Heterogeneity of Associations between Total and Types of Fish Intake and the Incidence of Type 2 Diabetes: Federated Meta-Analysis of 28 Prospective Studies Including 956,122 Participants
Abstract
1. Introduction
2. Materials and Methods
2.1. Populations
Dietary Assessment
2.2. Ascertainment of Incident Type 2 Diabetes
2.3. Potential Confounding Factors and Other Covariates
2.4. Statistical Analyses
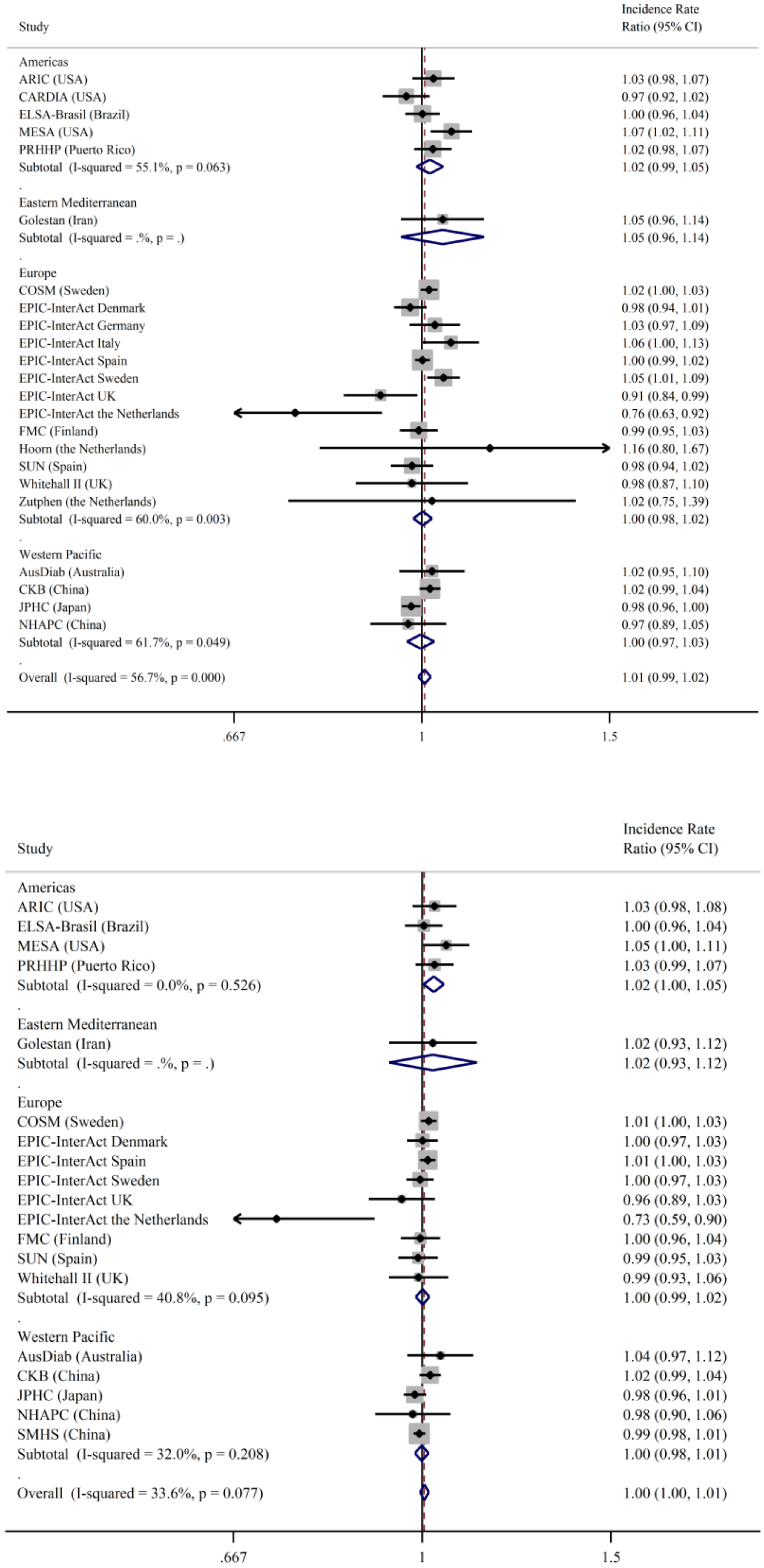
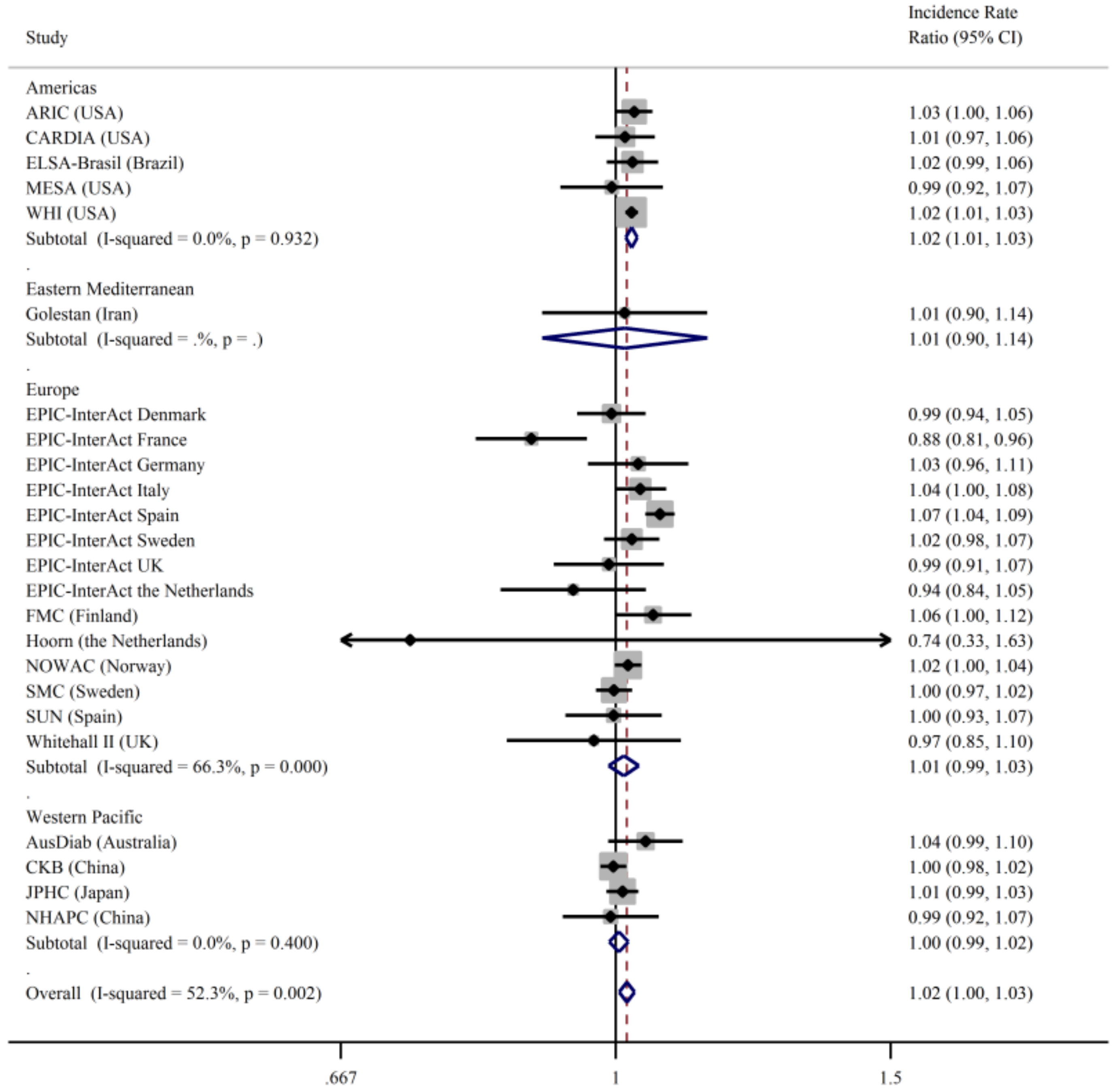
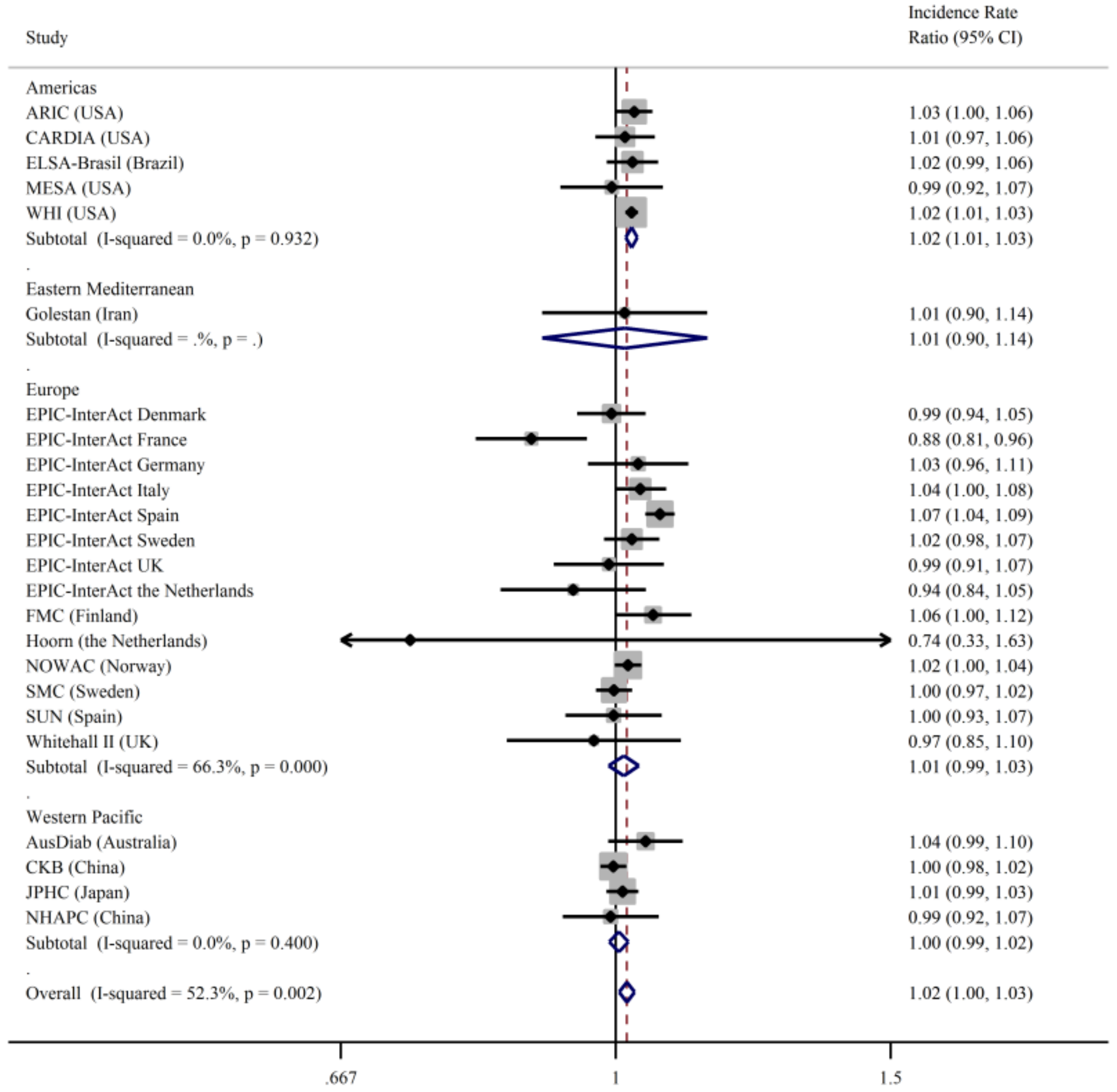
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Orsini, N.; Forouhi, N.G.; Wolk, A. Fish consumption in relation to myocardial infarction, stroke and mortality among women and men with type 2 diabetes: A prospective cohort study. Clin. Nutr. 2018, 37, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Nowlin, S.Y.; Hammer, M.J.; D’Eramo Melkus, G. Diet, inflammation, and glycemic control in type 2 diabetes: An integrative review of the literature. J. Nutr. Metab. 2012, 2012, 542698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tian, C.; Jia, C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: A meta-analysis of prospective studies. Br. J. Nutr. 2012, 108, 408–417. [Google Scholar] [CrossRef]
- Zheng, J.S.; Huang, T.; Yang, J.; Fu, Y.Q.; Li, D. Marine N-3 polyunsaturated fatty acids are inversely associated with risk of type 2 diabetes in Asians: A systematic review and meta-analysis. PLoS ONE 2012, 7, e44525. [Google Scholar] [CrossRef]
- Xun, P.; He, K. Fish Consumption and Incidence of Diabetes: Meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care 2012, 35, 930–938. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Micha, R.; Imamura, F.; Pan, A.; Biggs, M.L.; Ajaz, O.; Djousse, L.; Hu, F.B.; Mozaffarian, D. Omega-3 fatty acids and incident type 2 diabetes: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107 (Suppl. S2), S214–S227. [Google Scholar] [CrossRef]
- Wallin, A.; Di Giuseppe, D.; Orsini, N.; Patel, P.S.; Forouhi, N.G.; Wolk, A. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes: Systematic review and meta-analysis of prospective studies. Diabetes Care 2012, 35, 918–929. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Fan, M.; Cui, S.; Li, L. Meat and fish intake and type 2 diabetes: Dose-response meta-analysis of prospective cohort studies. Diabetes Metab. 2020. [Google Scholar] [CrossRef]
- Kaushik, M.; Mozaffarian, D.; Spiegelman, D.; Manson, J.E.; Willett, W.C.; Hu, F.B. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009, 90, 613–620. [Google Scholar] [CrossRef]
- Djousse, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am. J. Clin. Nutr. 2011, 93, 143–150. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.B.; Elasy, T.; Li, H.L.; Yang, G.; Cai, H.; Ye, F.; Fao, Y.T.; Shyr, Y.; Zheng, W.; et al. Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am. J. Clin. Nutr. 2011, 94, 543–551. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Matsushita, Y.; Poudel-Tandukar, K.; Kato, M.; Oba, S.; Inoue, M.; Tsugane, S.; et al. Fish intake and type 2 diabetes in Japanese men and women: The Japan Public Health Center-based Prospective Study. Am. J. Clin. Nutr. 2011, 94, 884–891. [Google Scholar] [CrossRef]
- Du, H.; on behalf of the China Kadoorie Biobank Collaborative Group; Guo, Y.; Bennett, D.A.; Bragg, F.; Bian, Z.; Chadni, M.; Yu, C.; Chen, Y.; Tan, Y.; et al. Red meat, poultry and fish consumption and risk of diabetes: A 9 year prospective cohort study of the China Kadoorie Biobank. Diabetology 2020, 63, 767–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, P.; Mao, L.; Chen, X.; Wang, J.; Cheng, L.; Ding, G.; Jiao, J. Current level of fish and omega-3 fatty acid intakes and risk of Type 2 diabetes in China. J. Nutr. Biochem. 2019, 74, 108249. [Google Scholar] [CrossRef]
- Patel, P.S.; Forouhi, N.G.; Kuijsten, A.; Schulze, M.B.; van Woudenbergh, G.J.; Ardanaz, E.; Amiano, P.; Arriola, L.; Balkau, B.; Barricarte, A.; et al. The prospective association between total and type of fish intake and type 2 diabetes in 8 European countries: EPIC-InterAct Study. Am. J. Clin. Nutr. 2012, 95, 1445–1453. [Google Scholar]
- Montonen, J.; Jarvinen, R.; Heliovaara, M.; Reunanen, A.; Aromaa, A.; Knekt, P. Food consumption and the incidence of type II diabetes mellitus. Eur. J. Clin. Nutr. 2005, 59, 441–448. [Google Scholar] [CrossRef]
- Van Woudenbergh, G.J.; van Ballegooijen, A.J.; Kuijsten, A.; Sijbrands, E.J.; van Rooij, F.J.; Geleijnse, J.M.; Hofman, A.; Witteman, J.C.M.; Feskens, E.J.M. Eating fish and risk of type 2 diabetes: A population-based, prospective follow-up study. Diabetes Care 2009, 32, 2021–2026. [Google Scholar] [CrossRef]
- Rylander, C.; Sandanger, T.M.; Engeset, D.; Lund, E. Consumption of lean fish reduces the risk of type 2 diabetes mellitus: A prospective population based cohort study of Norwegian women. PLoS ONE 2014, 9, e89845. [Google Scholar] [CrossRef]
- Patel, P.S.; Sharp, S.J.; Luben, R.N.; Khaw, K.T.; Bingham, S.A.; Wareham, N.J.; Forouhi, N.G. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: The European prospective investigation of cancer (EPIC)-Norfolk cohort study. Diabetes Care 2009, 32, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Di Giuseppe, D.; Orsini, N.; Akesson, A.; Forouhi, N.G.; Wolk, A. Fish consumption and frying of fish in relation to type 2 diabetes incidence: A prospective cohort study of Swedish men. Eur. J. Nutr. 2017, 56, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Sheehan, N.A.; Masca, N.; Wallace, S.E.; Murtagh, M.J.; Burton, P.R. DataSHIELD—Shared individual-level analysis without sharing the data: A biostatistical perspective. Norsk Epidemiol. 2012, 21, 231–239. [Google Scholar] [CrossRef]
- InterConnect. Gobal data for diabetes and obesity research. Available online: http://www.interconnect-diabetes.eu/ (accessed on 5 April 2021).
- DataShield. Available online: www.datashield.ac.uk (accessed on 5 April 2021).
- Michels, K.B.; Giovannucci, E.; Joshipura, K.J.; Rosner, B.A.; Stampfer, M.J.; Fuchs, C.S.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J. Natl. Cancer Inst. 2000, 92, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Onland-Moret, N.C.; van der A, D.L.; van der Schouw, Y.T.; Buschers, W.; Elias, S.G.; van Gils, C.H.; Koerselman, J.; Roest, M.; Grobbee, D.E.; Peeters, P.H. Analysis of case-cohort data: A comparison of different methods. J. Clin. Epidemiol. 2007, 60, 350–355. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition of regional groupings. Available online: https://www.who.int/healthinfo/global_burden_disease/definition_regions/en/ (accessed on 5 April 2021).
- The InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: The EPIC-InterAct study. Diabetologia 2013, 56, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Johansson, G.; Wikman, A.; Ahren, A.M.; Hallmans, G.; Johansson, I. Underreporting of energy intake in repeated 24-hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living. Public Health Nutr. 2001, 4, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Asbeck, I.; Mast, M.; Bierwag, A.; Westenhofer, J.; Acheson, K.J.; Muller, M.J. Severe underreporting of energy intake in normal weight subjects: Use of an appropriate standard and relation to restrained eating. Public Health Nutr. 2000, 5, 683–690. [Google Scholar] [CrossRef]
- Lafay, L.; Mennen, L.; Basdevant, A.; Charles, M.A.; Borys, J.M.; Eschwege, E.; Romon, M. Does energy intake underreporting involve all kinds of food or only specific food items? Results from the Fleurbaix Laventie Ville Sante (FLVS) study. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1500–1506. [Google Scholar] [CrossRef]
- Aminov, Z.; Haase, R.; Rej, R.; Schymura, M.J.; Santiago-Rivera, A.; Morse, G.; DeCaprio, A.; Carpenter, D.O.; Akweksasne Task Force on the Environment. Diabetes prevalence in relation to serum concentrations of polychlorinated biphenyl (PCB) congener groups and three chlorinated pesticides in a Native American population. Environ. Health Perspect. 2016, 124, 1376–1383. [Google Scholar] [CrossRef]
- He, K.; Xun, P.; Liu, K.; Morris, S.; Reis, J.; Guallar, E. Mercury exposure in young adulthood and incidence of diabetes later in life. Diabetes Care 2013, 36, 1584. [Google Scholar] [CrossRef]
- Taylor, K.W.; Novak, R.F.; Anderson, H.A.; Birnbaum, L.S.; Blystone, C.; DeVito, M.; Jacobs, D.; Köhrle, J.; Lee, D.H.; Rylander, L.; et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: A national toxicology program workshop review. Environ. Health Perspect. 2013, 121, 774–783. [Google Scholar] [CrossRef]
- Turyk, M.; Fantuzzi, G.; Persky, V.; Freels, S.; Lambertino, A.; Pini, M.; Rhodes, D.H.; Anderson, H.A. Persistent organic pollutants and biomarkers of diabetes risk in a cohort of Great Lakes sport caught fish consumers. Environ. Res. 2015, 140, 335–344. [Google Scholar] [CrossRef]
- Wu, H.; Bertrand, K.A.; Choi, A.L.; Hu, F.B.; Laden, F.; Grandjean, P.; Sun, Q. Persistent organic pollutants and type 2 diabetes: A prospective analysis in the Nurses’ Health Study and meta-analysis. Environ. Health Perspect. 2013, 121, 153–161. [Google Scholar] [CrossRef]
- Marushka, L.; Hu, X.; Batal, M.; Sadik, T.; Schwartz, H.; Ing, A.; Fediuk, K.; Tikhonov, C.; Chan, H.M. The relationship between persistent organic pollutants exposure and type 2 diabetes among First Nations in Ontario and Manitoba, Canada: A difference in difference Analysis. Int. J. Environ. Res. Public Health 2018, 15, 539. [Google Scholar] [CrossRef]
- Rylander, L.; Rignell-Hydbom, A.; Hagmar, L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ. Health 2005, 4, 28. [Google Scholar] [CrossRef]
- Silverstone, A.E.; Rosenbaum, P.F.; Weinstock, R.S.; Bartell, S.M.; Foushee, H.R.; Shelton, C.; Pavuk, M. Polychlorinated biphenyl (PCB) exposure and diabetes: Results from the Anniston Community Health Survey. Environ. Health Perspect. 2012, 120, 727–732. [Google Scholar] [CrossRef]
- Vasiliu, O.; Cameron, L.; Gardiner, J.; DeGuire, P.; Karmaus, W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology 2006, 17, 352–359. [Google Scholar] [CrossRef]
- Wang, S.L.; Tsai, P.C.; Yang, C.Y.; Leon Guo, Y. Increased risk of diabetes and polychlorinated biphenyls and dioxins: A 24-year follow-up study of the Youcheng cohort. Diabetes Care 2008, 31, 1574. [Google Scholar] [CrossRef]
- LaKind, J.S.; Berlin, C.M.; Sjodin, A.; Turner, W.; Wang, R.Y.; Needham, L.L.; Paul, I.M.; Stokes, J.L.; Naiman, D.Q.; Patterson, D.G., Jr. Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environ. Health Perspect. 2009, 117, 1625–1631. [Google Scholar] [CrossRef]
- Salihovi, S.; Lampa, E.; Lindström, G.; LInd, L.; Lind, P.M.; van Bavel, B. Circulating levels of persistent organic pollutants (POPs) among elderly men and women from Sweden: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Environ. Int. 2012, 44, 59–67. [Google Scholar] [CrossRef]
- UN Environment Programme. The 12 initial POPs under the Stockholm Copnvention. Available online: http://chm.pops.int/TheConvention/ThePOPs/The12InitialPOPs/tabid/296/Default.aspx (accessed on 5 April 2021).
- Wang, W.; Ye, S.; Qian, L.; Xing, X. Sex-Specific Association of Serum 25-Hydroxyvitamin D3 with Insulin Resistance in Chinese Han Patients with Newly Diagnosed Type 2 Diabetes Mellitus. J. Nutr. Sci. Vitaminol. 2018, 64, 173–178. [Google Scholar] [CrossRef]
- Esteghamati, A.; Aryan, Z.; Esteghamati, A.; Nakhjavani, M. Vitamin D deficiency is associated with insulin resistance in nondiabetics and reduced insulin production in type 2 diabetics. Horm. Metab. Res. 2015, 47, 273–279. [Google Scholar] [CrossRef]
- Sanghera, D.K.; Sapkota, B.R.; Aston, C.E.; Blackett, P.R. Vitamin D Status, Gender Differences, and Cardiometabolic Health Disparities. Ann. Nutr. Metab. 2017, 70, 79–87. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Hofer, D.; Munzker, J.; Schwetz, V.; Ulbing, M.; Hutz, K.; Stiegler, P.; Zigeuner, R.; Pieber, T.R.; Muller, H.; Obermay-er-Pietsch, B. Testicular synthesis and vitamin D action. J. Clin. Endocrinol. Metab. 2014, 99, 3766–3773. [Google Scholar] [CrossRef]
- Chang, E.M.; Kim, Y.S.; Won, H.J.; Yoon, T.K.; Lee, W.S. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J. Clin. Endocrinol. Metab. 2014, 99, 2526–2532. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; Busch, A.S.; et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim. Biophys. Acta 2009, 1790, 1453–1462. [Google Scholar] [CrossRef]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Chiang, C.H.; Yao, C.A.; Huang, K.C. Gender Differences with Dose-Response Relationship between Serum Selenium Levels and Metabolic Syndrome-A Case-Control Study. Nutrients 2019, 11, 477. [Google Scholar] [CrossRef]
- Fisk, H.; Irvine, M.; Miles, E.; Lietz, G.; Mathers, J.; Packard, C.; Armah, C.K.; Kofler, B.M.; Curtis, P.J.; Minihane, A.M.; et al. Association of oily fish intake, sex, age, BMI and APOE genotype with plasma long-chain n-3 fatty acid composition. Br. J. Nutr. 2018, 120, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Gooren, L.J.; Toorians, A.W.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.; Romeu-Nadal, M.; Burdge, G.; Calder, P. Gender differences in n-3 fatty acids content of tissues. Proc. Nutr. Soc. 2008, 67, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L.; PUFAH Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 366, l4697. [Google Scholar] [CrossRef]
| Cohort (Country) | Total (N) | Women (%) | New Type 2 Diabetes Cases (n) Primary | New Type 2 Diabetes Cases (n) Secondary | Mean (SD) Age (Years) | Median (IQR) Follow-Up Time (Years) | Mean (SD) BMI (kg/m2) |
|---|---|---|---|---|---|---|---|
| Americas | |||||||
| ARIC (US) | 9654 | 56 | 723 | 2003 | 53.7 (5.6) | 11.8 (8.8, 23.6) | 27.1 (4.9) |
| ELSA Brasil (Brazil) | 11,351 | 57 | 338 | 957 | 51.6 (8.9) | 3.8 (3.4, 4.0) | 26.7 (4.5) |
| CARDIA (US) | 3920 | 59 | 198 | 198 | 24.9 (3.5) | 25.0 (19.0, 25.0) | 24.3 (4.7) |
| MESA (US) | 4669 | 54 | 228 | 674 | 61.4 (10.1) | 4.0 (4.0, 5.0) | 27.9 (5.2) |
| PRHHP (Puerto Rico) | 6977 | 0 | 310 | 825 | 54.1 (6.5) | 5.0 (5.0, 5.0) | 24.9 (3.8) |
| WHI (US) | 86,296 | 100 | 10,233 | 10,233 | 63.6 (7.3) | 11.8 (7.8, 13.6) | 27.1 (5.6) |
| Eastern Mediterranean | |||||||
| Golestan (Iran) | 9932 | 52 | 532 | 1148 | 51.2 (7.8) | 4.2 (3.6, 5.6) | 26.7 (5.2) |
| Europe | |||||||
| EPIC-InterAct Denmark | 3896 | 44 | 1970 | 1970 | 56.9 (4.4) | 10.3 (6.3, 11.6) | 27.3 (4.5) |
| EPIC-InterAct France | 795 | 100 | 257 | 257 | 56.9 (6.5) | 9.2 (7.2, 10.5) | 24.5 (4.6) |
| EPIC-InterAct Germany | 3448 | 51 | 1505 | 1505 | 52.4 (8.3) | 9.5 (4.8, 11.2) | 27.6 (4.8) |
| EPIC-InterAct Italy | 3112 | 65 | 1271 | 1271 | 51.4 (7.7) | 10.8 (6.8, 12.9) | 27.3 (4.8) |
| EPIC-InterAct the Netherlands | 2067 | 83 | 741 | 741 | 54.1 (10.0) | 11.1 (6.4, 12.6) | 26.6 (4.5) |
| EPIC-InterAct Spain | 5584 | 57 | 2354 | 2354 | 50.3 (7.8) | 12.4 (8.9, 13.6) | 29.3 (4.5) |
| EPIC-InterAct Sweden | 3439 | 55 | 1574 | 1574 | 58.4 (7.4) | 12.0 (9.3, 13.6) | 26.8 (4.4) |
| EPIC-InterAct UK | 1858 | 53 | 608 | 608 | 58.3 (10.5) | 10.5 (6.3, 12.2) | 26.9 (4.4) |
| FMC Health Examination (Finland) | 9057 | 49 | 481 | 481 | 39.0 (15.5) | 24.2 (22.5, 25.7) | 24.7 (4.1) |
| Hoorn (the Netherlands) | 1206 | 54 | 16 | 93 | 60.0 (6.7) | 6.4 (6.1, 6.7) | 26.1 (3.1) |
| NOWAC (Norway) | 34,547 | 100 | 560 | 672 | 49.8 (5.8) | 6.0 (6.0, 7.0) | 22.4 (3.5) |
| COSM and SMC (Sweden) | 54,571 | 46 | 5339 | 5432 | 59.9 (9.0) | 18.0 (18.0, 18.0) | 25.2 (3.4) |
| SUN (Spain) | 19,261 | 60 | 142 | 142 | 37.6 (12.0) | 10.1 (5.9, 12.6) | 23.5 (3.5) |
| Whitehall II (UK) | 4554 | 29 | 368 | 632 | 49.7 (5.9) | 16.1 (15.4, 16.5) | 25.2 (3.6) |
| Zutphen Elderly (the Netherlands) | 475 | 0 | 11 | 62 | 70.9 (4.7) | 10.1 (5.3, 10.3) | 25.6 (2.8) |
| Western Pacific | |||||||
| AusDiab (Australia) | 6017 | 56 | 184 | 363 | 49.9 (12.3) | 11.7 (5.1, 12.2) | 27.5 (4.6) |
| CKB (China) | 482,588 | 59 | 9601 | 9601 | 51.1 (10.6) | 7.2 (6.3, 8.1) | 23.5 (3.3) |
| JPHC (Japan) | 50,054 | 55 | 801 | 801 | 56.1 (7.6) | 5.0 (5.0, 5.0) | 23.4 (2.9) |
| NHAPC (China) | 932 | 57 | 178 | 225 | 58.3 (6.0) | 6.0 (6.0, 6.0) | 24.5 (3.3) |
| SMHS (China) | 61,250 | 0 | 2976 | 2.976 | 55.3 (9.7) | 5.6 (5.0, 6.0) | 23.7 (3.0) |
| SWHS (China) | 74,710 | 100 | 4585 | 4585 | 52.6 (9.0) | 10.2 (9.2, 10.8) | 24.0 (3.4) |
| Cohort (Country) | Total Fish | Fatty Fish | Lean Fish | Seafood | Fried Fish g | Salted, Dried Smoked, Fish | Saltwater Fish | Freshwater Fish |
|---|---|---|---|---|---|---|---|---|
| Americas | ||||||||
| ARIC (US) | 26.9 (18.1, 48.5) | 1.9 (1.9, 7.7) | 7.7 (1.9, 16.4) | 1.8 (1.8, 7.6) | NA | NA | NA | NA |
| ELSA-Brasil (Brazil) | 33.0 (18.0, 58.0) | NA | NA | 0.0 (0.0, 3.0) | 0.0 (0.0, 12.0) | NA | NA | NA |
| CARDIA (US) | 34.4 (9.2, 80.5) | 0.0 (0.0, 0.0) | 19.0 (0.0, 46.0) | 3.5 (0.0, 23.0) | 0.0 (0.0, 0.0) | NA | NA | NA |
| MESA (US) | 24.3 (11.7, 47.2) | 3.5 (0.0, 9.2) | 3.5 (0.0, 9.2) | 1.7 (0.0, 4.6) | 3.5 (0.0, 9.2) | NA | NA | NA |
| PRHHP (Puerto Rico) | 0.0 (0.0, 0.0) | NA | NA | 0 (0, 0) | NA | NA | NA | NA |
| WHI (US) | 23.0 (11.8, 40.8) | 0.0 (0.0, 5.9) | 3.9 (0.0, 9.2) | 0.0 (0.0, 5.9) | 0.0 (0.0, 3.9) | NA | NA | NA |
| Eastern Mediterranean | ||||||||
| Golestan (Iran) | 3.7 (0.8, 10.2) | 0.0 (0.0, 1.6) | 2.2 (0.1, 7.4) | NA | 0.0 (0.0, 3.1) | 0.0 (0.0, 0.0) | 3.0 (0.6, 8.9) | 0.0 (0.0, 0.0) |
| Europe | ||||||||
| EPIC-InterAct Denmark | 36.6 (26.0, 57.3) | 11.7 (6.9, 18.5) | 15.4 (9.9, 23.4) | 1.7 (0.9, 4.2) | 6.1 (3.0, 12.9) | NA | NA | NA |
| EPIC-InterAct France | 30.9 (18.6, 47.2) | 8.9 (4.0, 16.2) | 10.2 (0.0, 20.1) | 0.0 (0.0, 4.6) | 0.0 (0.0, 0.0) | NA | NA | NA |
| EPIC-InterAct Germany | 17.2 (9.0, 29.0) | 0.0 (0.0, 1.2) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 4.2 (1.6, 6.9) | NA | NA | NA |
| EPIC-InterAct Italy | 25.3 (14.2, 40.8) | 8.1 (3.7, 14.9) | 5.1 (1.2, 12.2) | 2.8 (0.9, 6.8) | 0.0 (0.0, 0.0) | NA | NA | NA |
| EPIC-InterAct the Netherlands | 8.4 (3.4, 16.1) | 1.4 (0.6, 3.6) | 1.5 (0.5, 3.3) | 0.7 (0.3, 1.7) | 3.0 (1.0, 6.6) | NA | NA | NA |
| EPIC-InterAct Spain | 56.5 (35.2, 85.5) | 11.3 (2.8, 24.4) | 25.7 (10.2, 49.1) | 3.6 (0.0, 8.6) | 0.0 (0.0, 0.0) | NA | NA | NA |
| EPIC-InterAct Sweden | 36.7 (19.2, 57.6) | 2.3 (0.0, 16.3) | 0.0 (0.0, 16.6) | 1.7 (0.0, 6.1) | 2.4 (0.0, 8.3) | NA | NA | NA |
| EPIC-InterAct UK | 31.6 (18.1, 45.7) | 8.1 (0.0, 16.1) | 17.9 (8.1, 26.2) | 0.0 (0.0, 4.2) | 0.0 (0.0, 12) | NA | NA | NA |
| FMC (Finland) | 19.0 (9.0, 35.0) | 6.3 (2.0, 15.0) | 7.0 (2.0, 15.0) | 0.0 (0.0, 0.0) | 4.5 (0.6, 9.3) | 4.0 (0.7, 11.0) | 5.5 (1.7, 12.8) | 7.0 (2.0, 17.0) |
| Hoorn (the Netherlands) | 12.0 (1.0, 25.0) | 1.0 (0.0, 8.0) | 3.5 (0.0, 10.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | NA | NA | NA |
| NOWAC (Norway) | 86.1 (57.2, 123.5) | 11.4 (4.8, 21.4) | 23.6 (10.9, 40.7) | 3.5 (0.0, 3.5) | NA | NA | NA | NA |
| COSM and SMC (Sweden) | 29.0 (20.0, 41.0) | 8.0 (6.0, 15.0) | 10.0 (8.0, 25.0) | 4.0 (3.0, 5.0) | 12.3 (4.1, 16.4) | NA | NA | NA |
| SUN (Spain) | 85.7 (56.9, 128.6) | 21.4 (10.0, 64.3) | 31.4 (21.4, 74.3) | 16.7 (10.0, 20.7) | NA | 0.0 (0.0, 3.3) | NA | NA |
| Whitehall II (UK) | 35.0 (17.5, 52.5) | 8.7 (0.0, 17.5) | 17.5 (8.7, 26.2) | 0.0 (0.0, 8.7) | 0.0 (0.0, 8.7) | NA | NA | NA |
| Zutphen Elderly (the Netherlands) | 13.0 (0.0, 29.0) | 0.0 (0.0, 8.0) | 8.0 (0.0, 20.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 13.0) | 0.0 (0.0, 3.0) | 13.0 (0.0, 29.0) | 0.0 (0.0, 0.0) |
| Western Pacific | ||||||||
| AusDiab (Australia) | 25.3 (13.7, 44.0) | NA | NA | NA | 3.3 (1.5, 10.3) | NA | NA | NA |
| CKB (China) | 8.2 (1.2, 32.8) | NA | NA | NA | NA | NA | NA | NA |
| JPHC (Japan) | 79.1 (50.0, 121.2) | 27.0 (15.3, 48.9) | 8.0 (0.0, 20.0) | 10.7 (7.0, 18.3) | NA | 11.7 (4.4, 25.0) | NA | 40.1 (24.0, 65.6) |
| NHAPC (China) | 41.0 (20.7, 69.8) | NA | NA | 5.5 (1.9, 15.9) | NA | 1.4 (0.5, 3.6) | 9.8 (3.3, 21.4) | 14.3 (6.6, 28.6) |
| SMHS (China) | 38.4 (21.0, 66.1) | NA | NA | 11.5 (2.8, 15.0) | NA | NA | 21.5 (6.0, 26.2) | 16.5 (3.8, 21.0) |
| SWHS (China) | 8.9 (1.4, 35.7) | NA | NA | 10.0 (2.3, 12.5) | NA | NA | 20.4 (3.6, 26.2) | 17.5 (4.2, 21.0) |
| Cohort (Country) | Fatty Fish | Lean Fish | Seafood | Fried Fish | Salted, Dried Smoked, Fish | Saltwater Fish | Freshwater Fish |
|---|---|---|---|---|---|---|---|
| Americas | |||||||
| ARIC (US) | 1.04 (0.94, 1.16) | 1.08 (0.99, 1.18) | 1.00 (0.84, 1.17) | ||||
| ELSA-Brasil (Brazil) | 0.94 (0.74, 1.19) | 1.04 (0.92, 1.17) | |||||
| CARDIA (US) | |||||||
| MESA (US) | 0.86 (0.66, 1.12) | 0.97 (0.80, 1.18) | 0.98 (0.90, 1.08) | 1.05 (0.85, 1.28) | |||
| PRHHP (Puerto Rico) | 1.07 (0.98, 1.16) | ||||||
| WHI (US) | |||||||
| Eastern Mediterranean | |||||||
| Golestan (Iran) | 1.01 (0.82, 1.25) | 1.03 (0.91, 1.16) | 1.08 (0.95, 1.22) | 2.54 (0.04, 1.66) | 1.02 (0.91, 1.14) | 1.75 (0.84, 1.38) | |
| Europe | |||||||
| COSM (Sweden) | 1.01 (0.98, 1.04) | 1.01 (0.99, 1.04) | 1.08 (1.01, 1.16) | 1.04 (1.00, 1.08) | |||
| EPIC-InterAct Denmark | 0.99 (0.91, 1.07) | 1.01 (0.91, 1.08) | 1.02 (0.84, 1.24) | 0.99 (0.90, 1.08) | |||
| EPIC-InterAct France | |||||||
| EPIC-InterAct Germany | 0.93 (0.83, 1.05) | ||||||
| EPIC-InterAct Italy | |||||||
| EPIC-InterAct the Netherlands | 0.72 (0.41, 1.28) | 0.21 (0.08, 0.56) | 0.22 (0.05, 1.11) | 0.47 (0.29, 0.75) | |||
| EPIC-InterAct Spain | 1.00 (0.97, 1.04) | 1.01 (0.99, 1.04) | 1.03 (0.94, 1.12) | 1.01 (0.79, 1.30) | |||
| EPIC-InterAct Sweden | 0.98 (0.93, 1.03) | 1.10 (1.03, 1.17) | 1.33 (1.16, 1.53) | 0.95 (0.88, 1.03) | |||
| EPIC-InterAct UK | 0.93 (0.81, 1.06) | 0.91 (0.80, 1.03) | 1.26 (0.84, 1.89) | 1.07 (0.81, 1.43) | |||
| FMC (Finland) | 0.95 (0.87, 1.05) | 1.03 (0.97, 1.09) | 1.02 (0.93, 1.11) | 0.94 (0.84, 1.04) | 0.95 (0.84, 1.06) | 1.01 (0.96, 1.07) | |
| Hoorn (the Netherlands) | |||||||
| NOWAC (Norway) | |||||||
| SUN (Spain) | 0.97 (0.88, 1.07) | 0.90 (0.93, 1.06) | 0.97 (0.82, 1.15) | 1.28 (0.50, 3.29) | |||
| Whitehall II (UK) | 0.99 (0.89, 1.11) | 1.00 (0.89, 1.12) | 0.98 (0.78, 1.22) | 1.10 (0.94, 1.44) | |||
| Zutphen Elderly (the Netherlands) | |||||||
| Western Pacific | |||||||
| AusDiab (Australia) | 1.08 (0.90, 1.3) | ||||||
| CKB (China) | |||||||
| JPHC (Japan) | 0.96 (0.92, 1.01) | 0.91 (0.83, 1.00) | 0.97 (0.87, 1.07) | 1.01 (0.97, 1.06) | 0.96 (0.92, 1.00) | ||
| NHAPC (China) | 0.86 (0.65, 1.12) | 1.05 (0.92, 1.20) | 0.95 (0.76, 1.17) | 0.94 (0.77, 1.14) | |||
| SMHS (China) | 0.97 (0.93, 1.01) | 1.00 (0.98, 1.02) | 0.99 (0.97, 1.02) | ||||
| Overall IRR | 0.99 (0.98, 1.01) | 1.01 (0.99, 1.04) | 1.02 (0.97, 1.08) | 1.01 (0.97, 1.06) | 1.02 (0.98, 1.06) | 1.00 (0.98, 1.01) | 0.99 (0.97, 1.01) |
| Heterogeneity | I2 = 0% | I2 = 55% | I2 = 56% | I2 = 41% | I2 = 0% | I2 = 0% | I2 = 0% |
| Cohort (Country) | Fatty Fish | Lean Fish | Seafood | Fried Fish | Salted, Dried Smoked, Fish | Saltwater Fish | Freshwater Fish |
|---|---|---|---|---|---|---|---|
| Americas | |||||||
| ARIC (US) | 1.01 (0.91, 1.12) | 1.04 (0.98, 1.10) | 0.95 (0.77, 1.17) | ||||
| ELSA-Brasil (Brazil) | 1.09 (0.89, 1.34) | 0.99 (0.84, 1.16) | |||||
| CARDIA (US) | 1.01 (0.92, 1.10) | ||||||
| MESA (US) | 0.98 (0.77, 1.24) | 1.06 (0.83, 1.35) | 1,07 (0.81, 1.41) | 0.83 (0.57, 1.22) | |||
| PRHHP (Puerto Rico) | |||||||
| WHI (US) | 1.03 (1.00, 1.06) | 1.00 (0.98, 1.03) | 1.03 (0.99, 1.07) | 1.14 (1.11, 1.17) | |||
| Eastern Mediterranean | |||||||
| Golestan (Iran) | 0.88 (0.52, 1.47) | 1.02 (0.89, 1.17) | 1.08 (0.94, 1.25) | 1.29 (0.02, 93.6) | 0.97 (0.83, 1.13) | 1.17 (0.91, 1.52) | |
| Europe | |||||||
| EPIC-InterAct Denmark | 0.93 (0.84, 1.02) | 1.10 (1.01, 1.19) | 1.50 (1.15, 1.96) | 0.95 (0.84, 1.08) | |||
| EPIC-InterAct France | 1.01 (0.84, 1.22) | 1.00 (0.87, 1.14) | 0.46 (0.36, 0.58) | ||||
| EPIC-InterAct Germany | 1.09 (0.91, 1.30) | ||||||
| EPIC-InterAct Italy | 1.14 (0.98, 1.32) | 1.46 (0.95, 2.24) | |||||
| EPIC-InterAct the Netherlands | 1.32 (0.98, 1.78) | 0.62 (0.39, 1.00) | 1.78 (0.66, 4.81) | 0.79 (0.62, 1.00) | |||
| EPIC-InterAct Spain | 1.13 (1.08, 1.19) | 1.06 (1.03, 1.09) | 1.18 (0.99, 1.26) | 0.79 (0.53, 1.18) | |||
| EPIC-InterAct Sweden | 0.98 (0.92, 1.04) | 0.98 (0.90, 1.06) | 1.28 (1.09, 1.50) | 1.00 (0.88, 1.13) | |||
| EPIC-InterAct UK | 1.07 (0.94, 1.22) | 0.99 (0.89, 1.10) | 1.45 (0.90, 2.32) | 1.05 (0.77, 1.44) | |||
| FMC (Finland) | 1.06 (0.94, 1.20) | 1.09 (1.00, 1.19) | 1.09 (0.98, 1.21) | 1.09 (0.95, 1.26) | 0.99 (0.84, 1.15) | 1.11 (1.03, 1.19) | |
| Hoorn (the Netherlands) | |||||||
| NOWAC (Norway) | 1.04 (0.98, 1.12) | 1.00 (0.96, 1.05) | 1.09 (0.78, 1.52) | ||||
| SMC (Sweden) | 1.01 (0.95, 1.07) | 1.01 (0.98, 1.04) | 1.06 (0.96, 1.18) | 1.03 (0.98, 1.08) | |||
| SUN (Spain) | 1.12 (1.00, 1.24) | 0.94 (0.81, 1.10) | 1.06 (0.79, 1.43) | 0.41 (0.03, 4.76) | |||
| Whitehall II (UK) | 1.03 (0.88, 1.19) | 1.03 (0.87, 1.22) | 0.97 (0.74, 1.27) | 1.06 (0.67, 1.67) | |||
| Zutphen Elderly (the Netherlands) | |||||||
| Western Pacific | |||||||
| AusDiab (Australia) | 1.11 (0.97, 1.27) | ||||||
| CKB (China) | |||||||
| JPHC (Japan) | 1.04 (0.99, 1.10) | 1.04 (0.93, 1.16) | 1.05 (0.94, 1.16) | 1.02 (0.96, 1.09) | 1.04 (0.99, 1.08) | ||
| NHAPC (China) | 1.00 (0.81, 1.24) | 0.92 (0.57, 1.49) | 0.97 (0.82, 1.15) | 0.99 (0.86, 1.14) | |||
| SWHS (China) | 1.02 (0.98, 1.05) | 1.00 (0.98, 1.02) | 1.01 (0.99, 1.03) | ||||
| Overall IRR | 1.04 (1.01, 1.07) | 1.02 (1.00, 1.04) | 1.04 (0.98, 1.11) | 1.04 (0.98, 1.10) | 1.03 (0.97, 1.10) | 1.00 (0.98, 1.02) | 1.04 (1.00, 1.08) |
| Heterogeneity | I2 = 46% | I2 = 33% | I2 = 74% | I2 = 64% | I2 = 0% | I2 = 0% | I2 = 47% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorino, S.; Bishop, T.; Sharp, S.J.; Pearce, M.; Akbaraly, T.; Barbieri, N.B.; Bes-Rastrollo, M.; Beulens, J.W.J.; Chen, Z.; Du, H.; et al. Heterogeneity of Associations between Total and Types of Fish Intake and the Incidence of Type 2 Diabetes: Federated Meta-Analysis of 28 Prospective Studies Including 956,122 Participants. Nutrients 2021, 13, 1223. https://doi.org/10.3390/nu13041223
Pastorino S, Bishop T, Sharp SJ, Pearce M, Akbaraly T, Barbieri NB, Bes-Rastrollo M, Beulens JWJ, Chen Z, Du H, et al. Heterogeneity of Associations between Total and Types of Fish Intake and the Incidence of Type 2 Diabetes: Federated Meta-Analysis of 28 Prospective Studies Including 956,122 Participants. Nutrients. 2021; 13(4):1223. https://doi.org/10.3390/nu13041223
Chicago/Turabian StylePastorino, Silvia, Tom Bishop, Stephen J. Sharp, Matthew Pearce, Tasnime Akbaraly, Natalia B. Barbieri, Maira Bes-Rastrollo, Joline W. J. Beulens, Zhengming Chen, Huaidong Du, and et al. 2021. "Heterogeneity of Associations between Total and Types of Fish Intake and the Incidence of Type 2 Diabetes: Federated Meta-Analysis of 28 Prospective Studies Including 956,122 Participants" Nutrients 13, no. 4: 1223. https://doi.org/10.3390/nu13041223
APA StylePastorino, S., Bishop, T., Sharp, S. J., Pearce, M., Akbaraly, T., Barbieri, N. B., Bes-Rastrollo, M., Beulens, J. W. J., Chen, Z., Du, H., Duncan, B. B., Goto, A., Härkänen, T., Hashemian, M., Kromhout, D., Järvinen, R., Kivimaki, M., Knekt, P., Lin, X., ... Forouhi, N. G. (2021). Heterogeneity of Associations between Total and Types of Fish Intake and the Incidence of Type 2 Diabetes: Federated Meta-Analysis of 28 Prospective Studies Including 956,122 Participants. Nutrients, 13(4), 1223. https://doi.org/10.3390/nu13041223







