Abstract
Based on epidemiological and animal studies, the rationale for using polyunsaturated fatty acids (PUFAs) as a treatment for Attention Deficit Hyperactivity Disorder (ADHD) seems promising. Here, the objective was to systematically identify and critically assess the evidence from clinical trials. The primary outcome was ADHD core symptoms. The secondary outcomes were behavioral difficulties, quality of life, and side effects. We performed a systematic search in Medline, Embase, Cinahl, PsycInfo, and the Cochrane Library up to June 2020. The overall certainty of evidence was evaluated using Grades of Recommendation, Assessment, Development, and Evaluation (GRADE). We identified 31 relevant randomized controlled trials including 1755 patients. The results showed no effect on ADHD core symptoms rated by parents (k = 23; SMD: −0.17; 95% CI: −0.32, −0.02) or teachers (k = 10; SMD: −0.06; 95% CI: −0.31, 0.19). There was no effect on behavioral difficulties, rated by parents (k = 7; SMD: −0.02; 95% CI: −0.17, 0.14) or teachers (k = 5; SMD: −0.04; 95% CI: −0.35, 0.26). There was no effect on quality of life (SMD: 0.01; 95% CI: −0.29, 0.31). PUFA did not increase the occurrence of side effects. For now, there seems to be no benefit of PUFA in ADHD treatment; however, the certainty of evidence is questionable, and thus no conclusive guidance can be made. The protocol is registered in PROSPERO ID: CRD42020158453.
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a common neurodevelopmental disorder in children and adolescents, which may persist into adulthood. A meta-analysis of 102 prevalence studies found that the worldwide prevalence estimate for ADHD among children and adolescents under the age of 18 years is 3.4% (CI 95% 2.6–4.5) [1].
ADHD is characterized by three core symptoms, namely, inattention, hyperactivity, and impulsivity. The symptoms must be present in different settings and be both impairing and age inappropriate. ADHD is frequently comorbid with other psychiatric disorders and a substantial burden to the affected children and their families [2]. Long-term studies have revealed that a diagnosis of ADHD is associated with lower educational achievements and significant higher prevalence of, e.g., injury, substance abuse, unemployment, and delinquency [3,4,5,6,7,8].
A wide variety of treatments such as pharmacological and psychosocial interventions are used for the management of ADHD. There is some evidence for the short-term effectiveness of certain non-pharmacological interventions and pharmacological treatments [9,10,11]. However, some patients and parents are concerned with the use of pharmaceuticals for the treatment of ADHD, and initiation of pharmacological treatment may be linked with a certain degree of reluctance due to side-effects. Investigations of other interventions such as diet or nutritional supplements are therefore necessary.
Based on evidence from epidemiological studies and animal models, the rationale for using polyunsaturated fatty acids (PUFAs) as a treatment option for ADHD is promising. Studies have found that a deficiency of essential fatty acids positively correlates with ADHD symptoms [12]. Supporting the evidence that PUFAs may play a role in brain disorders, essential fatty acids have shown to regulate neurotransmitter and immune functions via the modulation of lipid rafts signaling platforms on the cell membrane in addition to having an anti-inflammatory inhibition of the free radical generation and oxidative stress [13,14,15].
The objective of this systematic review and meta-analysis was to systematically identify and critically assess the current evidence from clinical trials concerning the administration of supplementation with PUFAs for the treatment of ADHD among children and adolescents (6–18 years). Specifically, we sought to evaluate the effect of supplementation with PUFAs on ADHD core symptoms and behavioral difficulties, rated by both parent and teachers. Furthermore, we sought to investigate the impact on quality of life as well as the occurrence of side effects including diarrhea, gastrointestinal discomfort, and nausea.
The systematic review and meta-analysis were based on an update of the results from a Danish national clinical guideline published in 2018 by the Danish Health Authority.
2. Materials and Methods
Established methods recommended by the Cochrane Collaboration [16] and according to the principles described in the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) [17] approach were used to conduct this systematic review and meta-analysis. Moreover, the systematic review was structured according to Population, Intervention, Comparison, and Outcome (PICO) characterization [18]. Specifically, the PICO question was whether children and adolescents aged 6–18 years with ADHD should be offered PUFA supplementation. The definitions of population, intervention, comparator, outcomes, and study design are specified in Table 1. The research question is best addressed through a randomized study design in order to evaluate the effectiveness of the intervention so that issues regarding both measured and unmeasured confounding are minimized.

Table 1.
Population, Intervention, Comparison, and Outcome (PICO) criteria for inclusion and exclusion of studies.
The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020158453 (accepted 28 April 2020) and the systematic review was constructed in accordance to the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) statement [22,23] (PRISMA checklist is provided in the Supplementary Material).
2.1. Search Strategy
As a part of the conduction of the Danish national clinical guideline on ADHD by the Danish Health Authority, a literature search were performed by KB in September 2013 (with no restriction on date), in November 2017 (dates restricted to 2013–2017), and again in June 2020 (dates restricted to 2017–2020). The systematic literature search was performed in multiple databases including Medline, Embase, Cinahl, and PsycInfo and limited to randomized controlled trials. The original search strategy included a search for both systematic reviews and individual randomized controlled trials. The justification for the stepwise search strategy is that the Danish Health Authority prioritize to enrich existing guidelines with all available eligible literature approximately every third year. All search strategies for the 2013, 2017, and 2020 searches included medical subject heading (MeSH) and text words related to our eligibility criteria. As an example MeSH terms for the intervention were “unsaturated fatty acid” or “Diet therapy” or “diet supplementation” or “Fish oil” or “Carnitine “, or text words for the intervention ((fatty adj1 acid*) or ((Polyunsaturated or poly-unsaturated or unsaturated) adj1 (fat or fatty)) or omega-3 or omega3 or omega 3 or omega-6 or omega6 or omega 6 or (docosahexaenoic adj acid*) or (eicosapentaenoic adj acid*) or (arachidonic adj acid)) or ((fish adj1 oil*) or cod liver oil* or lax oil* or tuna oil* or carnitine or Levocarnitine or “L Carnitine” or L-carnitine or bicarnitine) or ((diet* or food or nutrition) adj1 (therapy or supplement*)) (for further details please see the example presented in Table 2 and in the search protocols provided in Supplementary Material). Both the original and updated searches were limited to literature written in English, Danish, Norwegian, and Swedish due to limitations in language proficiency in the author group. Moreover, to ensure that any relevant studies were not missed by the search, content experts from the guideline working group were conferred with, and reference lists of included articles and previous reviews were screened for potentially relevant studies. Conference abstracts were considered if data were not published elsewhere. Study authors were not contacted to identify additional studies.

Table 2.
Example of the search strategy in Embase.
2.2. Study Selection
The search results were imported to RefWorks (Refworks (online software) https://www.refworks.com, accessed on 2 March 2021), and duplicate references were removed. Subsequently, the records were imported into Covidence software (Covidence (Online software)). Covidence team https://www.covidence.org/home, accessed on 2 March 2021) was used for the screening process and data management.
The titles and abstracts of the identified references were screened by one reviewer (MLR), to assess according to the inclusion criteria (Table 1). Subsequently, the full text of potential studies was independently screened by two review authors out of a group of review authors (MLR, SL, HC, JFR, MNH) for eligibility. Disagreement was discussed or by consultation of a third reviewer (BT). Neither of the review authors were blinded to the journal titles, study authors/institutions, or year of publication.
2.3. Extraction of Individual Randomized Controlled Studies
Data extraction was conducted using a predefined template in Covidence software. The assessment of the studies included study settings, population demographics and baseline characteristics, details on intervention and control conditions, study design, outcome, and time of measurement. Two review authors out of a group of review authors (MLR, SL, HC, EB, JFR, MNH) independently extracted data in duplicate. Discrepancies were resolved through discussion in the review team.
Data for analysis were also extracted in Covidence (Online software: https://www.covidence.org/home, accessed on 2 March 2021) and afterwards exported to Review Manager (version 5.2) (Review Manager (RevMan) (Computer program)) (2014). None of the study authors were contacted by email to provide additional information to resolve uncertainties or to obtain missing data. If multiple reports of a single study were identified, the publication with most complete data was included.
2.4. Quality Assessment
Quality assessment of the evidence was evaluated using GRADE method [24]. There are four possible ratings of the quality: high, moderate, low, and very low. Downgrading was carried out for each outcome using the standard definition of risk of bias, inconsistency, indirectness, imprecision, and publication. The overall quality of evidence was based on the lowest quality of the primary outcome and reflected the extent to which we are confident that the effect estimates are correct.
Two review authors out of a group of review authors (MLR, SL, HC, EB, JFR, MNH) independently assessed risk of bias of the primary studies by using the Cochrane risk of bias tool [20] that includes the following characteristics: randomization sequence generation, reatment allocation concealment, blinding of patients and personnel, blinding of outcome assessors, completeness of outcome data, selective outcome reporting, other sources of bias. Discrepancies were resolved through discussion.
2.5. Meta-Analysis
For a dichotomous outcome, the relative risk (RR) was calculated, including the 95% confidence interval (CI). When few or zero events occurred in both the intervention and control group, a risk difference (RD) analysis was performed as sensitivity analysis. For a continuous outcome, since different measurement scales were used, we calculated effect size using the standardized mean difference (SMD), including the 95% confidence interval. Random effect models were used to calculate pooled estimates of effects. Furthermore, a funnel plot was produced to assess the publication bias across the studies, when more than 10 studies were included. Statistical heterogeneity was quantified using I2 statistic [25], with an I2 value greater than of 50% considered to be substantial heterogeneity.
Review Manager Software (version 5.3) (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used to produce the analyses and forest plots.
3. Results
3.1. Literature Search
In the 2013 search, one systematic review article was found [9], from which 12 randomized trials were identified among the included studies [26,27,28,29,30,31,32,33,34,35,36,37]. These were supplemented by 11 randomized trials from an updated literature search in 2017 [38,39,40,41,42,43,44,45,46,47,48] and a further eight randomized studies from the latest updated search in 2020 [49,50,51,52,53,54,55,56].
Thus, the evidence base consists of a total of 31 randomized trials.
A flowchart of included secondary and primary studies is presented in Figure 1; Figure 2, and a list of excluded studies after full-text screening including reasons for their exclusion is presented in Supplementary Material.
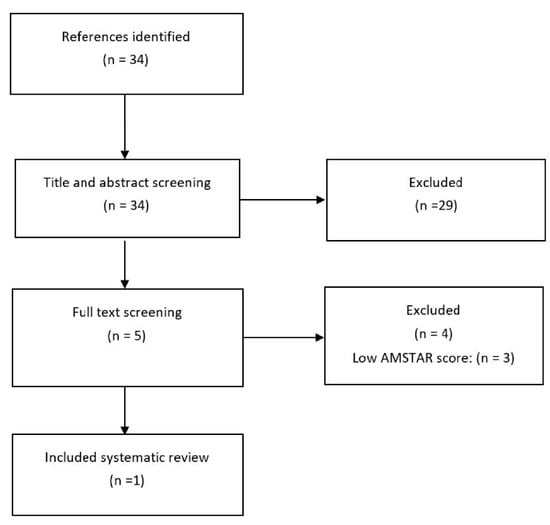
Figure 1.
Flowchart of the search for systematic reviews in 2013.
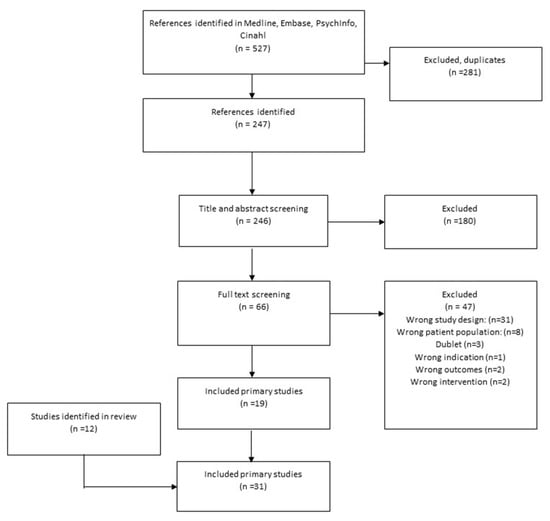
Figure 2.
Flowchart of the search for primary studies in 2017 and 2020.
3.2. Review of the Evidence
The populations in the included studies consisted of children with ADHD in the age group ranging from 6–18 years. The interventions consisted of supplements with polyunsaturated fatty acids with either omega 3, omega 6, or combined supplements with both types of fatty acids. The interventions lasted between 8 weeks to 12 months. In some studies [38,49,55], the children were also in medical treatment in both the intervention group and the placebo group, whereby fatty acid treatment was investigated as an active add-on treatment in the intervention group. Information on study identification, baseline characteristics, intervention, control, reported outcomes, and the authors’ conclusion is presented in Supplementary Material.
3.2.1. Primary Outcomes
The primary outcomes were reported in 24 studies [29,32,34,35,37,38,39,40,41,42,44,45,46,47,48,49,50,51,52,53,54,55,56] and were based on information from 1755 participants. The results showed no clinically relevant effect was found on the primary outcome parent-rated ADHD core symptoms (SMD: −0.17; 95% CI: −0.32, −0.02) (Figure 3), corresponding to a mean difference on the Parent ADHD rating scale of −1.85 (95% CI: −3.49, −0.22) calculated from the endpoint SD in the control group of Cornu et al., 2018 [51]. The Parent ADHD rating scale ranges from 0 to 54, and thus the result is equivalent to a decrease of 3.4% on the scale (MCID is estimated to a decrease of 30% [19,20,21]), nor was there any effect found on teacher-rated ADHD core symptoms (k = 10; SMD: −0.06; 95% CI: −0.31, 0.19) [27,29,32,33,35,41,45,50,53] (Figure 4).
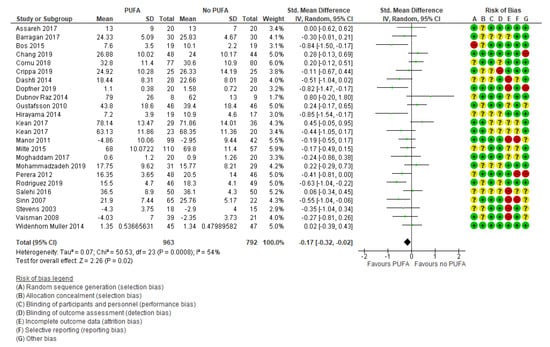
Figure 3.
Forest plot of comparison: PUFA vs. placebo, outcome: parent-reported core symptoms (end of treatment). Green square indicates summary estimates of the individual studies. Black diamond indicates total summary effect estimate.
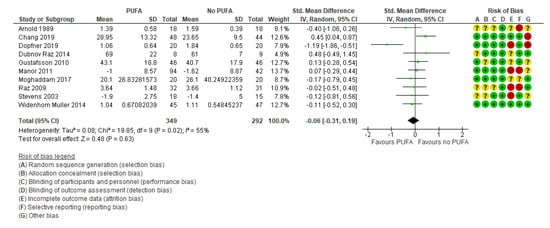
Figure 4.
Forest plot of comparison: polyunsaturated fatty acids (PUFA) vs. placebo, outcome: teacher-reported core symptoms (end of treatment). Green square indicates summary estimates of the individual studies. Black diamond indicates total summary effect estimate.
3.2.2. Secondary Outcomes
There was no clinically significant effect on behavioral difficulties, neither when the outcomes were rated by parents (k = 7; SMD: −0.02; 95% CI: −0.17, 0.14) [29,32,35,44,50,52,54] (Figure 5) nor by teachers (k = 5; SMD: −0.04; 95% CI: −0.35, 0.26) [29,32,35,39,50] (Figure 6).
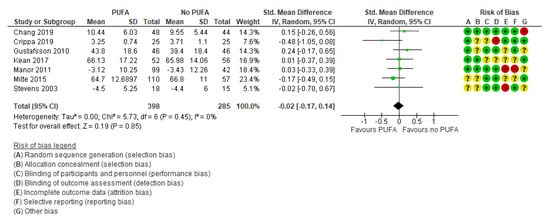
Figure 5.
Forest plot of comparison: PUFA vs. placebo, outcome: parent-reported behavioral difficulties (end of treatment). Green square indicates summary estimates of the individual studies. Black diamond indicates total summary effect estimate.
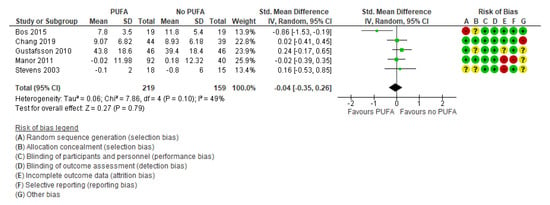
Figure 6.
Forest plot of comparison: PUFA vs. placebo, outcome: teacher-reported behavioral difficulties (end of treatment). Green square indicates summary estimates of the individual studies. Black diamond indicates total summary effect estimate.
Only two studies [32,52] examined the effect of the intervention on quality of life, with no significant effect (SMD: 0.01; 95% CI: −0.29, 0.31) (Figure 7).
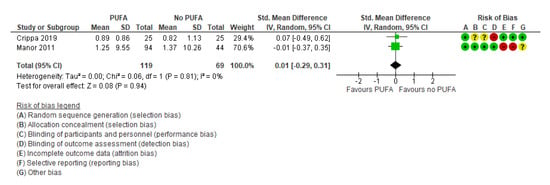
Figure 7.
Forest plot of comparison: PUFA vs. placebo, outcome: quality of life (longest follow-up time (minimum 3 months after end of treatment)). Green square indicates summary estimates of the individual studies. Black diamond indicates total summary effect estimate.
Additionally, there were no differences between the intervention group compared to the placebo group regarding the three selected side effects: diarrhea (k = 5, RR: 1.08; 95% CI: 0.32, 3.63 and RD: −0.00; 95% CI: −0.04, 0.03) [29,42,49,50,55], gastrointestinal discomfort (k = 4; RR: 0.72; 95% CI: 0.27, 1.88 and RD: −0.01; 95% CI: −0.04, 0.03) [42,43,44,55], and nausea (k = 6, RR: 0.99; 95% CI: 0.41, 2.38 and RD: 0.01; 95% CI: −0.02, 0.03) [29,32,33,42,43,49,55] (Figure 8).
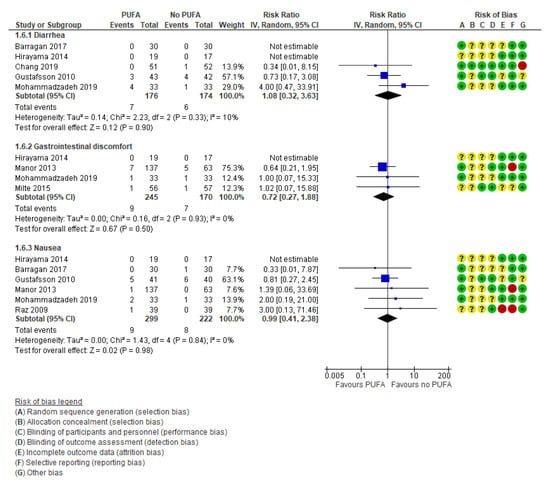
Figure 8.
Forest plot of comparison: PUFA vs. placebo, outcome: side effects (end of treatment). Blue square indicates summary estimates of the individual studies. Black diamond indicates total summary effect estimate.
3.3. Certainty of Evidence (GRADE)
The certainty of the evidence was overall low to very low, as there were problems with inaccurate effect estimates in small studies (very serious risk of imprecision) as well as serious risk of bias. The risk of bias was primarily due to problems with blinding of the observers that assessed the effect (parent and teachers). However, the studies generally suffered from methodological flaws and were largely poorly described, especially regarding how the randomization sequence was generated, how the allocation was concealed, and how incomplete data were handled.
4. Discussion
The objective of this systematic literature review and meta-analysis was to provide clinicians, caregivers and guideline panels with an updated overview and critical assessment of the evidence, investigating the effect of PUFA among children aged 6 to 18 years diagnosed with ADHD. Based on a substantial body of evidence, there is no indication that supplementation with PUFA has a positive effect on core symptoms of ADHD, behavioral difficulties, or quality of life. The present review suggests that there is no substantial increase in side effects following the use of PUFA, including the occurrence of diarrhea, gastrointestinal discomfort, or nausea.
Based on our findings, there is insufficient evidence to support patients, parents, clinicians, and caregivers in their decision on whether to use PUFA as a supplementation in the treatment of ADHD. Consequently, the patient preferences are expected to be unambiguous, in the sense that some parents will prefer dietary changes rather than pharmacological treatment, and other parents will find it difficult and relatively intrusive to implement dietary changes in children and adolescents. Effective treatment with PUFA would supposedly depend on the presence of an initial PUFA deficiency observed in the patient at baseline. A significant decrease in PUFA levels has indeed been observed in patients with ADHD as compared to healthy controls [57]. However, it still needs to be investigated what role PUFA plays in the pathology of ADHD. It remains unknown whether PUFA deficiency represents a neuropathological finding directly potentiating symptom outbreak, or rather a compensatory mechanism due to long-standing disease manifestation. In addition, an evaluation of the long-term effects of providing PUFA as a supplement is needed. For now, the effect has only been tested in patients over a time period ranging from 8 weeks to 12 months.
The amount of RCTs investigating the use of PUFA as a treatment for ADHD has increased since the latest Cochrane review by Gilles et al. was published in 2012 [58]. Despite an increase in the number of available studies, the conclusion and quality of the evidence remains unchanged. Thus, the evidence still indicates an inability of PUFA to effectively alter the symptomatology of ADHD. The evidence remains of a low to very low quality, thus reflecting a high degree of uncertainty of the effect estimates.
The risk of bias in the identified studies includes an inadequate random sequence generation and allocation concealment, which in RCTs is mandatory to ensure that intervention and control groups are kept as identical as possible. Thus, the inability to perform sufficient random allocation may induce systematic errors, which may have a considerable impact on the final results. Other major sources of bias were due to incomplete outcome reporting, thus reflecting an increased risk of reporting bias and attrition. Regarding incomplete data, a review has previously described in which problems with drop-out seem to be common in n-3 long chain PUFA supplementation trials in children and adolescents in general [59]. This indicates that dropouts may be a common, inherent issue when seeking to investigate the effect of PUFA in a research setting.
Blinding of the observers evaluating the effect is essential. The included studies generally displayed an unclear level of blinding, as blinding in the majority of studies was inadequately described. Blinding of participants may once again constitute an inherent problem when investigating the effect of PUFA due to the distinct smell and taste [60]. This may unmask the allocation to the respective groups, as patients and parents over time may become aware if they indeed are receiving the active PUFA component. Nevertheless, adequate blinding of observers not directly related to the child should be possible, including the researcher and teachers. Collectively, these problems lead to a high risk of bias, which may essentially have an impact on the results and thereby lead to a distortion in the final conclusion.
Our findings displaying an inadequate effect of PUFA in the treatment of ADHD is in line with many previous reviews on the subject [57,61,62,63,64,65], but not all [66,67,68,69].
When comparing our review to others reporting a beneficial effect, it becomes evident that there is a discrepancy in the methodology used to evaluate treatment effects, which may explain the discrepancy in results. In three of the reviews [66,67,69], no specific analysis to obtain pooled estimate of effects was performed, as results from the individual trials were only narratively described. This prevents a direct comparison between trials, including an assessment of the overall magnitude of effect. Common for all reviews reporting a positive effect was a lack of quality assessment of the included studies. This hinders an evaluation of the extent of trust in the data and may essentially mask some issues that otherwise would lead to a downscaling of the certainty in the evidence, and thereby the confidence in the results. It should be mentioned, apart from ADHD core symptoms, the reviews reporting a positive effect also include other outcomes not evaluated in the present review. The inclusion and exclusion criteria furthermore varied across the reviews. This may in part also explain some of the discrepancy in the reported results.
As mentioned above, several inherent methodological issues due indeed exist when it comes to investigating the effect of PUFA in research settings. Nevertheless, this does not unconditionally prevent the possibility of performing high-quality research on the matter. As such, future research should focus on conducting clinical trials of high-quality, following the CONSORT (Consolidated Standards of Reporting Trials) Statement [70]. This is essential if we wish to move forward and be able to conclusively evaluate the role of PUFA in the treatment of ADHD.
Strengths and Limitations of the Current Review
In order to ensure high methodological quality, this systematic review and meta-analyses followed the guidelines of the Cochrane Collaboration and PRISMA as well as pre-registering a protocol at PROSPERO. Moreover, a comprehensive search and duplicate full-text study selection, data extraction, and quality assessment were used. Amongst the limitations, we acknowledge that since the search was limited/restricted to English and Scandinavian languages, there might be relevant studies unidentified. Moreover, grey literature was not searched, and thus relevant studies may have been unidentified. Furthermore, the authors of the included studies were not contacted for further information, and the results are merely based on data published in peer-reviewed articles. The review authors were not blinded in the process of selecting literature.
5. Conclusions
Based on the current low to very low evidence, there seems to be no benefit of providing PUFA supplements to children and adolescents with ADHD concerning parent- or teacher-rated core symptoms, behavioral difficulties, or quality of life. Concerns on adverse effects of PUFA supplementation is limited. Conclusive guidance for patients, parent and clinical practice cannot be made due to the many limitations inherent to the included studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13041226/s1, Table S1: the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) checklist, Table S2: Search protocol, Table S3: list of excluded studies, Table S4: Study identification of the included randomized controlled trials, Table S5: The baseline characteristic of the included RCTs, Table S6: The intervention, control, reported outcomes, and authors’ conclusion of the included RCTs.
Author Contributions
Conceptualization, M.N.H., J.F.R., M.L.R., E.B., K.B., B.T., S.L., H.E.C.; methodology, M.N.H., J.F.R., M.L.R., E.B., K.B., B.T., S.L., H.E.C.; software, not applicable; validation, not applicable; formal analysis, H.E.C.; investigation, M.N.H., J.F.R., M.L.R., E.B., K.B., B.T., S.L., H.E.C.; resources, M.N.H., J.F.R., M.L.R., E.B., K.B., B.T., S.L., H.E.C.; data curation, not applicable; writing—original draft preparation, M.N.H., H.E.C. with contribution from J.F.R., M.L.R., E.B., K.B., B.T., S.L.; writing—review and editing, M.N.H., J.F.R., M.L.R., E.B., K.B., B.T., S.L., H.E.C.; visualization, M.N.H., H.E.C.; supervision, not applicable; project administration, M.N.H., H.E.C.; funding acquisition, M.N.H., J.F.R., M.L.R., E.B., K.B., B.T., S.L., H.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
The study was initiated and financed by the Danish Health Authority. The Parker Institute at Bispebjerg and Frederiksberg Hospital was supported by a core grant from the Oak Foundation (OCAY-13-309).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available at the Danish Health Authority website (www.sst.dk), accessed on 4 April 2021.
Acknowledgments
The study was initiated and financed by the Danish Health Authority. The Parker Institute at Bispebjerg and Frederiksberg Hospital was supported by a core grant from the Oak Foundation (OCAY-13-309). The authors would like to thank the work and reference group, as well as the secretary of the “National Clinical Guideline for the Treatment of ADHD in Children and Adolescents”, The Danish Health Authority.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef]
- Barkley, R.A.; Fischer, M. Hyperactive Child Syndrome and Estimated Life Expectancy at Young Adult Follow-Up: The Role of ADHD Persistence and Other Potential Predictors. J. Atten. Disord. 2019, 23, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Mohr-Jensen, C.; Steinhausen, H.C. A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations. Clin. Psychol. Rev. 2016, 48, 32–42. [Google Scholar] [CrossRef]
- Dalsgaard, S.; Leckman, J.F.; Mortensen, P.B.; Nielsen, H.S.; Simonsen, M. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: A prospective cohort study. Lancet Psychiatry 2015, 2, 702–709. [Google Scholar] [CrossRef]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef]
- Galera, C.; Melchior, M.; Chastang, J.F.; Bouvard, M.P.; Fombonne, E. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: The GAZEL Youth study. Psychol. Med. 2009, 39, 1895–1906. [Google Scholar] [CrossRef]
- Erskine, H.E.; Norman, R.E.; Ferrari, A.J.; Chan, G.C.; Copeland, W.E.; Whiteford, H.A.; Scott, J.G. Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 841–850. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Brandeis, D.; Cortese, S.; Daley, D.; Ferrin, M.; Holtmann, M.; Stevenson, J.; Danckaerts, M.; Van der Oord, S.; Döpfner, M.; et al. Nonpharmacological interventions for ADHD: Systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am. J. Psychiatry 2013, 170, 275–289. [Google Scholar] [CrossRef]
- De, C.F.; Cortese, S.; Adamo, N.; Janiri, L. Pharmacological and non-pharmacological treatment of adults with ADHD: A meta-review. Evid. Based Ment. Health 2017, 20, 4–11. [Google Scholar]
- Storebø, O.J.; Krogh, H.B.; Ramstad, E.; Moreira-Maia, C.R.; Holmskov, M.; Skoog, M.; Nilausen, T.D.; Magnusson, F.L.; Zwi, M.; Gillies, D.; et al. Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 2015, 351, h5203. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.-C.; Jingling, L.; Huang, Y.-T.; Lu, Y.-J.; Su, K.-P. Delay Aversion, Temporal Processing, and N-3 Fatty Acids Intake in Children with Attention-Deficit/Hyperactivity Disorder (ADHD). Clin. Psychol. Sci. 2016, 4, 1094–1103. [Google Scholar] [CrossRef]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. Fatty acids and immune function: New insights into mechanisms. Br. J. Nutr. 2007, 98 (Suppl. 1), S41–S45. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential fatty acids in health and chronic diseases. Forum Nutr. 2003, 56, 67–70. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Assessing risk of bias in cross-over trials. In Cochrane Handbook of Systematic Reviews of Interventions; The Cochrane Collaboration Version 5.1.0; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Schunemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Atkins, D.; Brozek, J.; Vist, G.; Alderson, P.; Glasziou, P.; Falck-Ytter, Y.; Schünemann, H.J. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 2011, 64, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Guanfacine Hydrochloride Extended Release (Intuniv XR) Tablets: For the Treatment of Attention-Deficit/Hyperactivity Disorder [Internet]: Appendix 5: Validity of Outcome Measures; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2015. [Google Scholar]
- Zhang, S.; Faries, D.E.; Vowles, M.; Michelson, D. ADHD Rating Scale IV: Psychometric properties from a multinational study as a clinician-administered instrument. Int. J. Methods Psychiatr. Res. 2005, 14, 186–201. [Google Scholar] [CrossRef]
- Buitelaar, J.K.; Montgomery, S.A.; van Zwieten-Boot, B.J. Attention deficit hyperactivity disorder: Guidelines for investigating efficacy of pharmacological intervention. Eur. Neuropsychopharmacol. 2003, 13, 297–304. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.G.; Mitchell, E.A.; Turbott, S.H. The effects of essential fatty acid supplementation by Efamol in hyperactive children. J. Abnorm. Child Psychol. 1987, 15, 75–90. [Google Scholar] [CrossRef]
- Arnold, L.E.; Kleykamp, D.; Votolato, N.A.; Taylor, W.A.; Kontras, S.B.; Tobin, K. Gamma-linolenic acid for attention-deficit hyperactivity disorder: Placebo-controlled comparison to D-amphetamine. Biol. Psychiatry 1989, 25, 222–228. [Google Scholar] [CrossRef]
- Bélanger, S.A.; Vanasse, M.; Spahis, S.; Sylvestre, M.P.; Lippé, S.; l’Heureux, F.; Ghadirian, P.; Vanasse, C.M.; Levy, E. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr. Child Health 2009, 14, 89–98. [Google Scholar] [CrossRef]
- Gustafsson, P.A.; Birberg-Thornberg, U.; Duchén, K.; Landgren, M.; Malmberg, K.; Pelling, H.; Strandvik, B.; Karlsson, T. EPA supplementationn improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010, 99, 1540–1549. [Google Scholar] [CrossRef]
- Johnson, M.; Ostlund, S.; Fransson, G.; Kadesjo, B.; Gillberg, C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: A randomized placebo-controlled trial in children and adolescents. J. Atten. Disord. 2009, 12, 394–401. [Google Scholar] [CrossRef]
- Hirayama, S.; Hamazaki, T.; Terasawa, K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder—a placebo-controlled double-blind study. Eur. J. Clin. Nutr. 2004, 58, 467–473. [Google Scholar] [CrossRef]
- Manor, I.; Magen, A.; Keidar, D.; Rosen, S.; Tasker, H.; Cohen, T.; Richter, Y.; Zaaroor-Regev, D.; Manor, Y.; Weizman, A. The effect of phosphatidylserine containing Omega3 fatty-acids on attention-deficit hyperactivity disorder symptoms in children: A double-blind placebo-controlled trial, followed by an open-label extension. Eur. Psychiatry 2012, 27, 335–342. [Google Scholar] [CrossRef]
- Raz, R.; Carasso, R.L.; Yehuda, S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: A double-blind placebo-controlled study. J. Child Adolesc. Psychopharmacol. 2009, 19, 167–177. [Google Scholar] [CrossRef]
- Sinn, N.; Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J. Dev. Behav. Pediatr. 2007, 28, 82–91. [Google Scholar] [CrossRef]
- Stevens, L.; Zhang, W.; Peck, L.; Kuczek, T.; Grevstad, N.; Mahon, A.; Zentall, S.S.; Eugene Arnold, L.; Burgess, J.R. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids 2003, 38, 1007–1021. [Google Scholar] [CrossRef]
- Voigt, R.G.; Llorente, A.M.; Jensen, C.L.; Fraley, J.K.; Berretta, M.C.; Heird, W.C. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2001, 139, 189–196. [Google Scholar] [CrossRef]
- Vaisman, N.; Kaysar, N.; Zaruk-Adasha, Y.; Pelled, D.; Brichon, G.; Zwingelstein, G.; Bodennec, J. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: Effect of dietary n-3 fatty acids containing phospholipids. Am. J. Clin. Nutr. 2008, 87, 1170–1180. [Google Scholar] [CrossRef]
- Assareh, M.; Davari Ashtiani, R.; Khademi, M.; Jazayeri, S.; Rai, A.; Nikoo, M. Efficacy of Polyunsaturated Fatty Acids (PUFA) in the Treatment of Attention Deficit Hyperactivity Disorder. J. Atten. Disord. 2017, 21, 78–85. [Google Scholar] [CrossRef]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.; Demmelmair, H.; Koletzko, B.; Eilander, A.; Hoeksma, M.; Durston, S. Reduced Symptoms of Inattention after Dietary Omega-3 Fatty Acid Supplementation in Boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef]
- Dashti, N.; Hekmat, H.; Soltani, H.R.; Rahimdel, A.; Javaherchian, M. Comparison of therapeutic effects of omega-3 and methylphenidate (ritalin®) in treating children with attention deficit hyperactivity disorder. Iran. J. Psychiatry Behav. Sci. 2014, 8, 7. [Google Scholar]
- Dubnov-Raz, G.; Khoury, Z.; Wright, I.; Raz, R.; Berger, I. The effect of alpha-linolenic acid supplementation on ADHD symptoms in children: A randomized controlled double-blind study. Front. Hum. Neurosci. 2014, 8, 780. [Google Scholar] [CrossRef]
- Hirayama, S.; Terasawa, K.; Rabeler, R.; Hirayama, T.; Inoue, T.; Tatsumi, Y.; Purpura, M.; Jäger, R. The effect of phosphatidylserine administration on memory and symptoms of attention-deficit hyperactivity disorder: A randomised, double-blind, placebo-controlled clinical trial. J. Hum. Nutr. Diet. 2014, 27, 284–291. [Google Scholar] [CrossRef]
- Manor, I.; Magen, A.; Keidar, D.; Rosen, S.; Tasker, H.; Cohen, T.; Richter, Y.; Zaaroor-Regev, D.; Manor, Y.; Weizman, A. Safety of phosphatidylserine containing omega3 fatty acids in ADHD children: A double-blind placebo-controlled trial followed by an open-label extension. Eur. Psychiatry 2013, 28, 386–391. [Google Scholar] [CrossRef]
- Milte, C.M.; Parletta, N.; Buckley, J.D.; Coates, A.M.; Young, R.M.; Howe, P.R.C. Increased Erythrocyte Eicosapentaenoic Acid and Docosahexaenoic Acid Are Associated with Improved Attention and Behavior in Children With ADHD in a Randomized Controlled Three-Way Crossover Trial. J. Atten. Disord. 2015, 19, 954–964. [Google Scholar] [CrossRef]
- Moghaddam, M.F.; Shamekhi, M.; Rakhshani, T. Effectiveness of methylphenidate and PUFA for the treatment of patients with ADHD: A double-blinded randomized clinical trial. Electron. Physician 2017, 9, 4412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salehi, B.; Mohammadbeigi, A.; Sheykholeslam, H.; Moshiri, E.; Dorreh, F. Omega-3 and Zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J. Res. Pharm. Pract. 2016, 5, 22. [Google Scholar]
- Widenhorn-Muller, K.; Schwanda, S.; Scholz, E.; Spitzer, M.; Bode, H. Effect of supplementation with long-chain omega-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Perera, H.; Jeewandara, K.C.; Seneviratne, S.; Guruge, C. Combined omega3 and omega6 supplementation in children with attention-deficit hyperactivity disorder (ADHD) refractory to methylphenidate treatment: A double-blind, placebo-controlled study. J. Child Neurol. 2012, 27, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Barragan, E.; Breuer, D.; Dopfner, M. Efficacy and Safety of Omega-3/6 Fatty Acids, Methylphenidate, and a Combined Treatment in Children with ADHD. J. Atten. Disord. 2017, 21, 433–441. [Google Scholar] [CrossRef]
- Chang, J.P.C.; Su, K.P.; Mondelli, V.; Satyanarayanan, S.K.; Yang, H.T.; Chiang, Y.J.; Chen, H.T.; Pariante, C.M. High-dose eicosapentaenoic acid (EPA) improves attention and vigilance in children and adolescents with attention deficit hyperactivity disorder (ADHD) and low endogenous EPA levels. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cornu, C.; Mercier, C.; Ginhoux, T.; Masson, S.; Mouchet, J.; Nony, P.; Kassai, B.; Laudy, V.; Berquin, P.; Franc, N.; et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur. Child Adolesc. Psychiatry 2018, 27, 377–384. [Google Scholar] [CrossRef]
- Crippa, A.; Tesei, A.; Sangiorgio, F.; Salandi, A.; Trabattoni, S.; Grazioli, S.; Agostoni, C.; Molteni, M.; Nobile, M. Behavioral and cognitive effects of docosahexaenoic acid in drug-naive children with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Eur. Child Adolesc. Psychiatry 2019, 28, 571–583. [Google Scholar] [CrossRef]
- Dopfner, M.; Dose, C.; Breuer, D.; Heintz, S.; Schiffhauer, S.; Banaschewski, T. Efficacy of Omega-3/Omega-6 Fatty Acids in Preschool Children at Risk of ADHD: A Randomized Placebo-Controlled Trial. J. Atten. Disord. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.D.; Sarris, J.; Scholey, A.; Silberstein, R.; Downey, L.A.; Stough, C. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524 R) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomised, double-blind, placebo-controlled trial. Psychopharmacology 2017, 234, 403–420. [Google Scholar] [PubMed]
- Mohammadzadeh, S.; Baghi, N.; Yousefi, F.; Yousefzamani, B. Effect of omega-3 plus methylphenidate as an alternative therapy to reduce attention deficit-hyperactivity disorder in children. Korean J. Pediatr. 2019, 62, 360. [Google Scholar] [CrossRef]
- Rodriguez, C.; Garcia, T.; Areces, D.; Fernandez, E.; Garcia-Noriega, M.; Domingo, J.C. Supplementation with high-content docosahexaenoic acid triglyceride in attention-deficit hyperactivity disorder: A randomized double-blind placebo-controlled trial. Neuropsychiatr. Dis. Treat. 2019, 15, 1193. [Google Scholar] [CrossRef]
- Tesei, A.; Crippa, A.; Ceccarelli, S.B.; Mauri, M.; Molteni, M.; Agostoni, C.; Nobile, M. The potential relevance of docosahexaenoic acid and eicosapentaenoic acid to the etiopathogenesis of childhood neuropsychiatric disorders. Eur. Child Adolesc. Psychiatry 2017, 26, 1011–1030. [Google Scholar] [CrossRef]
- Gillies, D.; Sinn, J.K.; Lad, S.S.; Leach, M.J.; Ross, M.J. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. 2012, 10, CD007986. [Google Scholar] [CrossRef]
- van der Wurff, I.S.M.; Meyer, B.J.; de Groot, R.H.M. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (n-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients 2020, 12, 3115. [Google Scholar] [CrossRef]
- Schachter, H.; Kourad, K.; Meraki, Z.; Lumb, A.; Tran, K.; Miguelez, M. Effects of Omega-3 FattyAcids on Mental Health. Evidence Report/TechnologyAssessment No 116 (Publication No 05-E022-2); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2005. [Google Scholar]
- Pelsser, L.M.; Frankena, K.; Toorman, J.; Rodrigues, P.R. Diet and ADHD, Reviewing the Evidence: A Systematic Review of Meta-Analyses of Double-Blind Placebo-Controlled Trials Evaluating the Efficacy of Diet Interventions on the Behavior of Children with ADHD. PLoS ONE 2017, 12, e0169277. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Lange, K.M.; Makulska-Gertruda, E.; Nakamura, Y.; Reissmann, A.; Sakaue, Y.; Takano, T.; Takeuchi, Y. The Role of Nutritional Supplements in the Treatment of ADHD: What the Evidence Says. Curr. Psychiatry Rep. 2017, 19, 8. [Google Scholar] [CrossRef]
- Catalá-López, F.; Hutton, B.; Núñez-Beltrán, A.; Page, M.J.; Ridao, M.; Macías Saint-Gerons, D.; Catalá, M.A.; Tabarés-Seisdedos, R.; Moher, D. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS ONE 2017, 12, e0180355. [Google Scholar] [CrossRef] [PubMed]
- Banaschewski, T.; Belsham, B.; Bloch, M.H.; Ferrin, M.; Johnson, M.; Kustow, J.; Robinson, S.; Zuddas, A. Supplementation with polyunsaturated fatty acids (PUFAs) in the management of attention deficit hyperactivity disorder (ADHD). Nutr. Health 2018, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Perez Carmona, M.P. Complementary/alternative medicine in adolescents with attention deficit hyperactivity disorder and mood disorders. Rev. Chil. Pediatr. 2017, 88, 294–299. [Google Scholar]
- Derbyshire, E. Do Omega-3/6 Fatty Acids Have a Therapeutic Role in Children and Young People with ADHD? J. Lipids 2017, 2017, 6285218. [Google Scholar] [CrossRef]
- Chang, J.P.; Su, K.P. Nutritional Neuroscience as Mainstream of Psychiatry: The Evidence- Based Treatment Guidelines for Using Omega-3 Fatty Acids as a New Treatment for Psychiatric Disorders in Children and Adolescents. Clin. Psychopharmacol. Neurosci. 2020, 18, 469–483. [Google Scholar] [CrossRef]
- Chang, J.P.; Su, K.P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Heilskov Rytter, M.J.; Andersen, L.B.B.; Houmann, T.; Bilenberg, N.; Hvolby, A.; Mølgaard, C.; Michaelsen, K.F.; Lauritzen, L. Diet in the treatment of ADHD in children—a systematic review of the literature. Nord. J. Psychiatry 2015, 69, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, MR000030. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).