digested (gastric or intestinal phases) pâté samples (µmol MDA/Kg).
2.7. Statistical Analysis
3. Results and Discussion
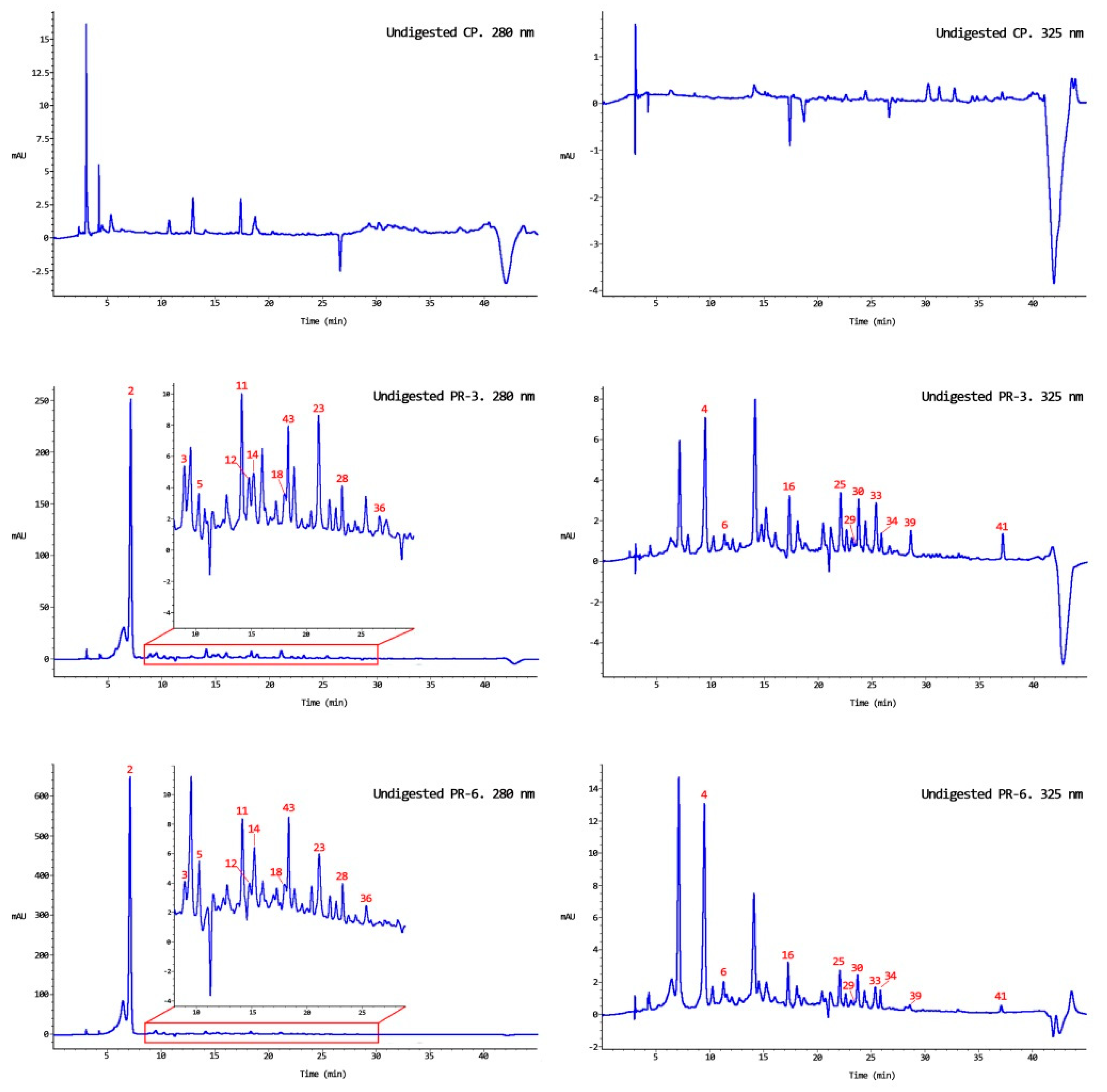
3.1. Bound and Free Phenolic Compounds in Persimmon Flour
3.2. Bound and Free Phenolic Compounds in Undigested Enriched Pâté
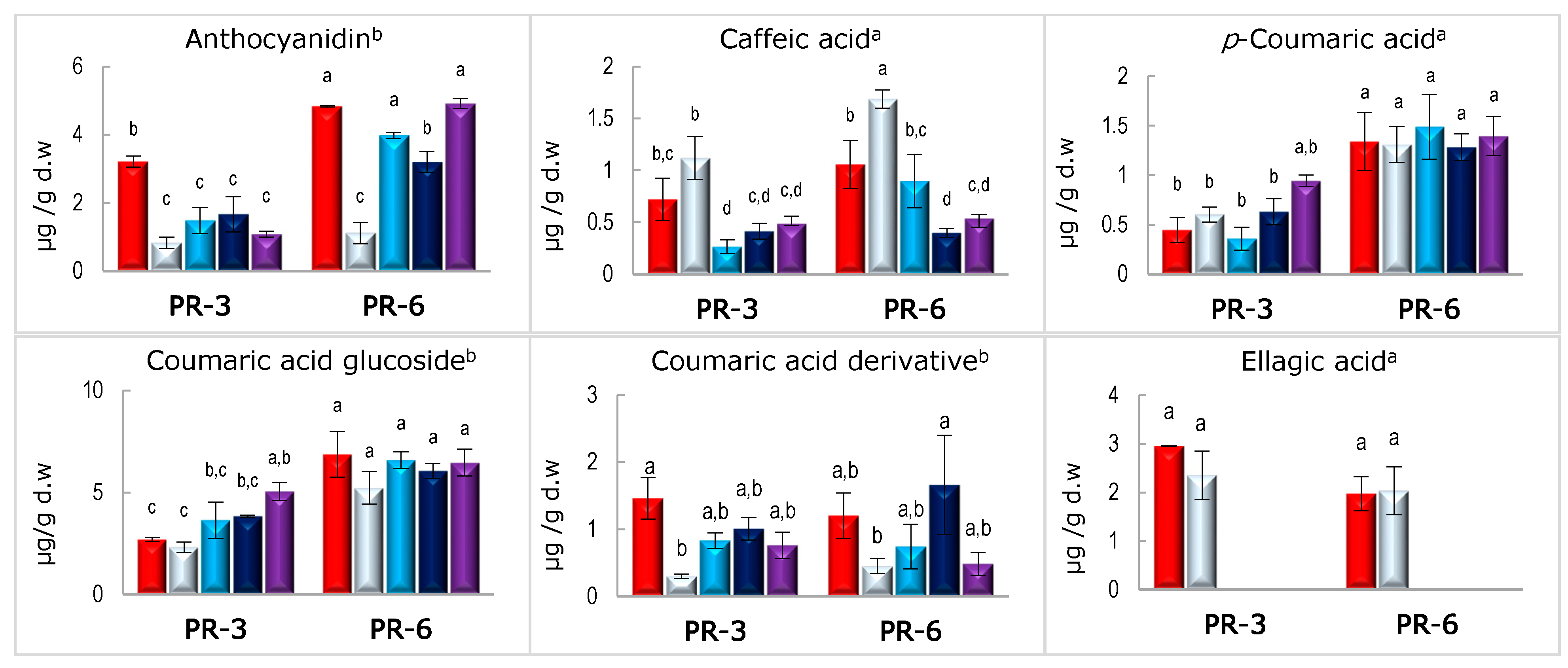
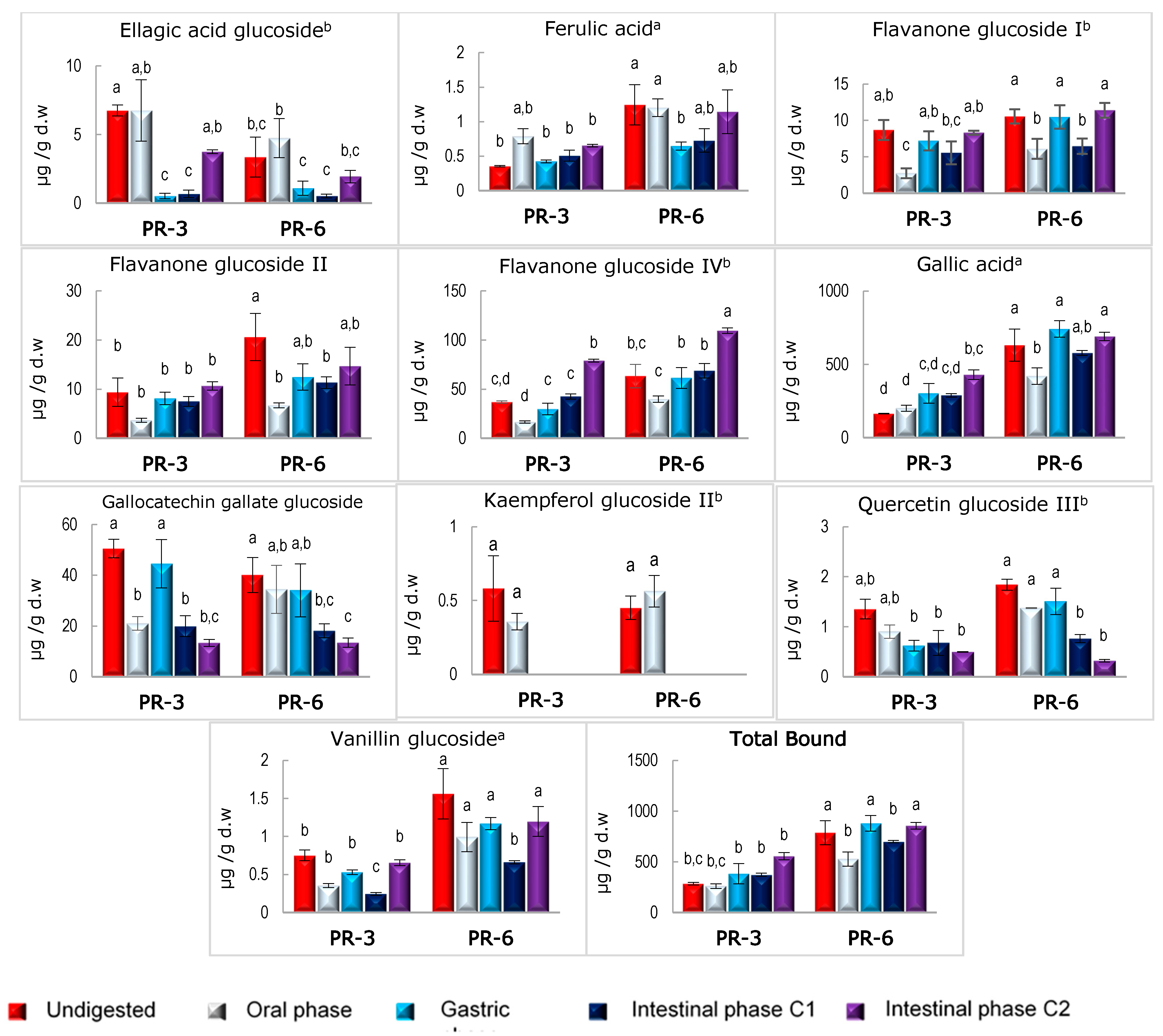
3.3. Stability of Bound and Free Phenolic Compounds in Enriched Pâté after In Vitro Digestion
3.4. Lipid Oxidation in Undigested and Digested Pâté Samples
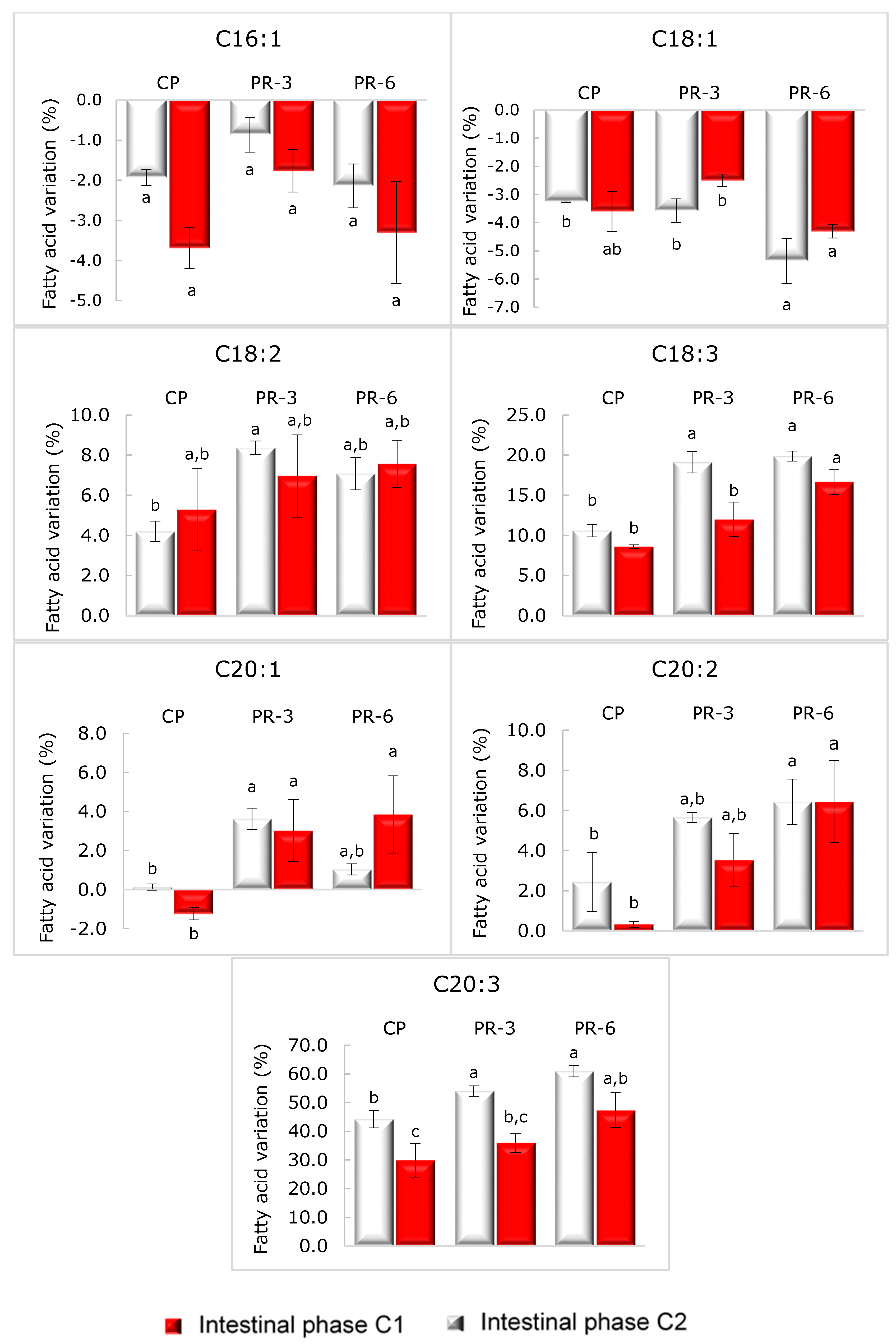
3.5. Fatty Acid Profile of Pâté and Its Stability after In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brito, G.; Andrade, J.M.; Havel, J.; Díaz, C.; García, F.J.; Peña-Méndez, E.M. Classification of some heat-treated liver pastes according to container type, using heavy metals content and manufacturer’s data, by principal components analysis and potential curves. Meat Sci. 2006, 74, 296–302. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Red Meat and Processed Meat; International Agency for Research on Cancer. World Health Organization: Lyon, France, 2018; p. 114. [Google Scholar]
- Viuda-Martos, M.; Fernandez-Lopez, J.; Sayas-Barbera, E.; Sendra, E.; Navarro, C.; Perez-Alvarez, J.A. Citrus co-products as technological strategy to reduce residual nitrite content in meat products. J. Food Sci. 2009, 74, R93–R100. [Google Scholar] [CrossRef] [PubMed]
- Steppeler, C.; Haugen, J.E.; Rødbotten, R.; Kirkhus, B. Formation of malondialdehyde, 4-hydroxynonenal, and 4-hydroxyhexenal during in vitro digestion of cooked beef, pork, chicken, and salmon. J. Agric. Food Chem. 2016, 64, 487–496. [Google Scholar] [CrossRef]
- Russell, E.A.; Lynch, A.; Lynch, P.B.; Kerry, J.P. Quality and shelf life of duck liver pâtés as influenced by dietary supplementation with a-tocopheryl acetate and various fat sources. J. Food Sci. 2003, 68, 799–802. [Google Scholar] [CrossRef]
- Grasso, S.; Brunton, N.P.; Lyng, J.G.; Lalor, F.; Monahan, F.J. Healthy processed meat products—Regulatory, reformulation and consumer challenges. Trends Food Sci. Technol. 2014, 39, 4–17. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Gullón, B.; Campagnol, P.C.B.; Franco, D. Novel strategy for developing healthy meat products replacing saturated fat with oleogels. Curr. Opin. Food Sci. 2021, 40, 40–45. [Google Scholar] [CrossRef]
- Thøgersen, R.; Bertram, H.C. Reformulation of processed meat to attenuate potential harmful effects in the gastrointestinal tract—A review of current knowledge and evidence of health prospects. Trends Food Sci. Technol. 2021, 108, 111–118. [Google Scholar] [CrossRef]
- Sirini, N.; Frizzo, L.S.; Aleu, G.; Soto, L.P.; Rosmini, M.R. Use of probiotic microorganisms in the formulation of healthy meat products. Curr. Opin. Food Sci. 2021, 38, 141–146. [Google Scholar] [CrossRef]
- Grasso, S.; Pintado, T.; Pérez-Jiménez, J.; Ruiz-Capillas, C.; Herrero, A.M. Potential of a sunflower seed by-product as animal fat replacer in healthier frankfurters. Foods 2020, 9, 445. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef]
- Peluzio, M.C.G.; Martinez, J.A.; Milagro, F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021, 108, 11–26. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant polyphenols-biofortified foods as a novel tool for the prevention of human gut diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef]
- Shan, S.; Lu, Y.; Zhang, X.; Shi, J.; Li, H.; Li, Z. Inhibitory effect of bound polyphenol from foxtail millet bran on miR-149 methylation increases the chemosensitivity of human colorectal cancer HCT-8/Fu cells. Mol. Cell Biochem. 2021, 476, 513–523. [Google Scholar] [CrossRef]
- Burri, S.; Granheimer, K.; Rémy, M.; Tannira, V.; So, Y.; Rumpunen, K.; Tornberg, E.; Paz, P.C.; Uhlig, E.; Oscarsson, E.; et al. Processed meat products with added plant antioxidants affect the microbiota and immune response in C57BL/6JRj mice with cyclically induced chronic inflammation. Biomed. Pharmacother. 2021, 135, 111133. [Google Scholar] [CrossRef]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.; Alves, P.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yokoyama, S.-I.; Gato, N. Bile acid-binding activity of young persimmon (Diospyros kaki) fruit and its hypolipidemic effect in mice. Phytother. Res. 2010, 24, 205–210. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Jesion, I.; Namiesnik, J.; Drzewiecki, J.; Park, Y.-S.; Ham, K.-S.; Giordani, E.; Trakhtenberg, S. Influence of two cultivars of persimmon on atherosclerosis indices in rats fed cholesterol-containing diets: Investigation in vitro and in vivo. Nutrition 2011, 27, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Hamauzu, Y.; Suwannachot, J. Non-extractable polyphenols and in vitro bile acid-binding capacity of dried persimmon (Diospyros kaki) fruit. Food Chem. 2019, 293, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.W.; Jeong, M.C.; Chung, S.K. The physicochemical properties and the antioxidant activities of persimmon peel powders with different particle sizes. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 442–446. [Google Scholar] [CrossRef]
- Akter, M.S.; Ahmed, M.; Eun, J.B. Dietary fibre components, antioxidant activities and hydration properties of ripe persimmon (Diospyros kaki L. cv. Daebong) peel powders as affected by different washing treatments. Int. J. Food Sci. Technol. 2010, 45, 1464–1471. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Álvarez, J.A.P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pellegrini, M.; Viuda-Martos, M.; Pérez-Álvarez, J.A.; Férnandez-López, J. Persimmon (Diospyros kaki Thunb.) coproducts as a new ingredient in pork liver pâté: Influence on quality properties. Int. J. Food Sci. Technol. 2019, 54, 1232–1239. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. From a Reductionist to a Holistic Approach in Preventive Nutrition to Define New and More Ethical Paradigms. Healthcare 2015, 3, 1054–1063. [Google Scholar] [CrossRef]
- Larsson, K.; Cavonius, L.; Alminger, M.; Undeland, I. Oxidation of cod liver oil during gastrointestinal in vitro digestion. J. Agric. Food Chem. 2012, 60, 7556–7564. [Google Scholar] [CrossRef]
- Zhu, X.; Ye, A.; Verrier, T.; Singh, H. Free fatty acid profiles of emulsified lipids during in vitro digestion with pancreatic lipase. Food Chem. 2013, 139, 398–404. [Google Scholar] [CrossRef]
- Martini, S.; Cavalchi, M.; Conte, A.; Tagliazucchi, D. The paradoxical effect of extra-virgin olive oil on oxidative phenomena during in vitro co-digestion with meat. Food Res. Int. 2018, 109, 82–90. [Google Scholar] [CrossRef]
- Goethals, S.; Van Hecke, T.; Vossen, E.; Vanhaecke, L.; Van Camp, J.; De Smet, S. Commercial luncheon meat products and their in vitro gastrointestinal digests contain more protein carbonyl compounds but less lipid oxidation products compared to fresh pork. Food Res. Int. 2020, 136, 109585. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Casal, S.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Influence of culinary practices on protein and lipid oxidation of chicken meat burgers during cooking and in vitro gastrointestinal digestion. Food Chem. Toxicol. 2020, 141, 111401. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Ke, Y.; Li, C.; Zhang, Z.; Liu, A.; Luo, Q.; Lin, B.; He, J.; Wu, W. Processing of four different cooking methods of Oudemansiella radicata: Effects on in vitro bioaccessibility of nutrients and antioxidant activity. Food Chem. 2021, 337, 128007. [Google Scholar] [CrossRef]
- Lucas-González, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Gullón, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In Vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Podio, N.S.; Baroni, M.V.; Pérez, G.T.; Wunderlin, D.A. Assessment of bioactive compounds and their in vitro bioaccessibility in whole-wheat flour pasta. Food Chem. 2019, 293, 408–417. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pérez-Álvarez, J.A.; Moscaritolo, S.; Fernández-López, J.; Sacchetti, G.; Viuda-Martos, M. Evaluation of polyphenol bioaccessibility and kinetic of starch digestion of spaghetti with persimmon (Dyospyros kaki) flours coproducts during in vitro gastrointestinal digestion. Food Chem. 2021, 338, 128142. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In vitro digestion models to assess lipolysis: The impact of the simulated conditions of gastric and intestinal pH, bile salts and digestive fluids. Food Res. Int. 2019, 125, 108511. [Google Scholar] [CrossRef] [PubMed]
- Tullberg, C.; Vegarud, G.; Undeland, I. Oxidation of marine oils during in vitro gastrointestinal digestion with human digestive fluids—Role of oil origin, added tocopherols and lipolytic activity. Food Chem. 2019, 270, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Lucas-Gonzalez, R.; Fernández-López, J.; Ricci, A.; Pérez-Álvarez, J.A.; Lo Sterzo, C.; Viuda-Martos, M. Bioaccessibility of polyphenolic compounds of six quinoa seeds during in vitro gastrointestinal digestion. J. Funct. Foods 2017, 38, 77–88. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Alvarez, J.A.; Fernández-López, J.; Delgado-Ospina, J.; Chaves-López, C.; Viuda-Martos, M. Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy 2021, 11, 401. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Marti, N.; Saura, D.; Valero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization of polyphenols, sugars, and other polar compounds in persimmon juices produced under different technologies and their assessment in terms of compositional variations. Food Chem. 2015, 182, 282–291. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Gratacós-Cubarsí, M.; Sárraga, C.; Guàrdia, M.D.; García-Regueiro, J.-A.; Castellari, M. Stability of phenolic compounds in dry fermented sausages added with cocoa and grape seed extracts. LWT 2014, 57, 329–336. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LCESI-LTQ-Orbitrap-MS). J. Funct. Foods 2016, 23, 370–377. [Google Scholar] [CrossRef]
- Sentandreu, E.; Cerdán-Calero, M.; Halket, J.M.; Navarro, J.L. Rapid screening of low-molecular-weight phenols from persimmon (Diospyros kaki) pulp using liquid chromatography/UV-visible/electrospray mass spectrometry analysis. J. Sci. Food Agric. 2015, 95, 1648–1654. [Google Scholar] [CrossRef]
- Kou, J.J.; Yan, X.; Zhao, Z.H.; Wang, W.J.; Liu, M.J.; Zhang, Y.X. Determination of flavonoid in pulp of different Japanese persimmon cultivars by HPLC. Acta Hortic. 2015, 1106, 117–122. [Google Scholar] [CrossRef]
- Ancillotti, C.; Caprini, C.; Scordo, C.; Renai, L.; Giordani, E.; Orlandini, S.; Furlanetto, S.; Del Bubba, M. Phenolic compounds in Rojo Brillante and Kaki Tipo persimmons at commercial harvest and in response to CO2 and ethylene treatments for astringency removal. LWT 2019, 100, 99–105. [Google Scholar] [CrossRef]
- Maulidiani, M.; Abdul-Hamid, N.A.; Abas, F.; Park, Y.S.; Park, Y.-K.; Kim, Y.M.; Gorinstein, S. Detection of bioactive compounds in persimmon (Diospyros kaki) using UPLC-ESI-Orbitrap-MS/MS and fluorescence analyses. Microchem 2019, 149, 103978. [Google Scholar] [CrossRef]
- Peng, J.; Li, K.; Zhu, W.; Deng, X.; Li, C. Separation and purification of four phenolic compounds from persimmon by high-speed counter-current chromatography. J. Chromatogr. B 2018, 1072, 78–85. [Google Scholar] [CrossRef]
- Chen, J.; Du, J.; Ge, Z.Z.; Zhu, W.; Nie, R.; Li, C.M. Comparison of sensory and compositions of five selected persimmon cultivars (Diospyros kaki L.) and correlations between chemical components and processing characteristics. J. Food Sci. Technol. 2016, 53, 1597–1607. [Google Scholar] [CrossRef]
- Chen, G.-L.; Zhang, X.; Chen, S.-G.; Han, M.-D.; Gao, Y.-Q. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the south of China. J. Funct. Foods 2017, 30, 290–302. [Google Scholar] [CrossRef]
- Suzuki, T.; Someya, S.; Hu, F.; Tanokura, M. Comparative study of catechin compositions in five Japanese persimmons (Diospyros kaki). Food Chem. 2005, 93, 149–152. [Google Scholar] [CrossRef]
- Kawakami, K.; Aketa, S.; Nakanami, M.; Iizuka, S.; Hirayama, M. Major Water-Soluble Polyphenols, Proanthocyanidins, in Leaves of Persimmon (Diospyros kaki) and Their α-Amylase Inhibitory Activity. Biosci. Biotechnol. Biochem. 2010, 74, 1380–1385. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Baltacioğlu, H.; Artik, N. Study of postharvest changes in the chemical composition of persimmon by HPLC. Turk. J. Agric. For. 2013, 37, 568–574. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J.A. Orange fibre as potential functional ingredient for dry-cured sausages. Eur. Food Res. Technol. 2007, 226, 1–6. [Google Scholar] [CrossRef]
- Zając, M.; Duda, I.; Skoczylas, Ł.; Tabaszewska, M. Potential use of hyssopus officinalis and borago officinalis as curing ingredients in pork meat formulations. Animals 2020, 10, 2327. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. In vitro bioaccessibility and functional properties of polyphenols from pomegranate peels and pomegranate peels-enriched cookies. Food Funct. 2016, 7, 4247–4258. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods. 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.P. Catabolism of raw and cooked green pepper (Capsicum annuum) (poly)phenolic compounds after simulated gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2016, 27, 201–213. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2017, 214, 486–496. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Hang Xiao, H. Designing food structure and composition to enhance nutraceutical bioactivity to support cancer inhibition. Semin. Cancer Biol. 2017, 46, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef]
- Swetha, M.P.; Radha, C.; Muthukumar, S.P. Bioaccessibility and bioavailability of Moringa oleifera seed flour polyphenols. Food Meas. 2018, 12, 1917–1926. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Q.; Hu, K.; Zhang, R.; Yuan, Y.; He, S.; Zeng, Q.; Su, D. Effects of in vitro simulated digestion on the free and bound phenolic content and antioxidant activity of seven species of seaweeds. Int. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomas-Barberan, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Crozier, A. Absorption, metabolism, and excretion of (–)-epicatechin in humans: An evaluation of recent findings. Am. J. Clin. Nutr. 2013, 98, 861–862. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Gao, T.; Li, L.; Chen, Y.; Xiao, D.; Liu, W.; Zou, B.; Lu, B.; Tian, X.; et al. A holistic view of gallic acid-induced attenuation in colitis based on microbiome-metabolomics analysis. Food Funct. 2019, 10, 4046–4061. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020, 11, 2231. [Google Scholar] [CrossRef]
- Martín-Sánchez, A.M.; Ciro-Gómez, G.L.; Zapata-Montoya, J.E.; Vilella-Esplá, J.; Pérez-Álvarez, J.A.; Sayas-Barberá, E. Effect of date palm coproducts and annatto extract on lipid oxidation and microbial quality in a pork liver pâté. J. Food Sci. 2014, 79, M2301–M2307. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Bertolín, J.R.; Joy, M.; Blanco, M. Malondialdehyde determination in raw and processed meat products by UPLC-DAD and UPLC-FLD. Food Chem. 2019, 298, 125009. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Shewfelt, R.L. Fish muscle lipolysis. A review. J. Food Biochem. 1981, 5, 79–100. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.J.; Decker, E.A. Impact of free fatty acid concentration and structure on lipid oxidation in oil-in-water emulsions. Food Chem. 2011, 129, 854–859. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Behaviour of non-oxidized and oxidized flaxseed oils, as models of omega-3 rich lipids, during in vitro digestion. Occurrence of epoxidation reactions. Food Res. Int. 2017, 97, 104–115. [Google Scholar] [CrossRef]
- Zając, M.; Świątek, R. The effect of hemp seed and linseed addition on the quality of liver pâtés. Acta Sci. Pol. Technol. Aliment. 2018, 17, 169–176. [Google Scholar] [CrossRef]
- Holman, R.T.; Elmer, O.C. The rates of oxidation of unsaturated fatty acids and esters. J. Am. Oil Chem. Soc. 1947, 24, 127–129. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 461–478. [Google Scholar] [CrossRef]
- Costa, S.; Afonso, C.; Cardoso, C.; Oliveira, R.; Alves, F.; Nunes, M.; Bandarra, N. Towards a deeper understanding of fatty acid bioaccessibility and its dependence on culinary treatment and lipid class: A case study of gilthead seabream (Sparus aurata). Br. J. Nutr. 2016, 116, 1816–1823. [Google Scholar] [CrossRef]

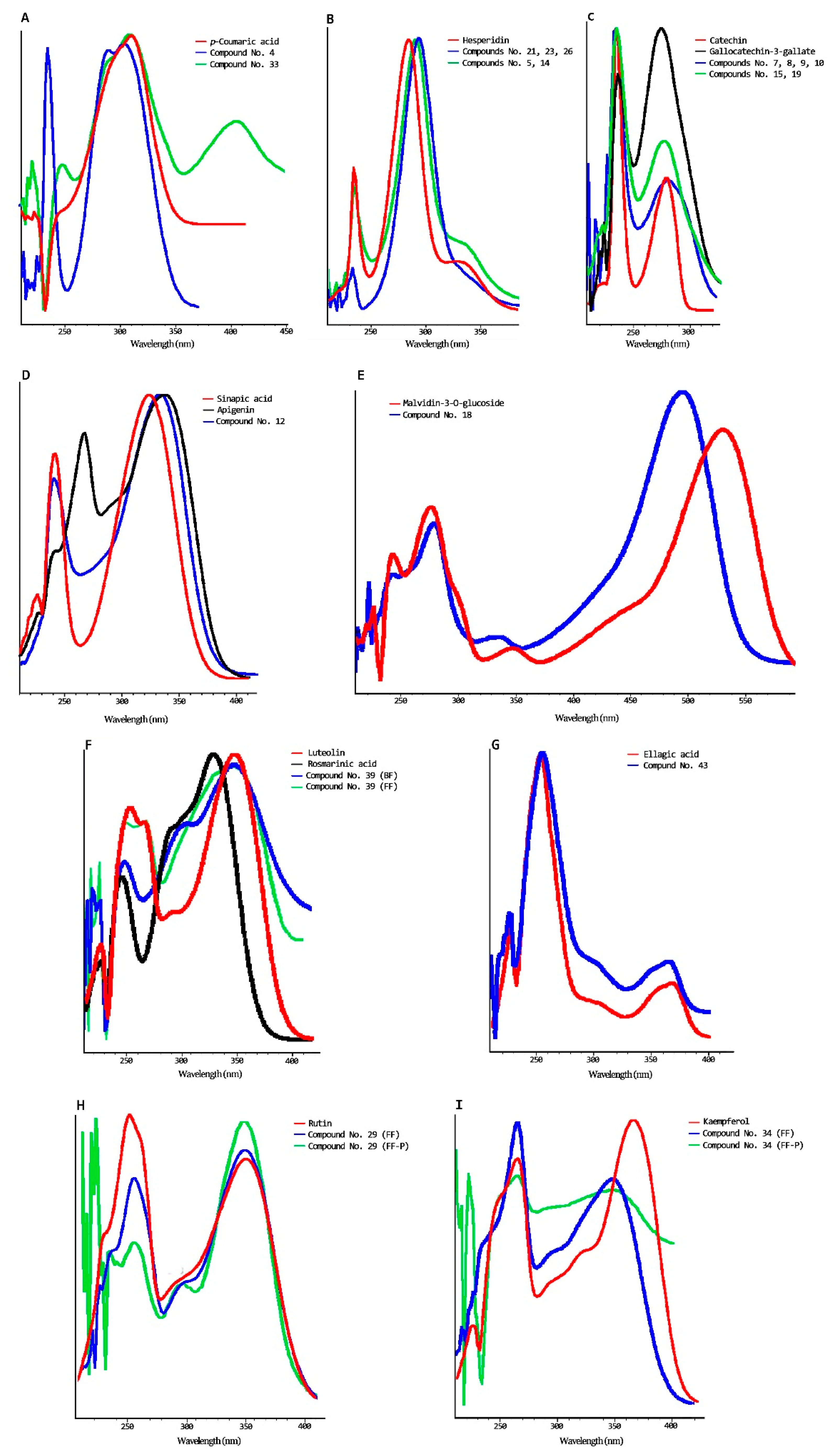

| No | Rt (min) | ʎmax (nm) | Tentative Identification | Fr. | Standard Use to Quantify |
|---|---|---|---|---|---|
| 1 | 4.2 | 234/268 | Gallic acid glucoside b | B | Gallic acid |
| 2 | 6.9 | 234/270 | Gallic acid a | B | Gallic acid |
| 3 | 8.7 | 236/270 | Gallocatechin glucoside b | B | Gallocatechin gallate |
| 4 | 9.5 | 236/300 | Coumaric acid glucoside c | B | p-Coumaric acid |
| 5 | 10.2 | 236/288sh338 | Flavanone glucoside I b | B | Hesperidin |
| 6 | 11.2 | 236/sh274/344/sh456 | Unknow | B | Not quantified |
| 7 | 12.3 | 236/280 | Catechin glucoside I b | B | Catechin |
| 8 | 12.8 | 236/280 | Catechin glucoside II b | B | Catechin |
| 9 | 13.1 | 236/280 | Catechin glucoside III b | B | Catechin |
| 10 | 13.5 | 236/280 | Catechin glucoside VI b | B | Catechin |
| 11 | 14.0 | 236/280/330 | Vanillin glucoside b | B | Vanillin |
| 12 | 14.5 | 244sh286/334 | Unknow | B | Not quantified |
| 13 | 15.0 | 236/280 | Catechin a | F | Catechin |
| 14 | 15.2 | 236/284sh338 | Flavanone glucoside II b | B | Hesperidin |
| 15 | 16.9 | 236/278 | Epigallocatechin-3-gallate glucoside b | B | Epigallocatechin-3-gallate |
| 16 | 17.3 | 246 sh298 324 | Caffeci acid a | B | Caffeic acid |
| 17 | 17.8 | 236/278 | Epigallocatechin-3-gallate a | B | Epigallocatechin-3-gallate |
| 18 | 17.9 | 242/272/sh334/494 | Anthocyanin c | B | Malvidin-3-O-glucose |
| 19 | 18.5 | 236/278 | Gallocatechin-3-gallate glucoside b | B | Gallocatechin-3-gallate |
| 20 | 19.2 | 236/278 | Gallocatechin-3-gallate a | B | Gallocatechin-3-gallate |
| 21 | 19.9 | 236/288sh334 | Flavanone glucoside III b | F | Hesperidin |
| 22 | 20.7 | 252/358 | Quercetin glucoside I b | F | Rutin |
| 23 | 21.0 | 236/288sh334 | Flavanone IV b | B | Hesperidin |
| 24 | 21.2 | 264/360 | Quercetin glucoside II b | F | Rutin |
| 25 | 22.0 | 240/sh300/310 | p-Coumaric acid a | B | p-Coumaric acid |
| 26 | 22.6 | 238/286sh334 | Flavanone glucoside V b | F | Hesperidin |
| 27 | 22.6 | 236/278 | Epicatechin-3-gallate a | B | Epicatechin-3-gallate |
| 28 | 23.2 | 254/362 | Ellagic acid a | F | Ellagic acid |
| 29 | 23.5 | 258/358 | Quercetin glucoside III b | F/B | Rutin |
| 30 | 23.7 | 242/sh296/324 | Ferulic acid a | B | Ferulic acid |
| 31 | 24.0 | 262/358 | Quercetin glucoside IV b | F | Rutin |
| 32 | 25.1 | 266/348 | Kaempferol glucoside I b | F | Kaempferol |
| 33 | 25.5 | 240/sh290/308/400 | p-Coumaric acid derivative c | B | p-Coumaric acid |
| 34 | 25.9 | 266/350 | Kaempferol glucoside II b | F/B | Kaempferol |
| 35 | 26.4 | 268/350 | Kaempferol glucoside III b | F | Kaempferol |
| 36 | 26.6 | 246/260/300 | Unknow | B | Not quantified |
| 37 | 27.6 | 266/344 | Kaempferol glucoside IV b | F | Kaempferol |
| 38 | 28.5 | 254/372 | Myrecetin a | F | Myrecetin |
| 39 | 28.8 | 238sh302/346 | Unknow | F/B | Not quantified |
| 40 | 33.2 | 256/370 | Quercetin a | F/B | Quercetin |
| 41 | 37.1 | 254sh264/312sh374 | Unknow | B | Not quantified |
| 42 | 37.8 | 258/372 | Kaempferol a | F | Kaempferol |
| Family | No. | Compound | Free Fraction | Bound Fraction |
|---|---|---|---|---|
| Phenolic acids | 16 | Caffeic acid | 23.80 ± 4.09 | |
| 25 | p-Coumaric acid | 39.40 ± 6.06 | ||
| 4 | Coumaric acid glucoside | 77.35 ± 5.37 | ||
| 33 | Coumaric acid derivative | 15.71 ± 3.93 | ||
| 28 | Ellagic acid | 7.30 ± 1.88 | ||
| 30 | Ferulic acid | 21.08 ± 3.65 | ||
| 2 | Gallic acid | 10,074.64 ± 1049.51 | ||
| 1 | Gallic acid glucoside | 6.24 ± 0.56 | ||
| 11 | Vanillin glucoside | 80.93 ± 25.09 | ||
| Total phenolic acids | 7.30 ± 1.88 | 10,339.15 ± 1098.26 | ||
| Flavanones | 5 | Flavanone glucoside I | 221.08± 50.98 | |
| 14 | Flavanone glucoside II | 222.41 ± 15.49 | ||
| 21 | Flavanone glucoside III | 28.21 ± 6.74 | ||
| 23 | Flavanone glucoside IV | 1233.34 ± 104.81 | ||
| 26 | Flavanone glucoside V | 23.65 ± 3.38 | ||
| Total flavanones | 51.86 ± 10.12 | 1676.83 ± 171.29 | ||
| Flavanols | 13 | Catechin | 37.10 ± 5.42 | |
| 7 | Catechin glucoside I | 540.52 ± 46.86 | ||
| 8 | Catechin glucoside II | 122.69 ± 4.75 | ||
| 9 | Catechin glucoside III | 158.00 ± 39.70 | ||
| 10 | Catechin glucoside IV | 133.59 ± 32.04 | ||
| 27 | Epicatechin-3-gallate | 30.84 ± 2.16 | ||
| 17 | Epigallocatechin-3-gallate | 38.81 ± 6.37 | ||
| 3 | Gallocatechin glucoside | 327.24 ± 1.23 | ||
| 20 | Gallocatechin-3-gallate | 34.17 ± 8.85 | ||
| 19 | Gallocatechin-3-gallate glucoside | 50.00 ± 9.49 | ||
| 15 | Epigallocatechin-3-galato glucoside | 173.24 ± 43.80 | ||
| Total flavanols | 37.10 ± 5.42 | 1628.47 ± 185.46 | ||
| Flavonols | 42 | Kaempferol | 2.96 ± 1.29 | |
| 32 | Kaempferol glucoside I | 4.03 ± 0.34 | ||
| 34 | Kaempferol glucoside II | 11.84 ± 1.10 | 9.94 ± 2.19 | |
| 35 | Kaempferol glucoside III | 2.47 ± 0.48 | ||
| 37 | Kaempferol glucoside IV | 2.00 ± 0.64 | ||
| 40 | Quercetin | 7.40 ± 5.64 | 7.67 ± 1.24 | |
| 22 | Quercetin glucoside I | 6.07 ± 0.66 | ||
| 24 | Quercetin glucoside II | 6.30 ± 0.47 | ||
| 29 | Quercetin glucoside III | 29.76 ± 4.82 | 32.73 ± 1.02 | |
| 31 | Quercetin glucoside IV | 10.74 ± 0.11 | ||
| 38 | Myricetin | 3.61 ± 0.54 | ||
| Total flavonols | 87.18 ± 13.09 | 50.34 ± 4.46 | ||
| Anthocyanins | 18 | Anthocyanin | 76.67 ± 6.59 | |
| Total flavonoids | 176.14 ± 28.63 | 3432.31 ± 367.79 | ||
| Total | 183.44 ± 30.51 | 13,958.7 ± 1489.2 |
| Bound Polyphenols | PR-3 | PR-6 | Free Polyphenols | PR-3 | PR-6 |
|---|---|---|---|---|---|
| Gallic | 302.2 | 604.5 | Catechin | 1.11 | 2.23 |
| Flavanone glucoside IV | 37.0 | 74.0 | Quercetin glucoside III | 0.89 | 1.79 |
| Catechin glucoside I | 16.2 | 32.4 | Flavanone glucoside III | 0.85 | 1.69 |
| Gallocatechin glucoside | 9.8 | 19.6 | Flavanone glucoside V | 0.71 | 1.42 |
| Flavanone glucoside II | 6.7 | 13.3 | Kaempferol glucoside II | 0.36 | 0.71 |
| Flavanone glucoside I | 6.6 | 13.3 | Quercetin glucoside IV | 0.32 | 0.64 |
| Epigallocatechin-gallate glucoside | 5.2 | 10.4 | Ellagic acid | 0.22 | 0.44 |
| Catechin glucoside III | 4.7 | 9.5 | Hydroxycinnamic acid | 0.09 | 0.18 |
| Catechin glucoside IV | 4.0 | 8.0 | Quercetin | 0.22 | 0.44 |
| Catechin glucoside II | 3.7 | 7.4 | Quercetin glucoside II | 0.19 | 0.38 |
| Vanillin glucoside | 2.4 | 4.9 | Quercetin glucoside I | 0.18 | 0.36 |
| Coumaric acid glucoside | 2.3 | 4.6 | Kaempferol glucoside I | 0.12 | 0.24 |
| Anthocyanin | 2.3 | 4.6 | Myricetin | 0.11 | 0.22 |
| Gallocatechin-3-gallate glucoside | 1.5 | 3.0 | Kaempferol | 0.09 | 0.18 |
| p coumaric acid | 1.2 | 2.4 | Kaempferol glucoside III | 0.07 | 0.15 |
| Epigallocatechin-3-gallate | 1.2 | 2.3 | Kaempferol glucoside IV | 0.06 | 0.12 |
| Gallocatechin-3-gallate | 1.0 | 2.1 | |||
| Quercetin glucoside III | 1.0 | 2.0 | |||
| Epicatechin-3-gallate | 0.9 | 1.9 | |||
| Caffeic acid | 0.7 | 1.4 | |||
| Ferulic acid | 0.6 | 1.3 | |||
| Coumaric acid derivative | 0.5 | 0.9 | |||
| Kaempferol glucoside II | 0.3 | 0.6 | |||
| Quercetin | 0.2 | 0.5 | |||
| Gallic acid glucoside | 0.2 | 0.4 | |||
| PR-3 | PR-6 | |||
|---|---|---|---|---|
| Colon Available Index (%) | Colon Available Index (%) | |||
| Polyphenolic Compound | IP-C1 | IP-C2 | IP-C1 | IP-C2 |
| Caffeic acid | 57.53 ± 11.25 a | 67.30 ± 2.10 a | 37.58 ± 4.09 b | 44.47 ± 1.52 b |
| p-Coumaric acid | 140.7 ± 29.0 b | 210.6 ± 13.2 a | 95.74 ± 9.99 b | 104.06 ± 14.73 b |
| Coumaric acid glucoside | 198.75 ± 2.31 b | 268.09 ± 24.94 a | 95.26 ± 6.35 a | 102.20 ± 11.03 a |
| Coumaric acid derivative | 68.90 ± 11.43 b | 52.08 ± 13.43 b | 138.36 ± 61.75 a | 40.12 ± 13.88 b |
| Ellagic acid | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Ellagic acid glucoside | 9.98 ± 4.15 b | 55.44 ± 2.05 a | 15.73 ± 4.08 b | 57.73 ± 13.34 a |
| Ferulic acid | 143.54 ± 22.05 a | 184.50 ± 5.16 a | 58.32 ± 13.66 b | 91.87 ± 25.36 b |
| Gallic acid | 176.25 ± 7.29 b | 261.66 ± 20.04 a | 91.56 ± 2.67 c | 109.52 ± 4.58 c |
| Vanillin glucoside | 32.54 ± 2.63 b | 87.09 ± 4.90 a | 42.34 ± 1.32 b | 76.67 ± 12.59 a |
| Flavanone glucoside I | 63.88 ± 18.17 b | 95.84 ± 3.31 a | 61.15 ± 9.97 b | 108.08 ± 9.65 a |
| Flavanone glucoside II | 80.20 ± 29.50 a | 113.51 ± 9.32 a | 55.13 ± 5.73 a | 71.31 ± 18.62 a |
| Flavanone glucoside IV | 115.5 ± 7.0 c | 214.1 ± 3.9 a | 108.68 ± 11.70 c | 173.01 ± 4.46 b |
| Gallocatechin gallate glucoside | 39.53 ± 8.22 a | 26.38 ± 2.67 a | 45.27 ± 6.83 a | 33.59 ± 4.49 a |
| Quercetin glucoside III | 50.283 ± 18.448 a | 36.80 ± 0.57 a | 41.51 ± 4.51 a | 17.43 ± 1.49 a |
| Kaempferol glucoside II | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Anthocyanidin | 51.8 ± 16.0 bc | 33.7 ± 2.8 c | 66.17 ± 6.26 b | 101.61 ± 3.09 a |
| Total phenolic acids | 170.25 ± 6.91 b | 253.98 ± 19.03 a | 91.15 ± 2.35 c | 108.99 ± 4.35 c |
| Total flavonols | 50.28 ± 18.448 a | 36.80 ± 0.57 a | 41.51 ± 4.51 a | 17.43 ± 1.49 a |
| Total flavanone | 105.80 ± 10.04 c | 194.96 ± 1.28 a | 91.70 ± 7.57 c | 139.57 ± 4.82 b |
| Total flavan-3-ols | 39.53 ± 8.22 a | 26.38 ± 2.67 a | 45.27 ± 6.83 a | 33.59 ± 4.49 a |
| Total anthocyanidins | 51.8 ± 16.0 bc | 33.7 ± 2.8 c | 66.17 ± 6.26 b | 101.61 ± 3.09 a |
| Total polyphenols | 131.39 ± 5.49 b | 195.78 ± 12.46 a | 88.73 ± 1.55 d | 108.74 ± 4.08 c |
| Undigested | Gastric Phase | Intestinal Phase C1 | Intestinal Phase C2 | |
|---|---|---|---|---|
| CP | 3.19 ± 0.33 d | 2.54 ± 0.48 d | 5.33 ± 0.68 b | 7.80 ± 0.50 a |
| PR-3 | 4.20 ± 0.33 c,d | 3.52 ± 0.40 c,d | 5.53 ± 0.35 b | 8.08 ± 0.88 a |
| PR-6 | 4.63 ± 0.42 b,c | 4.86 ± 0.45 b,c | 5.77 ± 0.58 b | 8.66 ± 0.85 a |
| Fatty Acids (FA), g/100 g of FA | PC | PR-3 | PR-6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Undigested | IP-C1 | IP-C2 | Undigested | IP-C1 | IP-C2 | Undigested | IP-C1 | IP-C2 | |
| C10:0 | 0.07 ± 0.00 b | 0.07 ± 0.00 b | 0.07 ± 0.00 b | 0.08 ± 0.00 a | 0.07 ± 0.00 a,b | 0.07 ± 0.01 a | 0.08 ± 0.00 a | 0.08 ± 0.00 a | 0.08 ± 0.00 a |
| C12:0 | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.09 ± 0.00 b |
| C14:0 | 1.31 ± 0.00 a | 1.30 ± 0.00 a | 1.28 ± 0.00 b | 1.33 ± 0.01 a | 1.32 ± 0.00 a | 1.30 ± 0.02 b | 1.30 ± 0.04 a | 1.30 ± 0.00 a | 1.28 ± 0.02 b |
| C15:0 | 0.07 ± 0.00 c | 0.07 ± 0.00 c | 0.07 ± 0.00 c | 0.08 ± 0.00 b | 0.08 ± 0.00 b | 0.08 ± 0.00 b | 0.09 ± 0.00 a | 0.09 ± 0.00 a | 0.08 ± 0.00 a,b |
| C16:0 | 22.59 ± 0.50 a | 22.68 ± 0.04 a | 22.40 ± 0.15 a | 23.26 ± 0.39 a | 22.59 ± 0.03 a | 22.75 ± 0.07 a | 22.62 ± 0.72 a | 22.52 ± 0.10 a | 22.53 ± 0.06 a |
| C16:1 | 2.22 ± 0.01 b | 2.18 ± 0.00 b | 2.16 ± 0.03 b | 2.47 ± 0.03 a | 2.45 ± 0.01 a | 2.43 ± 0.01 a | 2.42 ± 0.07 a | 2.42 ± 0.01 a | 2.39 ± 0.03 a |
| C17:0 | 0.39 ± 0.01 c | 0.39 ± 0.00 c | 0.38 ± 0.00 c | 0.43 ± 0.01 b | 0.42 ± 0.00 b | 0.42 ± 0.00 b | 0.46 ± 0.01 a | 0.46 ± 0.00 a | 0.46 ± 0.00 a |
| C18:0 | 11.98 ± 0.44 a | 12.24 ± 0.03 a | 11.94 ± 0.13 a | 12.12 ± 0.47 a | 11.61 ± 0.02 a | 11.71 ± 0.12 a | 11.57 ± 0.35 a | 11.58 ± 0.10 a | 11.78 ± 0.20 a |
| C18:1 | 41.16 ± 0.43 b, c | 39.82 ± 0.01 b,c | 39.68 ± 0.29 c | 42.45 ± 1.29 a,b | 40.93 ± 0.18 b,c | 42.02 ± 0.90 a,b | 42.16 ± 1.92 a | 41.06 ± 0.35 b | 41.51 ± 0.10 a,b |
| C18:2 (n 6,9) | 13.43 ± 0.36 b | 13.99 ± 0.07 a | 14.14 ± 0.28 a, b | 11.92 ± 0.15 d | 12.92 ± 0.04 c | 12.75 ± 0.24 c | 11.49 ± 0.31 d | 12.54 ± 0.09 c | 12.59 ± 0.14 c |
| C18:3 (n 3,6,9) | 0.71 ± 0.03 b | 0.79 ± 0.01 a | 0.75 ± 0.02 b | 0.64 ± 0.01 c | 0.76 ± 0.01 a, b | 0.72 ± 0.01 b | 0.63 ± 0.02 c | 0.77 ± 0.00 a, b | 0.75 ± 0.01 b |
| C20:0 | 0.16 ± 0.00 b | 0.16 ± 0.00 b | 0.17 ± 0.00 b | 0.18 ± 0.01 a | 0.17 ± 0.00 a,b | 0.18 ± 0.01 a | 0.19 ± 0.01 a | 0.18 ± 0.00 a | 0.20 ± 0.01 a |
| C20:1 | 0.92 ± 0.01 a | 0.92 ± 0.00 a | 0.91 ± 0.00 a | 0.86 ± 0.01 b | 0.89 ± 0.00 a | 0.89 ± 0.01 a,b | 0.85 ± 0.03 b | 0.88 ± 0.00 a,b | 0.90 ± 0.02 a |
| C20:2 (n 11,14) | 0.62 ± 0.01 a | 0.63 ± 0.01 a | 0.61 ± 0.00 a | 0.55 ± 0.00 c | 0.58 ± 0.00 b | 0.57 ± 0.01 b,c | 0.52 ± 0.01 c | 0.56 ± 0.01 b | 0.56 ± 0.01 b |
| C20:3 (n 8,14,17) | 0.57 ± 0.05 c | 0.82 ± 0.01 a | 0.74 ± 0.03 a | 0.52 ± 0.02 c | 0.81 ± 0.01 a | 0.71 ± 0.02 b | 0.51 ± 0.03 c | 0.83 ± 0.01 a | 0.76 ± 0.03 a |
| Others | 3.71 ± 0.24 a | 3.86 ± 0.16 a | 4.62 ± 0.74 a | 3.01 ± 0.94 a | 4.30 ± 0.27 a | 3.30 ± 0.79 a | 2.69 ± 0.99 a | 4.66 ± 0.69 a | 4.03 ± 8.03 a |
| SFA | 36.66 ± 0.97 a | 37.00 ± 0.07 a | 36.38 ± 0.30 a | 37.57 ± 0.88 a | 36.36 ± 0.06 a | 36.61 ± 0.22 a | 36.39 ± 1.14 a | 36.29 ± 0.21 a | 36.50 ± 0.29 a |
| UFA | 59.62 ± 0.91 a | 59.15 ± 0.12 a | 58.99 ± 0.67 a | 59.42 ± 1.51 a | 59.34 ± 0.25 a | 60.09 ± 1.21 a | 58.60 ± 2.38 a | 59.05 ± 0.48 a | 59.47 ± 0.34 a |
| MFA | 44.30 ± 0.45 b | 42.92 ± 0.02 c | 42.75 ± 0.33 c | 45.78 ± 1.33 a | 44.27 ± 0.19 b | 45.34 ± 0.93 b | 45.44 ± 2.02 a | 44.36 ± 0.36 b | 44.81 ± 0.15 a |
| PFA | 15.32 ± 0.46 b | 16.23 ± 0.10 a | 16.25 ± 0.34 a | 13.64 ± 0.18 c | 15.07 ± 0.06 b | 14.75 ± 0.28 b | 13.16 ± 0.37 c | 14.69 ± 0.11 b | 14.66 ± 0.19 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas-González, R.; Pérez-Álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability. Nutrients 2021, 13, 1332. https://doi.org/10.3390/nu13041332
Lucas-González R, Pérez-Álvarez JÁ, Viuda-Martos M, Fernández-López J. Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability. Nutrients. 2021; 13(4):1332. https://doi.org/10.3390/nu13041332
Chicago/Turabian StyleLucas-González, Raquel, José Ángel Pérez-Álvarez, Manuel Viuda-Martos, and Juana Fernández-López. 2021. "Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability" Nutrients 13, no. 4: 1332. https://doi.org/10.3390/nu13041332
APA StyleLucas-González, R., Pérez-Álvarez, J. Á., Viuda-Martos, M., & Fernández-López, J. (2021). Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of In Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability. Nutrients, 13(4), 1332. https://doi.org/10.3390/nu13041332