Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Dietary Interventions
2.3. Dietary Assessment
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Adherence to the Dietary Interventions
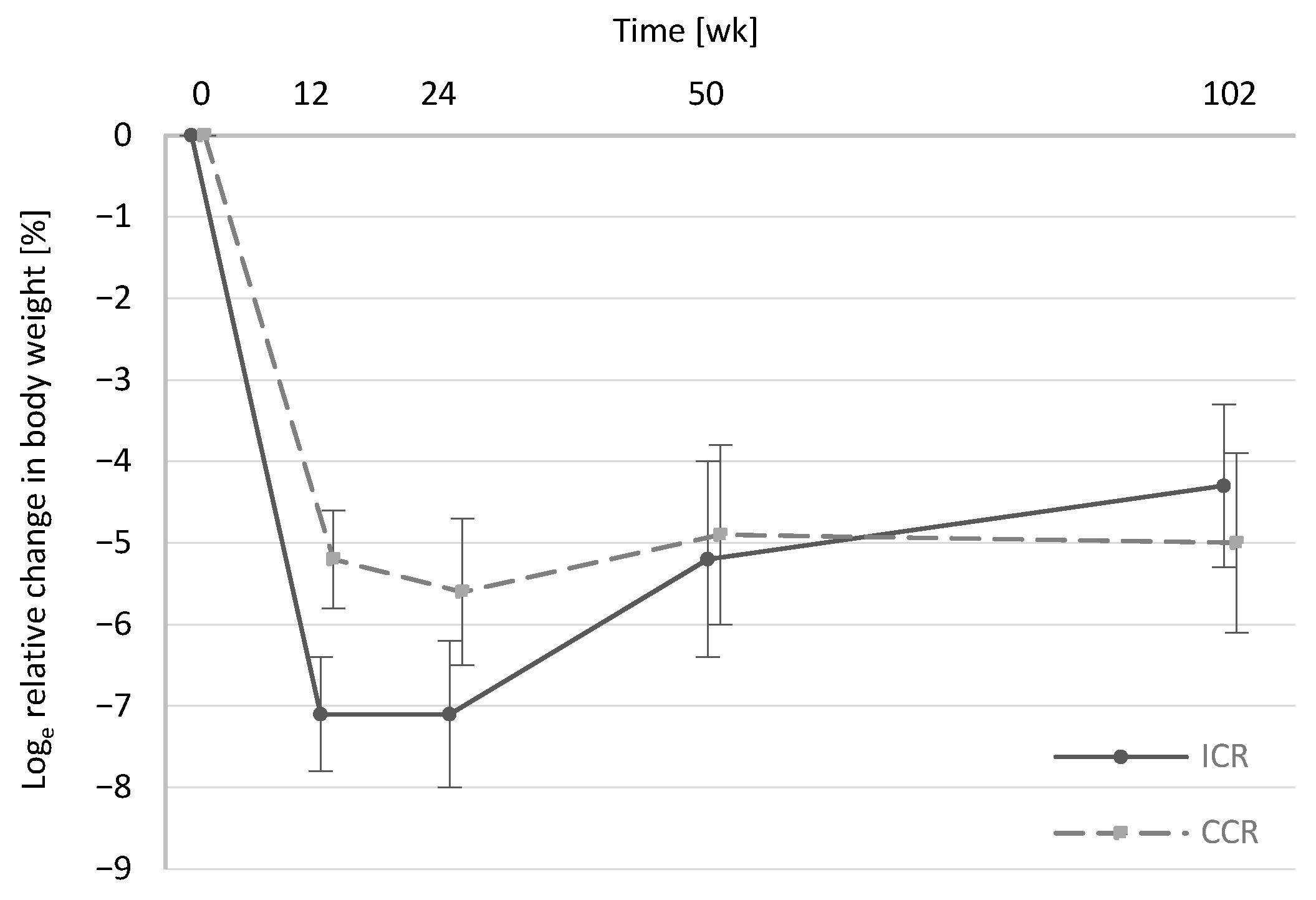
3.2.1. Energy Intake and Body Weight
3.2.2. Self-Reported Adherence
3.3. Dietary Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noncommunicable Diseases Country Profiles 2018. Available online: https://apps.who.int/iris/handle/10665/274512 (accessed on 1 May 2020).
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef]
- Johnstone, A. Fasting for weight loss: An effective strategy or latest dieting trend? Int. J. Obes. 2015, 39, 727–733. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, S.; Kim, S.; Bersamin, A.; King, A.C.; Gardner, C.D. Dietary adherence and weight loss success among overweight women: Results from the A TO Z weight loss study. Int. J. Obes. 2008, 32, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Headland, M.L.; Clifton, P.M.; Keogh, J.B. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int. J. Obes. 2018, 43, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Intermittent calorie restriction-a more effective approach to weight loss? Am. J. Clin. Nutr. 2018, 108, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.V.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.P.; Wood, R.E.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol. Cell. Endocrinol. 2015, 418, 153–172. [Google Scholar] [CrossRef]
- Harris, L.; McGarty, A.; Hutchison, L.; Ells, L.; Hankey, C. Short-term intermittent energy restriction interventions for weight management: A systematic review and meta-analysis. Obes. Rev. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Schübel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; Von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of Intermittent Compared With Continuous Energy Restricted Diet on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw. Open 2018, 1, e180756. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.N.; Howell, T. Could Intermittent Energy Restriction and Intermittent Fasting Reduce Rates of Cancer in Obese, Overweight, and Normal-Weight Subjects? A Summary of Evidence. Adv. Nutr. 2016, 7, 690–705. [Google Scholar] [CrossRef]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef]
- Panizza, C.E.; Lim, U.; Yonemori, K.M.; Cassel, K.D.; Wilkens, L.R.; Harvie, M.N.; Maskarinec, G.; Delp, E.J.; Lampe, J.W.; Shepherd, J.A.; et al. Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients 2019, 11, 1386. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Tonstad, S.; Svendsen, M. Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. Eur. J. Clin. Nutr. 2018, 73, 1006–1014. [Google Scholar] [CrossRef]
- Schübel, R.; Graf, M.E.; Nattenmuller, J.; Nabers, D.; Sookthai, D.; Gruner, L.F.; Johnson, T.; Schlett, C.L.; von Stackelberg, O.; Kirsten, R.; et al. The effects of intermittent calorie restriction on metabolic health: Rationale and study design of the HELENA Trial. Contemp. Clin. Trials 2016, 51, 28–33. [Google Scholar] [CrossRef]
- Schrimpf, D.; Plotnicki, L.; Pilz, L.R. Web-based open source application for the randomization process in clinical trials: RANDI2. Int. J. Clin. Pharmacol. Ther. 2010, 48, 465–467. [Google Scholar] [CrossRef]
- Jungvogel, A.; Wendt, I.; Schäbethal, K.; Leschik-Bonnet, E.; Oberritter, H. Überarbeitet: Die 10 regeln der dge. ErnUm 2013, 59, 644–645. [Google Scholar]
- Oberritter, H.; Schäbethal, K.; Von Ruesten, A.; Boeing, H. The DGE nutrition circle—Presentation and basis of the food-related recommendations from the German Nutrition Society (DGE). ErnUm 2013, 60, 24–29. [Google Scholar]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Penningten Biomedical Research Center Weight Loss Predictor. Available online: https://www.pbrc.edu/research-and-faculty/calculators/weight-loss-predictor/about/ (accessed on 22 March 2021).
- Headland, M.L.; Clifton, P.M.; Keogh, J.B. Impact of intermittent vs. continuous energy restriction on weight and cardiometabolic factors: A 12-month follow-up. Int. J. Obes. 2020, 44, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res. Clin. Pract. 2019, 151, 11–19. [Google Scholar] [CrossRef]
- Jospe, M.R.; Roy, M.; Brown, R.C.; Haszard, J.J.; Meredith-Jones, K.; Fangupo, L.J.; Osborne, H.; Fleming, E.A.; Taylor, R.W. Intermittent fasting, Paleolithic, or Mediterranean diets in the real world: Exploratory secondary analyses of a weight-loss trial that included choice of diet and exercise. Am. J. Clin. Nutr. 2020, 111, 503–514. [Google Scholar] [CrossRef]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: A randomized controlled trial; the Met-IER study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef]
- Thomas, D.M.; Ivanescu, A.E.; Martin, C.K.; Heymsfield, S.B.; Marshall, K.; Bodrato, V.E.; Williamson, D.A.; Anton, S.D.; Sacks, F.M.; Ryan, D.; et al. Predicting successful long-term weight loss from short-term weight-loss outcomes: New insights from a dynamic energy balance model (the POUNDS Lost study). Am. J. Clin. Nutr. 2015, 101, 449–454. [Google Scholar] [CrossRef]
- Unick, J.L.; Hogan, P.E.; Neiberg, R.H.; Cheskin, L.J.; Dutton, G.R.; Evans-Hudnall, G.; Jeffery, R.; Kitabchi, A.E.; Nelson, J.A.; Pi-Sunyer, F.X.; et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity 2014, 22, 1608–1616. [Google Scholar] [CrossRef]
- Wadden, T.A.; West, D.S.; Neiberg, R.H.; Wing, R.R.; Ryan, D.H.; Johnson, K.C.; Foreyt, J.P.; Hill, J.O.; Trence, D.L.; Vitolins, M.Z. One-year weight losses in the Look AHEAD study: Factors associated with success. Obesity 2009, 17, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Faber, P.; Gibney, E.R.; Elia, M.; Horgan, G.; Golden, B.E.; Stubbs, R.J. Effect of an acute fast on energy compensation and feeding behaviour in lean men and women. Int. J. Obes. 2002, 26, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br. J. Nutr. 2016, 115, 951–959. [Google Scholar] [CrossRef]
- Harvey, J.; Howell, A.; Morris, J.; Harvie, M. Intermittent energy restriction for weight loss: Spontaneous reduction of energy intake on unrestricted days. Food Sci. Nutr. 2018, 6, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Klempel, M.C.; Bhutani, S.; Fitzgibbon, M.; Freels, S.; Varady, K.A. Dietary and physical activity adaptations to alternate day modified fasting: Implications for optimal weight loss. Nutr. J. 2010, 9, 35–42. [Google Scholar] [CrossRef]
- Referenzwerte Kohlenhydrate, Ballaststoffe. Available online: https://www.dge.de/wissenschaft/referenzwerte/kohlenhydrate-ballaststoffe/ (accessed on 15 May 2020).
- Kroeger, C.M.; Trepanowski, J.F.; Klempel, M.C.; Barnosky, A.; Bhutani, S.; Gabel, K.; Varady, K.A. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutr. Health 2018, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef]
| ICR n = 49; 49% Females | CCR n = 49; 49% Females | |
|---|---|---|
| Age (years) | 49.4 ± 9.0 | 50.5 ± 8.0 |
| Weight (kg) | 96.4 ± 15.8 | 92.5 ± 15.7 |
| BMI (kg/m2) | 32.0 ± 3.8 | 31.2 ± 4.0 |
| Waist (cm) | 104.7 ± 12.3 | 103.7 ± 11.9 |
| Professional degree 2 | ||
| Higher education entrance qualification | 31 (62.3) | 28 (59.6) |
| Primary school | 7 (14.3) | 5 (10.6) |
| Secondary school certificate | 11 (22.5) | 14 (29.8) |
| Blood pressure | ||
| Systolic (mmHg) | 139.4 ± 18.7 | 136.0 ± 16.7 |
| Diastolic (mmHg) | 87.2 ± 9.9 | 87.3 ± 8.7 |
| Glucose (mg/dL) | 92.7 ± 7.5 | 93.9 ± 7.5 |
| HbA1c (%) | 5.4 ± 0.3 | 5.5 ± 0.4 |
| HDL cholesterol (mg/dL) | 54.1 ± 14.4 | 56.2 ± 16.3 |
| LDL cholesterol(mg/dL) | 124.5 ± 22.4 | 122.5 ± 31.5 |
| Triglycerides (mg/dL) | 130.0 ± 83.8 | 121.2 ± 66.3 |
| Energy Requirement (kcal/d) 2 | Reported Intake wk0 (kcal/d) | Prescribed Intake (kcal/d) 3 | Reported Intake wk2 (kcal/d) | Change wk0–wk2 (%) 1 | p-Value 4 | Reported Intake wk12 (kcal/d) | Change wk0–wk12 (%) 1 | p-Value 4 | Reported Intake wk50 (kcal/d) | Change wk0–wk50 (%) 1 | p-Value 4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICR (daily) | 2630.8 ± 490.9 | 2053.3 ± 746.0 | 2067.1 ± 385.7 | 1469.9 ± 454.5 | −30.2 ± 5.3 * | <0.001 | 1438.4 ± 486.1 | −32.1 ± 5.2 * | <0.001 | 1689.8 ± 624.4 | −16.2 ± 5.4 * | <0.001 |
| CCR (daily) | 2507.3 ± 378.5 | 1981.0 ± 476.5 | 1970.1 ± 297.3 | 1537.4 ± 342.1 | −25.4 ± 3.4 * | <0.001 | 1529.4 ± 364.8 | −25.8 ± 2.8 * | <0.001 | 1768.8 ± 553.2 | −14.2 ± 5.0 * | <0.001 |
| p-value 5 | 0.23 | 0.15 | 0.51 | |||||||||
| ICR NR day 6 | - | - | 2630.8 ± 490.9 | 1783.0 ± 641.4 | - | <0.001 | 1699.9 ± 568.3 | - | <0.001 | 1765.9 ± 633.6 | - | <0.001 |
| ICR R day 6 | - | - | 657.7 ± 122.7 | 706.0 ± 220.6 | - | 0.45 | 700.7 ± 246.5 | - | 0.74 | 566.8 ± 161.7 | - | 0.09 |
| wk0 | wk2 | Change wk0–wk2 (%) 1 | wk12 | Change Wk0–wk12 (%) 1 | wk50 | Change wk0–wk50 (%) 1 | ||
|---|---|---|---|---|---|---|---|---|
| Protein (E%) | ICR | 15.8 ± 2.5 | 20.4 ± 3.3 | 25.7 ± 3.1 * | 19.6 ± 4.0 | 20.9 ± 3.6 * | 17.1 ± 3.0 | 9.3 ± 2.7 * |
| CCR | 15.6 ± 3.2 | 17.7 ± 2.7 | 11.7 ± 3.3 * | 17.2 ± 2.6 | 8.5 ± 2.9 * | 16.3 ± 2.5 | 3.3 ± 4.0 | |
| p-value 2,3 | 0.83 2 | <0.001 2 | <0.001 3 | <0.001 2 | 0.002 3 | 0.20 2 | 0.26 3 | |
| Fat (E%) | ICR | 38.1 ± 5.6 | 29.4 ± 5.9 | −26.7 ± 3.4 * | 30.6 ± 6.3 | −22.6 ± 3.4 * | 34.0 ± 6.3 | −10.7 ± 3.8 * |
| CCR | 37.2 ± 5.2 | 29.5 ± 5.6 | −23.2 ± 3.3 * | 31.5 ± 6.5 | −17.1 ± 3.6 * | 32.9 ± 8.2 | −13.0 ± 5.4 * | |
| p-value 2,3 | 0.39 2 | 0.89 2 | 0.48 3 | 0.51 2 | 0.27 3 | 0.50 2 | 0.89 3 | |
| SFA (E%) | ICR | 15.3 ± 3.1 | 11.8 ± 2.9 | −24.8 ± 4.0 * | 11.4 ± 3.0 | −30.0 ± 4.1 * | 13.0 ± 3.4 | −14.7 ± 4.7 * |
| CCR | 14.3 ± 2.9 | 10.8 ± 2.8 | −29.0 ± 3.7 * | 10.9 ± 2.8 | −28.8 ± 4.9 * | 12.5 ± 4.1 | −17.3 ± 6.4 * | |
| p-value 2,3 | 0.12 2 | 0.08 2 | 0.73 3 | 0.41 2 | 0.62 3 | 0.55 2 | 0.83 3 | |
| MUFA (E%) | ICR | 11.0 ± 2.8 | 9.5 ± 2.2 | −14.0 ± 4.3 * | 9.2 ± 3.0 | −20.7 ± 6.3 * | 9.9 ± 2.7 | −9.4 ± 5.5 * |
| CCR | 11.0 ± 2.5 | 10.1 ± 2.5 | −8.6 ± 4.4 | 9.8 ± 2.3 | −11.6 ± 4.7 | 10.4 ± 3.5 | −8.0 ± 6.5 | |
| p-value 2,3 | 0.91 2 | 0.24 2 | 0.34 3 | 0.28 2 | 0.41 3 | 0.50 2 | 0.60 3 | |
| PUFA (E%) | ICR | 4.9 ± 1.7 | 4.6 ± 1.3 | −5.5 ± 5.2 | 4.7 ± 1.6 | −5.2 ± 6.3 | 4.9 ± 2.1 | −0.8 ± 6.3 |
| CCR | 4.9 ± 1.6 | 5.5 ± 2.1 | 9.9 ± 7.0 | 5.1 ± 1.6 | 5.6 ± 5.0 | 4.4 ± 1.3 | −2.8 ± 6.1 | |
| p-value 2,3 | 0.87 2 | 0.027 2 | 0.10 3 | 0.28 2 | 0.37 3 | 0.21 2 | 0.38 3 | |
| Carbohydrates (E%) | ICR | 44.4 ± 6.4 | 47.0 ± 5.5 | 6.0 ± 2.7 * | 46.9 ± 6.9 | 5.3 ± 2.4 | 46.4 ± 6.7 | 2.6 ± 2.8 |
| CCR | 45.4 ± 6.5 | 49.7 ± 5.3 | 9.5 ± 2.3 * | 48.5 ± 5.7 | 7.1 ± 2.9 * | 48.3 ± 8.2 | 5.1 ± 4.0 | |
| p-value 2,3 | 0.43 2 | 0.021 2 | 0.27 3 | 0.23 2 | 0.69 3 | 0.26 2 | 0.65 3 | |
| Fiber (g/d) 4 | ICR | 17.9 ± 8.1 | 21.8 ± 7.4 | 25.1 ± 6.4 * | 18.9 ± 7.9 | 8.4 ± 7.2 | 19.8 ± 8.9 | 12.0 ± 7.4 |
| CCR | 17.5 ± 6.1 | 24.4 ± 7.7 | 33.6 ± 4.9 * | 21.8 ± 9.1 | 22.3 ± 5.0 * | 21.9 ± 12.4 | 19.3 ± 6.9 * | |
| p-value 2,3 | 0.79 2 | 0.11 2 | 0.07 3 | 0.11 2 | 0.08 3 | 0.39 2 | 0.31 3 |
| wk0 | wk2 | Change wk0–wk2 (%) 1 | wk12 | Change wk0–wk12 (%) 1 | wk50 | Change wk0–wk50 (%) 1 | |
|---|---|---|---|---|---|---|---|
| Vegetables and vegetable products (g/d) | |||||||
| ICR | 135.1 ± 93.8 | 283.2 ± 133.4 | 80.9 ± 12.4 * | 282.0 ± 207.7 | 63.1 ± 13.2 * | 189.1 ± 123.5 | 33.6 ± 11.9 * |
| CCR | 154.3 ± 89.7 | 254.4 ± 118.8 | 56.5 ± 10.9 * | 234.2 ± 162.7 | 42.3 ± 10.8 * | 204.2 ± 136.0 | 28.7 ± 13.2 * |
| p-value 2,3 | 0.30 2 | 0.28 2 | 0.13 3 | 0.22 2 | 0.11 3 | 0.60 2 | 0.76 3 |
| Fruits and fruit products (g/d) | |||||||
| ICR | 180.6 ± 219.1 | 172.3 ± 86.8 | 18.1 ± 12.3 | 168.5 ± 96.1 | 10.0 ± 17.5 | 176.0 ± 151.0 | 5.3 ± 15.8 |
| CCR | 195.8 ± 144.0 | 258.8 ± 138.0 | 50.5 ± 16.3 * | 225.7 ± 123.2 | 36.3 ± 14.5 * | 245.1 ± 175.4 | 40.5 ± 18.5 * |
| p-value 2,3 | 0.68 2 | <0.001 2 | 0.08 3 | 0.014 2 | 0.27 3 | 0.06 2 | 0.18 3 |
| Bread (g/d) | |||||||
| ICR | 126.9 ± 68.5 | 101.8 ± 61.9 | −22.0 ± 9.7 * | 98.1 ± 53.7 | −22.3 ± 9.0 * | 114.5 ± 74.6 | −12.6 ± 7.6 |
| CCR | 97.5 ± 50.0 | 102.8 ± 46.9 | 9.9 ± 8.4 | 107.1 ± 55.0 | 12.1 ± 9.9 | 102.8 ± 50.0 | −0.8 ± 10.2 |
| p-value 2,3 | 0.017 2 | 0.94 2 | 0.023 3 | 0.43 2 | 0.002 3 | 0.42 2 | 0.27 3 |
| Grains and grain products, rice (g/d) | |||||||
| ICR | 20.9 ± 22.2 | 36.0 ± 31.6 | 39.0 ± 19.5 | 22.3 ± 20.4 | 7.9 ± 16.4 | 24.6 ± 24.9 | −7.8 ± 19.8 |
| CCR | 31.9 ± 32.3 | 51.7 ± 44.1 | 46.1 ± 14.4 * | 39.1 ± 31.8 | 37.1 ± 17.2 * | 27.6 ± 26.5 | −1.2 ± 21.4 |
| p-value 2,3 | 0.05 2 | 0.06 2 | 0.64 3 | 0.003 2 | 0.35 3 | 0.61 2 | 0.27 3 |
| Potatoes and starchy foods, mushrooms (g/d) | |||||||
| ICR | 28.0 ± 30.5 | 43.5 ± 40.3 | 69.5 ± 26.5 * | 36.5 ± 38.1 | 32.5 ± 25.6 | 40.9 ± 36.5 | 54.7 ± 23.9 * |
| CCR | 31.5 ± 33.7 | 42.0 ± 38.9 | 42.3 ± 14.8 * | 42.2 ± 35.0 | 16.7 ± 21.4 | 35.6 ± 30.4 | −0.6 ± 30.1 |
| p-value 2,3 | 0.59 2 | 0.85 2 | 0.66 3 | 0.45 2 | 0.79 3 | 0.48 2 | 0.35 3 |
| Milk, dairy products and cheese (g/d) | |||||||
| ICR | 197.9 ± 156.6 | 194.9 ± 141.9 | 9.1 ± 10.2 | 199.8 ± 143.2 | 12.8 ± 10.3 | 176.8 ± 153.8 | −10.0 ± 10.2 |
| CCR | 187.5 ± 160.2 | 181.9 ± 96.6 | 18.0 ± 15.6 | 198.5 ± 121.4 | 14.6 ± 13.4 | 183.9 ± 119.7 | 18.5 ± 17.2 |
| p-value 2,3 | 0.75 2 | 0.61 2 | 0.96 3 | 0.97 2 | 0.88 3 | 0.82 2 | 0.31 3 |
| Beef, veal, pork, mutton (g/d) | |||||||
| ICR | 11.9 ± 16.3 | 14.7 ± 16.2 | −33.2 ± 28.2 | 14.2 ± 24.6 | 30.2 ± 30.8 | 14.3 ± 23.6 | 3.2 ± 23.4 |
| CCR | 21.4 ± 28.0 | 19.5 ± 21.4 | −20.6 ± 21.6 | 13.0 ± 18.9 | −12.3 ± 28.6 | 22.0 ± 26.6 | −16.6 ± 18.3 |
| p-value 2,3 | 0.043 2 | 0.23 2 | 0.35 3 | 0.79 2 | 0.10 3 | 0.17 2 | 0.57 3 |
| Game, poultry, offal (g/d) | |||||||
| ICR | 17.4 ± 21.9 | 21.8 ± 21.0 | −1.1 ± 18.2 | 21.2 ± 25.8 | −13.9 ± 22.1 | 15.3 ± 21.1 | −10.9 ± 26.2 |
| CCR | 18.3 ± 23.4 | 17.0 ± 19.7 | −10.2 ± 23.4 | 15.9 ± 17.2 | −9.4 ± 18.8 | 16.6 ± 24.4 | 45.8 ± 19.0 * |
| p-value 2,3 | 0.84 2 | 0.27 2 | 0.29 3 | 0.26 2 | 0.28 3 | 0.80 2 | 0.91 3 |
| Sausage and processed meat (g/d) | |||||||
| ICR | 51.1 ± 44.4 | 26.0 ± 20.4 | ‑58.0 ± 15.4 * | 23.6 ± 23.0 | −64.9 ± 14.4 * | 40.0 ± 33.1 | −7.0 ± 13.8 |
| CCR | 31.5 ± 33.1 | 20.1 ± 24.7 | −16.6 ± 20.2 | 21.5 ± 24.2 | −24.8 ± 21.1 | 22.0 ± 19.1 | −17.6 ± 19.3 |
| p-value 2,3 | 0.015 2 | 0.21 2 | 0.08 3 | 0.68 2 | 0.016 3 | 0.004 2 | 0.79 3 |
| Fish and seafood (g/d) | |||||||
| ICR | 22.6 ± 40.3 | 22.3 ± 27.2 | −38.7 ± 26.0 | 13.5 ± 17.1 | −57.8 ± 23.8 * | 11.5 ± 16.8 | −46.3 ± 40.7 |
| CCR | 18.7 ± 24.6 | 17.9 ± 17.4 | 20.2 ± 25.4 | 16.9 ± 18.0 | 4.1 ± 28.3 | 16.9 ± 24.5 | 4.2 ± 26.0 |
| p-value 2,3 | 0.57 2 | 0.36 2 | 0.97 3 | 0.36 2 | 0.25 3 | 0.26 2 | 0.27 3 |
| Eggs and egg products, pasta (g/d) | |||||||
| ICR | 41.0 ± 35.4 | 30.0 ± 27.0 | −24.9 ± 16.9 | 32.4 ± 31.7 | −18.0 ± 18.3 | 34.4 ± 37.7 | −34.1 ± 21.0 |
| CCR | 52.5 ± 59.7 | 39.1 ± 35.8 | −6.2 ± 19.1 | 34.9 ± 26.3 | −22.7 ± 19.7 | 32.2 ± 25.5 | −72.5 ± 28.2 * |
| p-value 2,3 | 0.25 2 | 0.17 2 | 0.88 3 | 0.68 2 | 0.47 3 | 0.77 2 | 0.25 3 |
| Fats and oils (g/d) | |||||||
| ICR | 18.1 ± 14.8 | 8.2 ± 5.8 | −68.0 ± 14.3 * | 9.3 ± 7.6 | −58.3 ± 15.6 * | 13.2 ± 11.8 | −29.3 ± 18.5 |
| CCR | 17.3 ± 10.9 | 11.3 ± 6.4 | −38.9 ± 13.3 * | 11.0 ± 7.1 | −35.6 ± 13.5 * | 11.1 ± 8.5 | −47.8 ± 13.8 * |
| p-value 2,3 | 0.76 2 | 0.015 2 | 0.11 3 | 0.26 2 | 0.33 3 | 0.38 2 | 0.71 3 |
| Legumes, nuts and seeds (g/d) | |||||||
| ICR | 9.2 ± 14.7 | 10.8 ± 17.0 | −7.9 ± 31.4 | 12.6 ± 17.4 | 26.0 ± 31.7 | 6.7 ± 11.8 | −20.4 ± 21.7 |
| CCR | 13.3 ± 15.8 | 20.7 ± 41.2 | 5.5 ± 32.1 | 12.6 ± 16.0 | 9.6 ± 30.9 | 15.5 ± 29.7 | 19.8 ± 36.5 |
| p-value 2,3 | 0.19 2 | 0.14 2 | 0.42 3 | 1.00 2 | 0.35 3 | 0.08 2 | 0.38 3 |
| Bakery products, cakes and pastry (g/d) | |||||||
| ICR | 48.9 ± 41.5 | 18.4 ± 23.5 | −79.7 ± 24.2 * | 23.7 ± 25.5 | −59.2 ± 18.3 * | 46.2 ± 39.6 | 7.4 ± 15.2 |
| CCR | 52.7 ± 52.0 | 15.6 ± 17.5 | −96.7 ± 23.2 * | 25.5 ± 30.8 | −53.4 ± 18.8 * | 34.1 ± 27.5 | −35.8 ± 20.7 |
| p-value 2,3 | 0.68 2 | 0.51 2 | 0.45 3 | 0.76 2 | 0.72 3 | 0.12 2 | 0.07 3 |
| Sweets, sugar and ice cream (g/d) | |||||||
| ICR | 40.8 ± 37.2 | 19.6 ± 25.8 | −78.3 ± 21.4 * | 20.2 ± 20.4 | −77.4 ± 19.8 * | 25.7 ± 33.3 | −86.7 ± 17.7 * |
| CCR | 38.1 ± 34.8 | 14.3 ± 16.1 | −92.5 ± 18.4 * | 11.2 ± 11.2 | −79.2 ± 23.9 * | 26.7 ± 30.5 | −37.6 ± 23.8 |
| p-value 2,3 | 0.71 2 | 0.24 2 | 0.78 3 | 0.010 2 | 0.39 3 | 0.88 2 | 0.56 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannen, S.T.; Maldonado, S.G.; Nonnenmacher, T.; Sowah, S.A.; Gruner, L.F.; Watzinger, C.; Nischwitz, K.; Ulrich, C.M.; Kaaks, R.; Schübel, R.; et al. Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity. Nutrients 2021, 13, 1195. https://doi.org/10.3390/nu13041195
Pannen ST, Maldonado SG, Nonnenmacher T, Sowah SA, Gruner LF, Watzinger C, Nischwitz K, Ulrich CM, Kaaks R, Schübel R, et al. Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity. Nutrients. 2021; 13(4):1195. https://doi.org/10.3390/nu13041195
Chicago/Turabian StylePannen, Sarah T., Sandra González Maldonado, Tobias Nonnenmacher, Solomon A. Sowah, Laura F. Gruner, Cora Watzinger, Karin Nischwitz, Cornelia M. Ulrich, Rudolf Kaaks, Ruth Schübel, and et al. 2021. "Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity" Nutrients 13, no. 4: 1195. https://doi.org/10.3390/nu13041195
APA StylePannen, S. T., Maldonado, S. G., Nonnenmacher, T., Sowah, S. A., Gruner, L. F., Watzinger, C., Nischwitz, K., Ulrich, C. M., Kaaks, R., Schübel, R., Grafetstätter, M., & Kühn, T. (2021). Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity. Nutrients, 13(4), 1195. https://doi.org/10.3390/nu13041195







