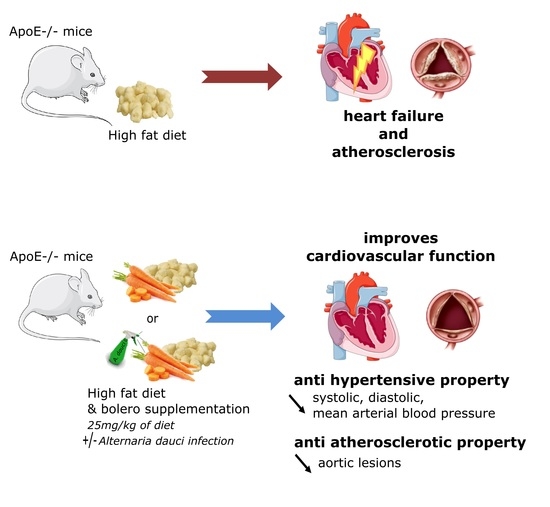
Carrot Supplementation Improves Blood Pressure and Reduces Aortic Root Lesions in an Atherosclerosis-Prone Genetic Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Products
2.2. Ethics Statement
2.3. Animals
2.4. Cardiovascular Parameters Measurements
2.5. Echocardiography
2.6. Biochemical Parameters
2.7. Hepatic Secretion of Triglycerides
2.8. Liver Histology
2.9. RNA Extraction and Real-Time RT-qPCR
2.10. Short Chain Fatty Acids Analysis and Quantification by Gas Liquid Chromatography
2.11. Atherosclerotic Lesions Analysis
2.12. Statistical Analysis
3. Results
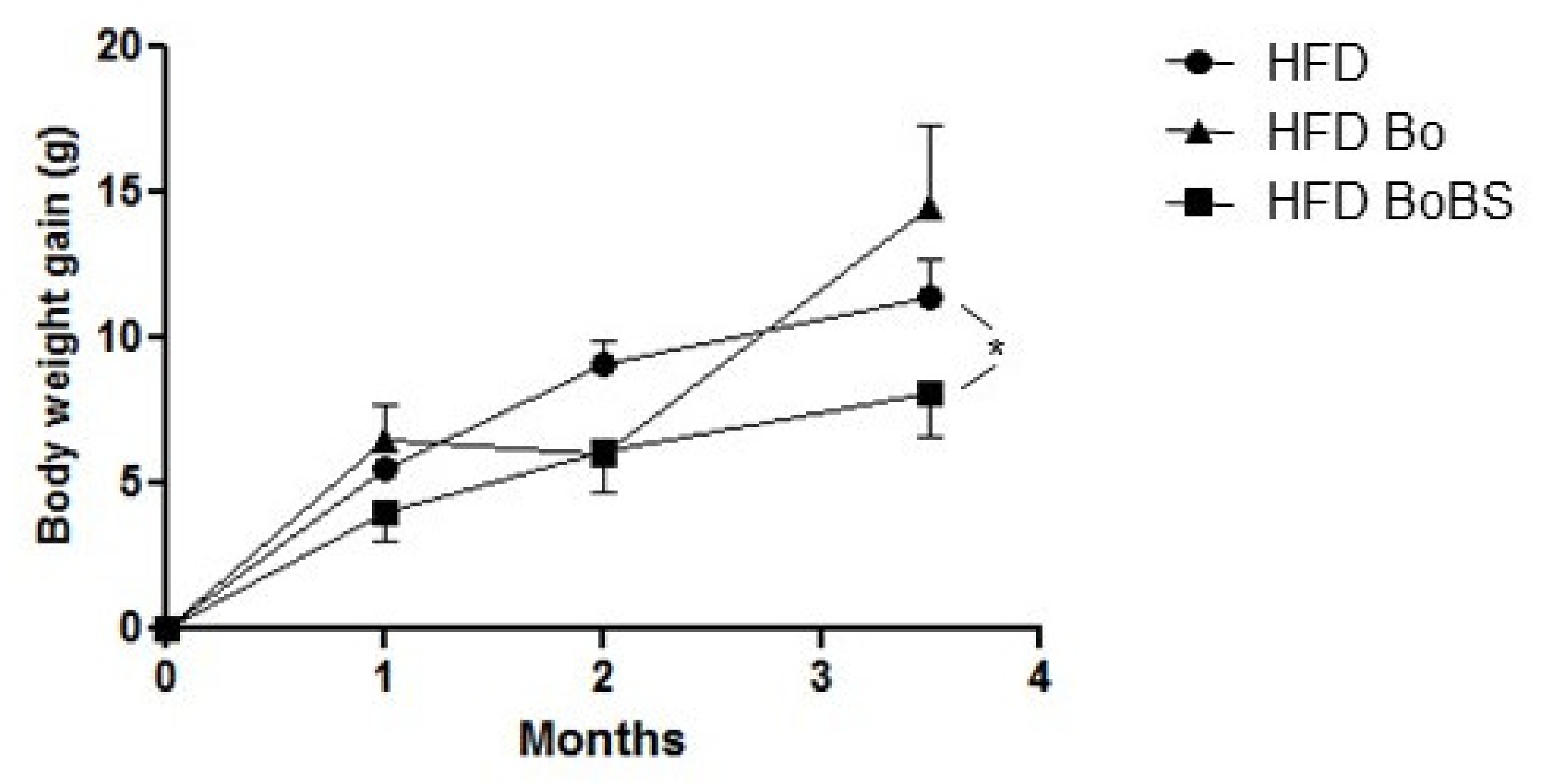
3.1. Carrot Supplementation Effects on Body Weight Gain
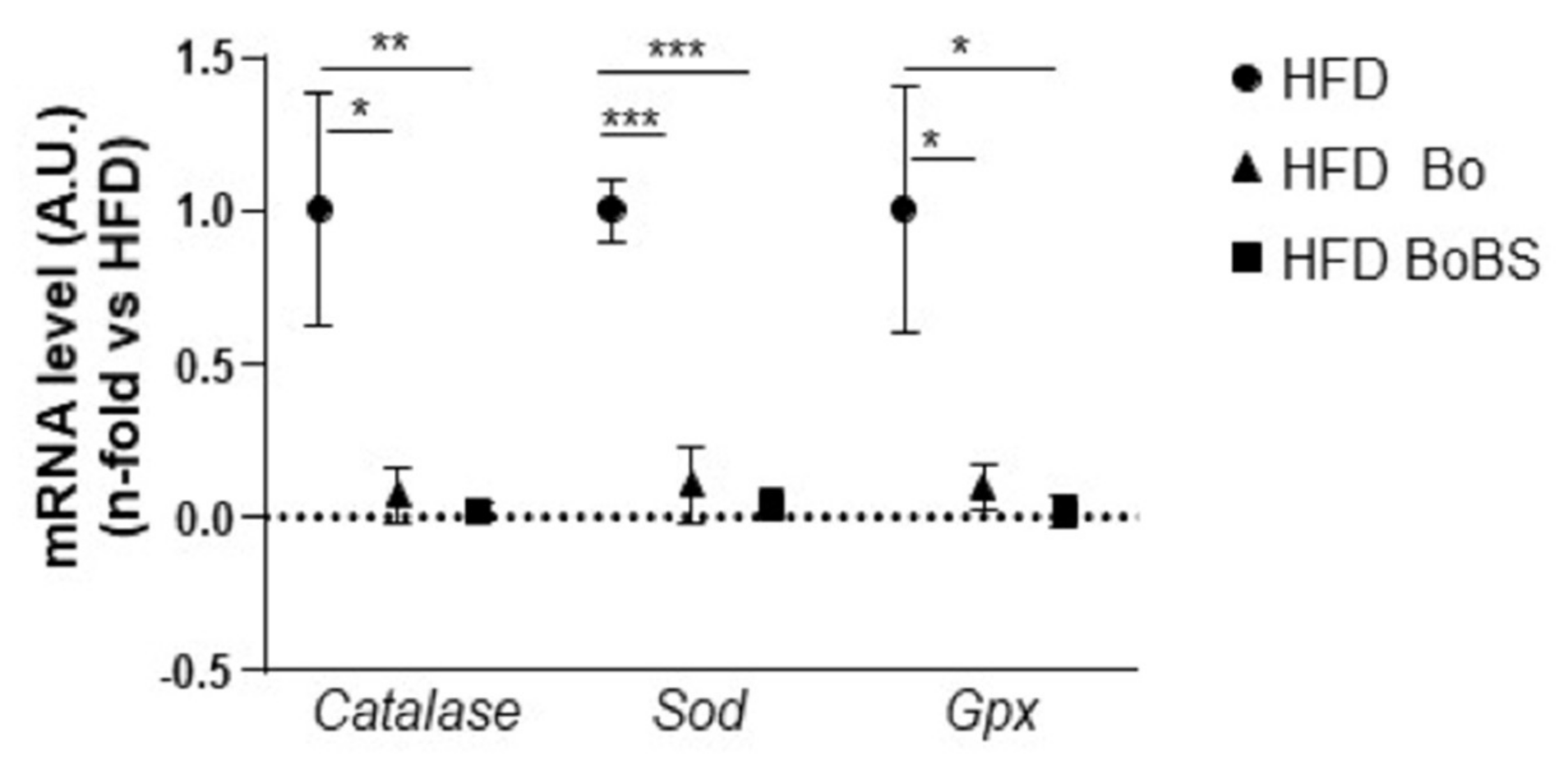
3.2. Effects of Carrot Supplementation on Hepatic Antioxidant Enzymes
3.3. Carrot Supplementation Effects on Plasma Glucido-Lipidic Parameters
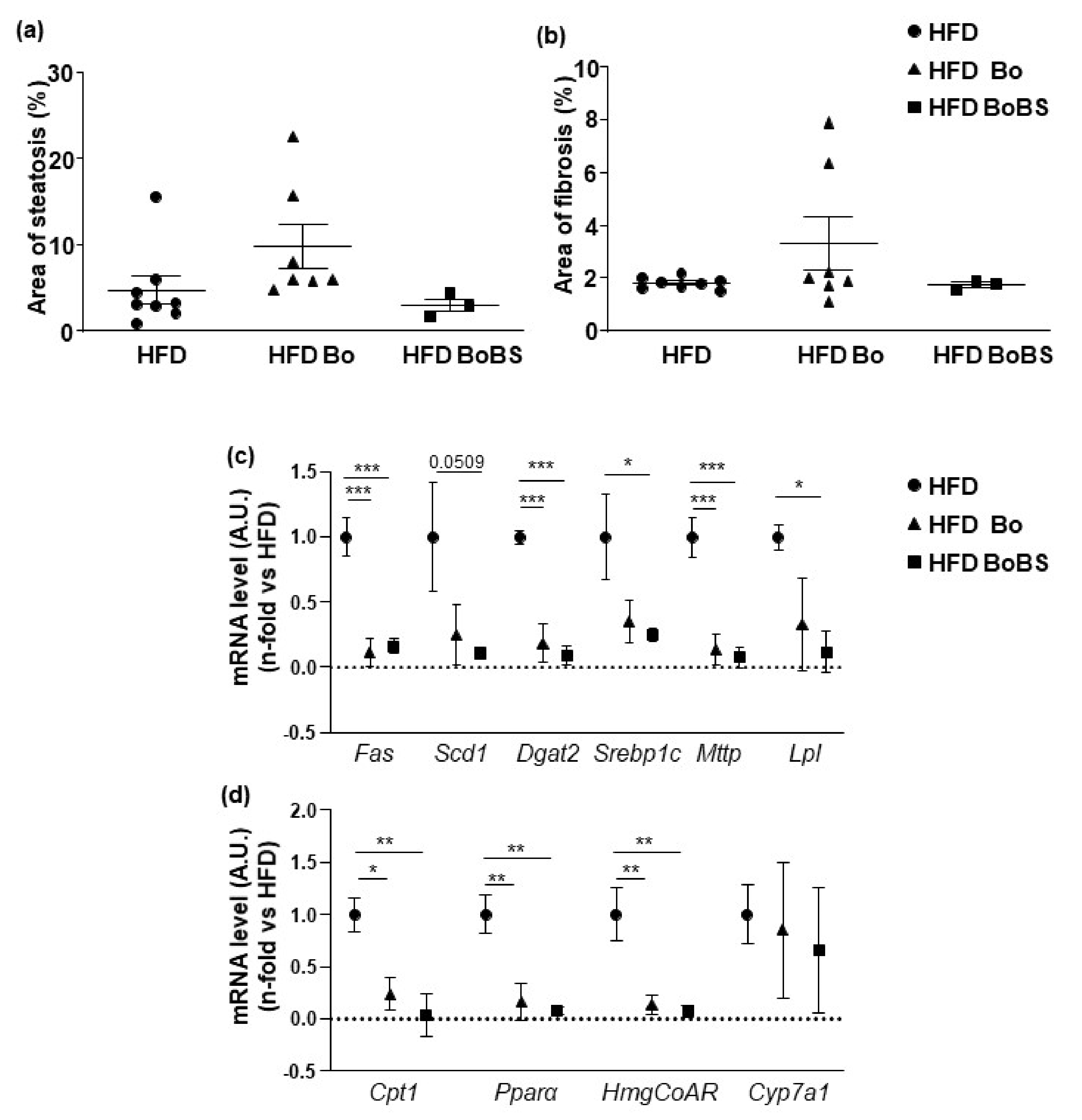
3.4. Effects of Carrot on Hepatic Function and Structure
3.4.1. Triglycerides Hepatic Secretion
3.4.2. Steatosis and Fibrosis Quantification
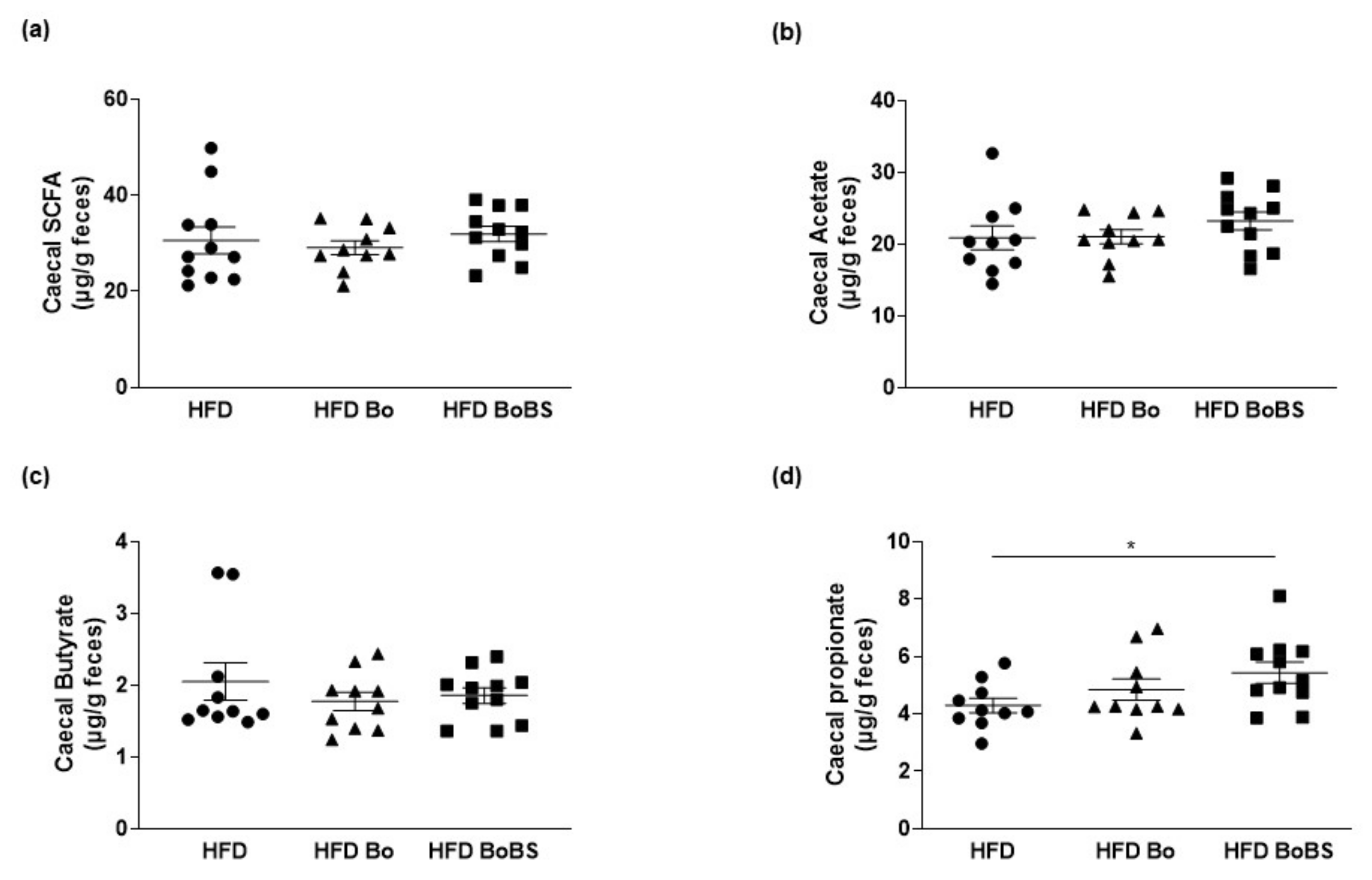
3.5. Effects of Carrot Supplementation on Short Chain Fatty Acid Composition
3.6. Effects of Carrot on Heart Function
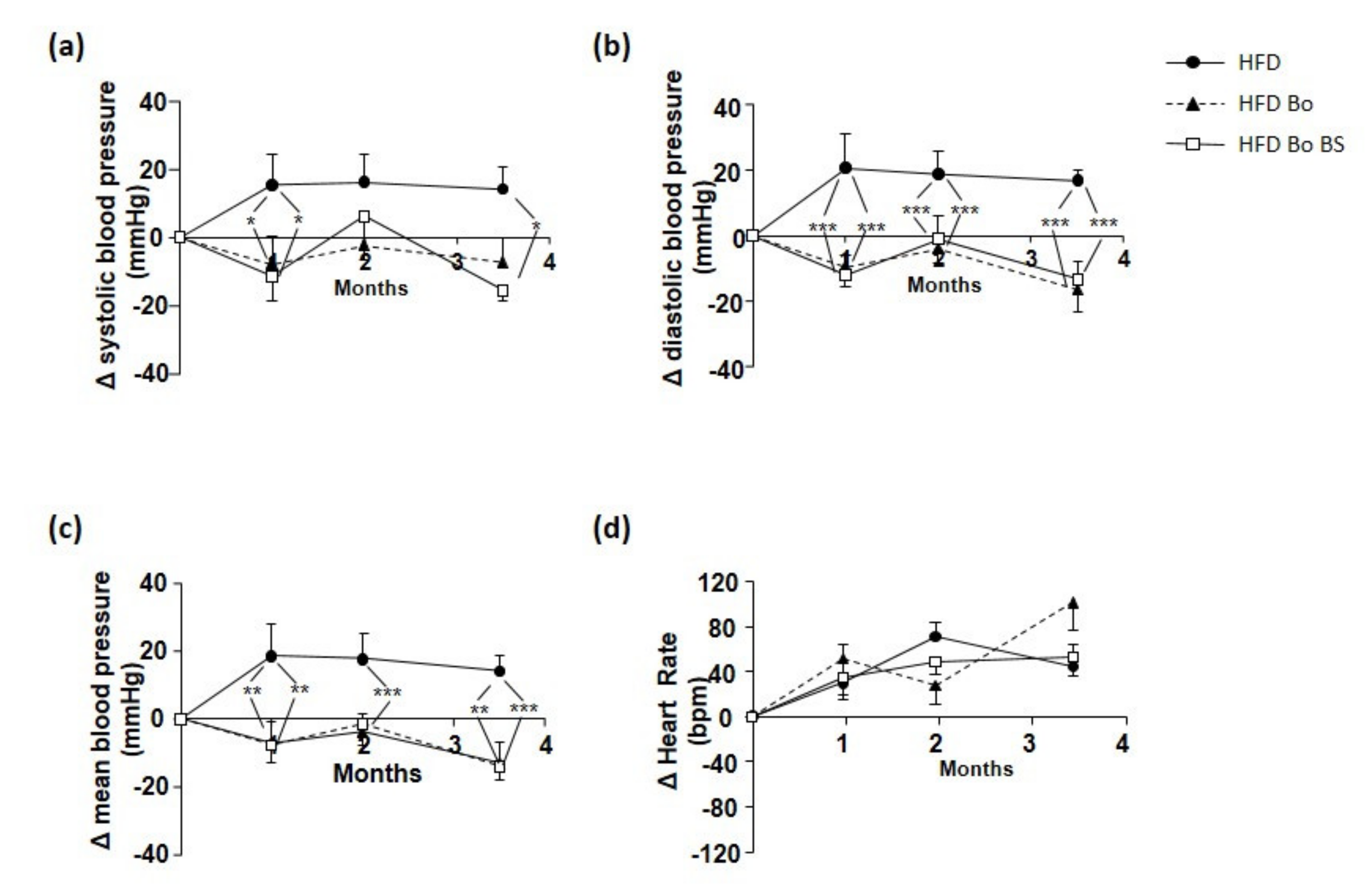
3.7. Effects of Carrot on Blood Pressure and Heart Rate
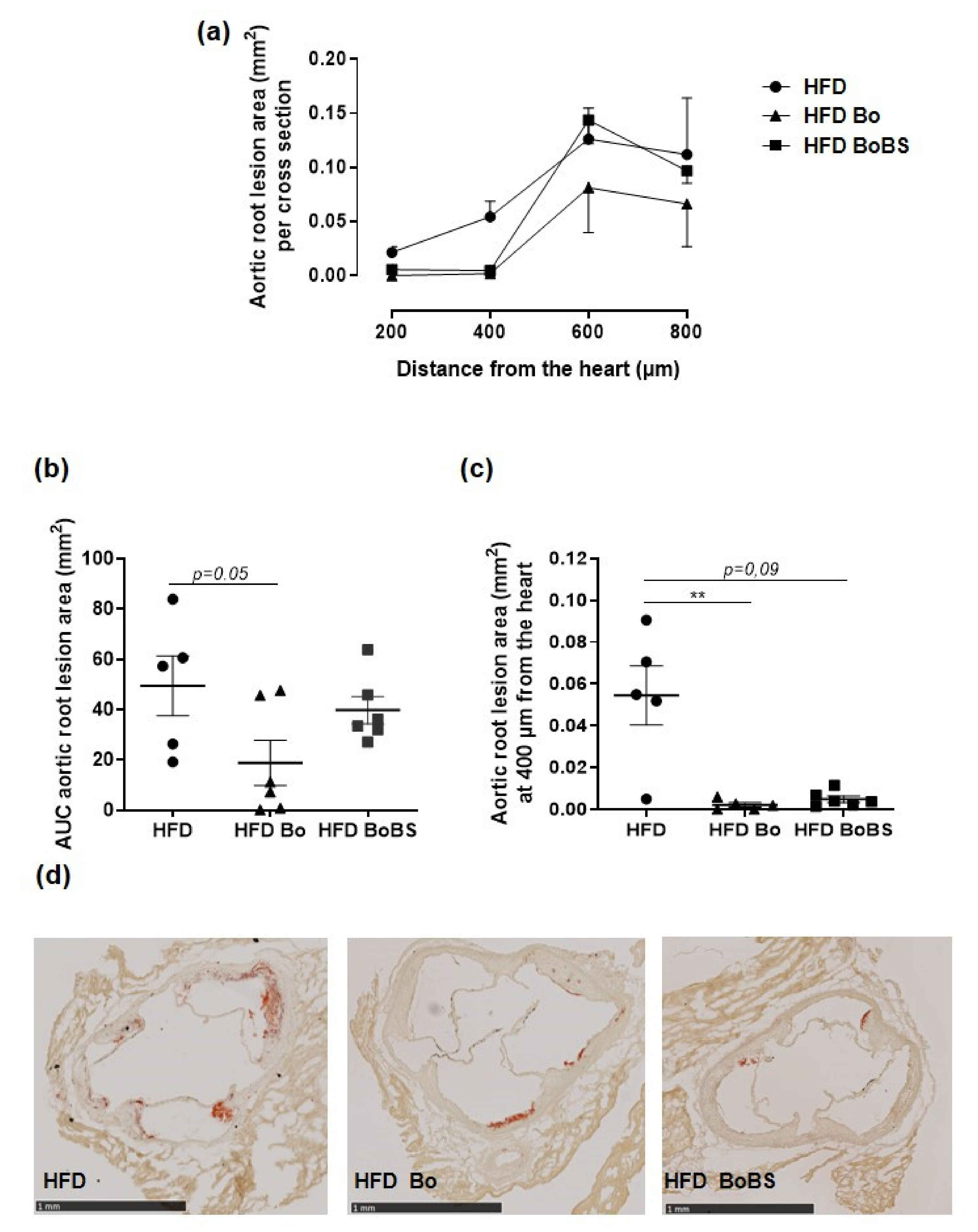
3.8. Carrot Supplementation Incidence on Atherosclerotic Lesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Nicolle, C.; Simon, G.; Rock, E.; Amouroux, P.; Rémésy, C. Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J. Am. Soc. Hortic. Sci. 2004, 129, 523–529. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotene and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Nicolle, C.; Cardinault, N.; Aprikian, O.; Busserolles, J.; Grolier, P.; Rock, E.; Demigné, C.; Mazur, A.; Scalbert, A.; Amouroux, P.; et al. Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. Eur. J. Nutr. 2003, 42, 254–261. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.; Brown, L. Comparison of purple carrot juice and beta-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1322–1332. [Google Scholar] [CrossRef]
- Potter, A.S.; Foroudi, S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr. J. 2011, 10, 96. [Google Scholar] [CrossRef]
- Soleti, R.; Mallegol, P.; Hilairet, G.; Frifra, M.; Perrin, F.; Dubois-Laurent, C.; Huet, S.; Pignon, P.; Basset, L.; Geoffriau, E.; et al. Carrot genotypes contrasted by root color and grown under different conditions displayed differential pharmacological profiles in vascular and metabolic cells. Nutrients 2020, 12, 337. [Google Scholar] [CrossRef]
- Perrin, F.; Dubois-Laurent, C.; Gibon, Y.; Citerne, S.; Huet, S.; Suel, A.; Le Clerc, V.; Briard, M.; Hamama, L.; Peltier, D.; et al. Combined Alternaria dauci infection and water stresses impact carotenoid content of carrot leaves and roots. Environ. Exp. Bot. 2017, 143, 125–134. [Google Scholar] [CrossRef]
- Schotz, M.C.; Scanu, A.; Page, I.H. Effect of triton on lipoprotein lipase of rat plasma. Am. J. Physiol. 1957, 188, 399–402. [Google Scholar] [CrossRef]
- Siri, P.; Candela, N.; Zhang, Y.L.; Ko, C.; Eusufzai, S.; Ginsberg, H.N.; Huang, L.S. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity, and insulin resistance. J. Biol. Chem. 2001, 276, 46064–46072. [Google Scholar] [CrossRef] [PubMed]
- Gühnemann-Schäfer, K.; Kindl, H. Fatty acid beta-oxidation in glyoxysomes. Characterization of a new tetrafunctional protein (MFP III). Biochim. Biophys. Acta 1995, 1256, 181–186. [Google Scholar] [CrossRef]
- Nicoletti, A.; Kaveri, S.; Caligiuri, G.; Bariéty, J.; Hansson, G.K. Immunoglobulin treatment reduces atherosclerosis in apo E knockout mice. JCI 1998, 102, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, H.; Otomo, Y.; Karaki, S.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Dobrzyn, A.; Ntambi, J.M. The role of stearoyl-CoA desaturase in the control of metabolism. PLEFA 2005, 73, 35–41. [Google Scholar] [CrossRef]
- Vijayakumar, R.S.; Surya, D.; Nalini, N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004, 9, 105–110. [Google Scholar] [CrossRef]
- Louis, X.L.; Raj, P.; McClinton, K.J.; Yu, L.; Suh, M.; Netticadan, T. Supplementation of type 1 diabetic rats with carrot powder lowers blood glucose without improving cardiac structure and function. Prev. Nutr. Food Sci. 2018, 23, 115–121. [Google Scholar] [CrossRef]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 28, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Étienne-Selloum, N.; Li, H.; Martínez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Carrot (Daucuscarota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2013, 97, 574–581. [Google Scholar] [CrossRef]
- Borgi, L.; Muraki, I.; Satija, A.; Willett, W.C.; Rimm, E.B.; Forman, J.P. Fruit and vegetable consumption and the incidence of hypertension in three prospective cohort studies. Hypertension 2016, 67, 288–293. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Ausman, L.M.; Jalbert, S.M.; Vilella-Bach, M.; Jauhiainen, M.; McGladdery, S.; Erkkila, A.T.; Ehnholm, C.; Frohlich, J.; Schaefer, E.J. Efficacy of a therapeutic lifestyle change/Step 2 diet in moderately hypercholesterolemic middle-aged and elderly female and male subjects. J. Lipid Res. 2002, 43, 264–273. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Zhang, D. Association between dietary carotenoid intakes and hypertension in adults: National Health and Nutrition Examination Survey 2007–2014. J. Hypertens. 2019, 37, 2371–2379. [Google Scholar] [CrossRef]
- Nicolle, C.; Gueux, E.; Lab, C.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Lyophilized carrot ingestion lowers lipemia and beneficially affects cholesterol metabolism in cholesterol-fed C57BL/6J mice. Eur. J. Nutr. 2004, 43, 237–245. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, R.; Cao, Y.; Ouyang, W.F.; Li, H.B.; Ling, W.H.; Chen, Y.M. Higher dietary and serum carotenoid levels are associated with lower carotid intima-media thickness in middle-aged and elderly people. Br. J. Nutr. 2018, 119, 590–598. [Google Scholar] [CrossRef]
- Luo, M.; Tian, R.; Lu, N. Quercetin inhibited endothelial dysfunction and atherosclerosis in apolipoprotein E-deficient mice: Critical roles for NADPH oxidase and heme oxygenase-1. J. Agric. Food Chem. 2020, 68, 10875–10883. [Google Scholar] [CrossRef]

| Diet | High Fat Diet | High Fat Diet + Bolero (Bo or BoBS) |
|---|---|---|
| Composition, g/kg | ||
| Bo or BoBS | without | 25 |
| Sucrose | 340 | 340 |
| Dairy butter | 200 | 200 |
| Casein | 180.5 | 180.5 |
| Pregelatinized cornstarch | 145 | 145 |
| Premixture of minerals | 70 | 70 |
| Crude cellulose | 50 | 46.77 |
| Premixture of vitamins | 10 | 10 |
| DL-methionine | 3 | 3 |
| Cholesterol | 1.43 | 1.43 |
| Energy, % | ||
| Protein | 17.7 | 17.7 |
| Fat | 41.7 | 41.7 |
| Carbohydrate | 40.6 | 40.6 |
| HFD | HFD Bo | HFD BoBS | |
|---|---|---|---|
| Glucose (g/L) | 1.81 ± 0.34 | 1.23 ± 0.22 | 0.89 ± 0.21 |
| Insulin (g/L) | 0.58 ± 0.03 | 0.86 ± 0.15 | 0.83 ± 0.09 |
| Triglycerides (g/L) | 1.34 ± 0.17 | 0.77 ± 0.14 * | 0.44 ± 0.06 *** |
| Cholesterol (g/L) | 7.70 ± 2.25 | 7.41 ± 1.06 | 6.37 ± 1.14 |
| HDL/LDL | 0.09 ± 0.02 | 0.24 ± 0.06 | 0.19 ± 0.05 |
| HFD | HFD Bo | HFD BoBS | |
|---|---|---|---|
| LVESD (mm) | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.8 ± 0.1 * |
| LVEDD (mm) | 3.6 ± 0.1 | 3.5 ± 0.1 | 4 ± 0.1 * |
| LVESV (mL) | 19.4 ± 2.2 | 16.2 ± 1.4 | 29.8 ± 3.7 * |
| LVEDV (mL) | 55.3 ± 2.4 | 52.5 ± 4 | 73 ± 6 * |
| Stroke volume (mL) | 35.9 ± 2.4 | 36.3 ± 3.4 | 43.2 ± 3 |
| Ejection fraction (%) | 65.1 ± 3.6 | 68.9 ± 2 | 59 ± 5 |
| Shortening fraction (%) | 35.5 ± 2.7 | 38 ± 1.6 | 31 ± 2.1 |
| Cardiac output (mL/min) | 17.4 ± 2.5 | 28.5 ± 4 ** | 25.5 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleti, R.; Coué, M.; Trenteseaux, C.; Hilairet, G.; Fizanne, L.; Kasbi-Chadli, F.; Mallegol, P.; Chaigneau, J.; Boursier, J.; Krempf, M.; et al. Carrot Supplementation Improves Blood Pressure and Reduces Aortic Root Lesions in an Atherosclerosis-Prone Genetic Mouse Model. Nutrients 2021, 13, 1181. https://doi.org/10.3390/nu13041181
Soleti R, Coué M, Trenteseaux C, Hilairet G, Fizanne L, Kasbi-Chadli F, Mallegol P, Chaigneau J, Boursier J, Krempf M, et al. Carrot Supplementation Improves Blood Pressure and Reduces Aortic Root Lesions in an Atherosclerosis-Prone Genetic Mouse Model. Nutrients. 2021; 13(4):1181. https://doi.org/10.3390/nu13041181
Chicago/Turabian StyleSoleti, Raffaella, Marine Coué, Charlotte Trenteseaux, Gregory Hilairet, Lionel Fizanne, Fatima Kasbi-Chadli, Patricia Mallegol, Julien Chaigneau, Jerome Boursier, Michel Krempf, and et al. 2021. "Carrot Supplementation Improves Blood Pressure and Reduces Aortic Root Lesions in an Atherosclerosis-Prone Genetic Mouse Model" Nutrients 13, no. 4: 1181. https://doi.org/10.3390/nu13041181
APA StyleSoleti, R., Coué, M., Trenteseaux, C., Hilairet, G., Fizanne, L., Kasbi-Chadli, F., Mallegol, P., Chaigneau, J., Boursier, J., Krempf, M., Geoffriau, E., Andriantsitohaina, R., & Ouguerram, K. (2021). Carrot Supplementation Improves Blood Pressure and Reduces Aortic Root Lesions in an Atherosclerosis-Prone Genetic Mouse Model. Nutrients, 13(4), 1181. https://doi.org/10.3390/nu13041181