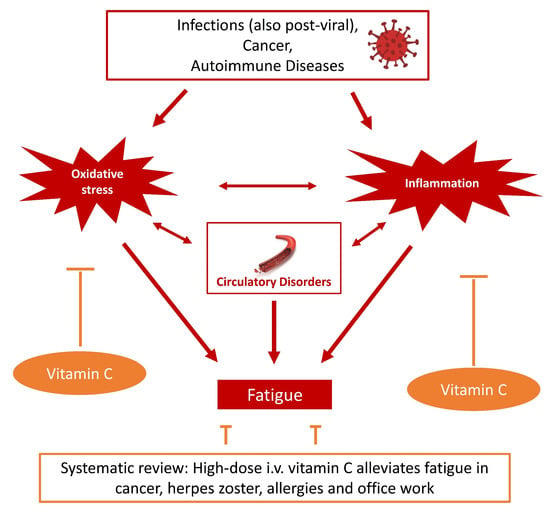
Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue
Abstract
1. Introduction
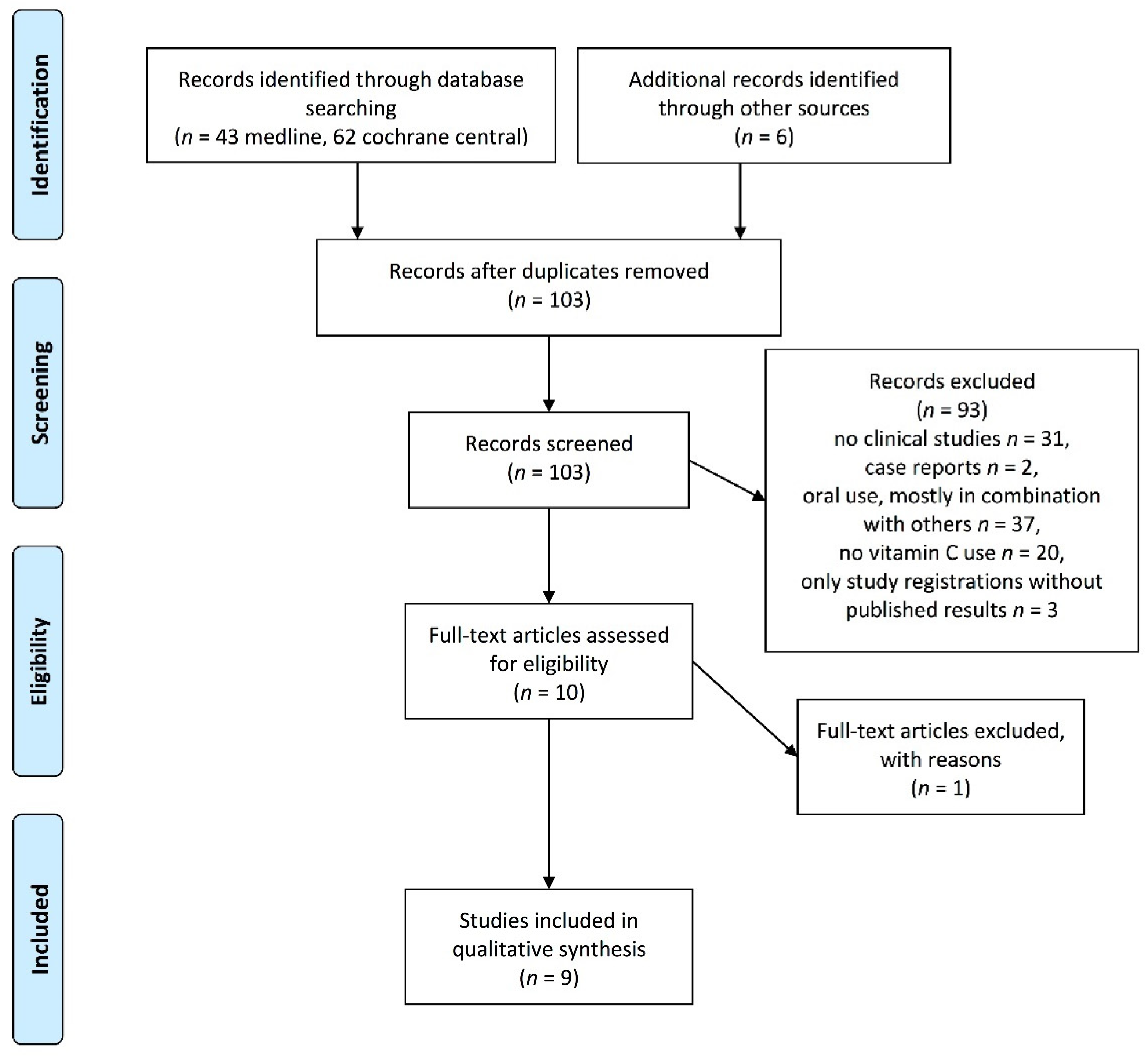
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. (Lausanne) 2020, 7, 606824. [Google Scholar] [CrossRef]
- Bleijenberg, G.; van der Meer, J.W.M. Chapter 442: Chronic Fatigue Syndrome. In Harrison’s Principles of Internal Medicine, 20e; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- NIHR. Living with Covid19. Available online: https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ (accessed on 25 February 2021).
- NIH. COVID-19 Treatment Guidlines: Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 15 March 2021).
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-term effects of COVID-19: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Schonrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Chiscano-Camon, L.; Ruiz-Rodriguez, J.C.; Ruiz-Sanmartin, A.; Roca, O.; Ferrer, R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit. Care 2020, 24, 522. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Dixon, L.; Chambers, S.T. Patients with Community Acquired Pneumonia Exhibit Depleted Vitamin C Status and Elevated Oxidative Stress. Nutrients 2020, 12, 1318. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, B.; Yin, L.; Guo, M.; Shi, H.; Zhu, Z.; Zhang, L.; He, J.; Ling, Y.; Gao, M.; et al. Vitamin C supplementation is necessary for patients with coronavirus disease: An ultra-high-performance liquid chromatography-tandem mass spectrometry finding. J. Pharm Biomed. Anal. 2021, 196, 113927. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. Pilot Study Antioxid. 2021, 10, 257. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum Levels of Vitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study. Med. Drug Discov. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczynski, P.; Zhitkovich, A.; Rubis, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nistico, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Dietary Antioxidants and Related Compounds. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: Concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Kuiper, C.; Vissers, M.C.; Hicks, K.O. Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue. Free Radic. Biol. Med. 2014, 77, 340–352. [Google Scholar] [CrossRef]
- Patterson, T.; Isales, C.M.; Fulzele, S. Low level of Vitamin C and dysregulation of Vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2021, 12, 14–26. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T.; et al. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef]
- Stephenson, C.M.; Levin, R.D.; Spector, T.; Lis, C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharm. 2013, 72, 139–146. [Google Scholar] [CrossRef]
- Takahashi, H.; Mizuno, H.; Yanagisawa, A. High-dose intravenous vitamin C improves quality of life in cancer patients. Pers. Med. Universe 2012, 1, 49–53. [Google Scholar] [CrossRef]
- Yeom, C.H.; Jung, G.C.; Song, K.J. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 25, 983–990. [Google Scholar]
- Schencking, M.; Vollbracht, C.; Weiss, G.; Lebert, J.; Biller, A.; Goyvaerts, B.; Kraft, K. Intravenous vitamin C in the treatment of shingles: Results of a multicenter prospective cohort study. Med. Sci. Monit. 2012, 18, CR215. [Google Scholar] [CrossRef] [PubMed]
- Vollbracht, C.; Raithel, M.; Krick, B.; Kraft, K.; Hagel, A.F. Intravenous vitamin C in the treatment of allergies: An interim subgroup analysis of a long-term observational study. J. Int. Med. Res. 2018, 46, 3640–3655. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, J.S.; Moon, S.; Yeo, J. Effect of Intravenous High Dose Vitamin C on Postoperative Pain and Morphine Use after Laparoscopic Colectomy: A Randomized Controlled Trial. Pain Res. Manag. 2016, 2016, 9147279. [Google Scholar] [CrossRef]
- Suh, S.Y.; Bae, W.K.; Ahn, H.Y.; Choi, S.E.; Jung, G.C.; Yeom, C.H. Intravenous Vitamin C Administration Reduces Fatigue in Office Workers: A Double-blind Randomized Controlled Trial. Nutr. J. 2012, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Vissers, M.C.; Cook, J.S. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat Care 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef]
- Elera-Fitzcarrald, C.; Rocha, J.; Burgos, P.I.; Ugarte-Gil, M.F.; Petri, M.; Alarcon, G.S. Measures of Fatigue in Patients With Rheumatic Diseases: A Critical Review. Arthritis Care Res. 2020, 72 (Suppl. 10), 369–409. [Google Scholar] [CrossRef]
- Mohandas, H.; Jaganathan, S.K.; Mani, M.P.; Ayyar, M.; Rohini Thevi, G.V. Cancer-related fatigue treatment: An overview. J. Cancer Res. Ther. 2017, 13, 916–929. [Google Scholar] [CrossRef]
- Repka, C.P.; Hayward, R. Effects of an Exercise Intervention on Cancer-Related Fatigue and Its Relationship to Markers of Oxidative Stress. Integr. Cancer Ther. 2018, 17, 503–510. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.G.; Lee, D.S.; Son, C.G. Oxidative Stress is a Convincing Contributor to Idiopathic Chronic Fatigue. Sci. Rep. 2018, 8, 12890. [Google Scholar] [CrossRef]
- Morris, G.; Stubbs, B.; Kohler, C.A.; Walder, K.; Slyepchenko, A.; Berk, M.; Carvalho, A.F. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med. Rev. 2018, 41, 255–265. [Google Scholar] [CrossRef]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.M.; Thomas, W.; Zhu, X.; Diebes, A.; McElvain, G.; Baechler, E.; Gross, M. Oxidative stress and fatigue in systemic lupus erythematosus. Lupus 2012, 21, 984–992. [Google Scholar] [CrossRef]
- Pearson, E.J.M.; Morris, M.E.; di Stefano, M.; McKinstry, C.E. Interventions for cancer-related fatigue: A scoping review. Eur. J. Cancer Care 2018, 27. [Google Scholar] [CrossRef]
- Korte, S.M.; Straub, R.H. Fatigue in inflammatory rheumatic disorders: Pathophysiological mechanisms. Rheumatology 2019, 58, v35–v50. [Google Scholar] [CrossRef]
- Lunec, J.; Blake, D.R. The determination of dehydroascorbic acid and ascorbic acid in the serum and synovial fluid of patients with rheumatoid arthritis (RA). Free Radic. Res. Commun. 1985, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, S.; Mehta, H.C.; Sood, A.K.; Kaur, J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin. Chim. Acta 2003, 338, 123–129. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy-Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Subramenium, G.A.; Marchant, J.S.; Said, H.M. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: A role for NF-kappaB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G241–G248. [Google Scholar] [CrossRef]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Sturzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13. [Google Scholar] [CrossRef]
- Bhadelia, N.; Belkina, A.C.; Olson, A.; Winters, T.; Urick, P.; Lin, N.; Rifkin, I.; Kataria, Y.; Yuen, R.R.; Sagar, M.; et al. Distinct Autoimmune Antibody Signatures Between Hospitalized Acute COVID-19 Patients, SARS-CoV-2 Convalescent Individuals, and Unexposed Pre-Pandemic Controls. medRxiv 2021. [Google Scholar] [CrossRef]
- Rottoli, M.; La Gioia, S.; Frigeni, B.; Barcella, V. Pathophysiology, assessment and management of multiple sclerosis fatigue: An update. Expert Rev. Neurother. 2017, 17, 373–379. [Google Scholar] [CrossRef]
- Cramp, F. The role of non-pharmacological interventions in the management of rheumatoid-arthritis-related fatigue. Rheumatology 2019, 58, v22–v28. [Google Scholar] [CrossRef]
- Griggs, S.; Morris, N.S. Fatigue Among Adults With Type 1 Diabetes Mellitus and Implications for Self-Management: An Integrative Review. Diabetes Educ. 2018, 44, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Type; Number of Patients (n); Underlying Disease | IV Vitamin C Dose | Additional Interventions | Estimation of Fatigue | Impact on Fatigue and Related Parameters |
|---|---|---|---|---|---|
| Oncology | |||||
| [21] | Single-center, phase II, randomized clinical trial; n = 97; extensively pretreated patients with advanced, refractory non-small-cell lung cancer | 1 g/kg bw, 3 times/week, 25 treatments in total | Vitamin C group received concurrently modulated electro-hyperthermia; both groups received best supportive care | EORTC QLQ-C30 | Fatigue (mean ± SD) Verum group: pre: 46.48 ± 17.52, post: 20.63 ± 18.14 (* p < 0.0001) Control group: pre: 39.93 ± 20.59, post: 61.34 ± 25.32 (* p < 0.0001) (** p< 0.0001) Physical function ↑ (** p < 0.0001) Cognitive function (** p = 0.1026) Dyspnea ↓ (** p < 0.0001) Insomnia (** p = 0.0772 Pain ↓(p** p < 0.0001) |
| [22] | Single-center phase I clinical trial; n = 17; patients with refractory, advanced solid tumors (stage III-IV; colon, pancreas, breast, etc.) | 0.8–3 g/kg bw, 4 times/week for 4 weeks | None | EORTC QLQ-C30 | Fatigue ↓ (pre: 49/ post 11) Physical function ↑ (pre 69/post 87) Cognitive function ↑ (pre 75/post 83) Dyspnea ↓ (pre 24/post 0) Insomnia ↓ (pre 31/post 17) Pain ↓ (pre 36/ post 0) |
| [23] | Multi-center, prospective observational trial; n = 60; patients with advanced tumors (lung, breast, stomach, colonm etc.) | Increasing dosages up to 50 g and more to achieve plasma levels of 350–400 mg/dL 2 times/week for 4 weeks | +/− chemotherapy | EORTC QLQ-C30 | Fatigue (mean ± SD) Pre: 42.4 ± 28.7 post: 28.4 25.7 (* p < 0.01) Physical function ↑ (* p < 0.05) Cognitive function ↑ (* p < 0.01) Dyspnea (not significant) Insomnia ↓ (* p < 0.01) Pain ↓ (* p < 0.05) |
| [24] | Single-center, prospective before-and-after study; n = 39, terminal cancer patients (stomach, colon, lungs, breast, gall bladder, etc.) | 10 g 2 times/week for one week | None | EORTC QLQ-C30 | Fatigue (mean ± SD) Pre: 52 ± 24, post: 40 ± 19 (* p = 0.001) Physical function ↑ (* p = 0.037) Cognitive function ↑ (* p = 0.002) Dyspnea (p = 0.051) Insomnia ↓ (* p = 0.029) Pain ↓ (* p = 0.013) |
| [25] | Multi-center, retrospective, cohort study; n = 125, patients with breast cancer UICC IIa-IIIb | ≥7.5 g at least 1 time/week for at least 4 weeks | +/− chemotherapy, radiation | 3-point Likert scale | Fatigue (mean ± SD) During adjuvant therapy (first 6 months after operation): Verum: pre: 1.53 ± 1.11, post: 0.71 ± 0.89 Control: pre 1.68 ± 1.004, post: 1.24 ± 0.936 (** p = 0.004) During after care (6–12 month after operation): Verum: 0.34 ± 0.58 Control: 0.64 ± 0.718 (** p = 0.023) Sleep disorders ↓ (** p = 0.005) Depression ↓ (** p = 0.01) |
| Infection, allergies | |||||
| [26] | Multi-center, prospective observational trial; n = 67; patients with herpes zoster infection | 7.5 or 15 g; on average 8 infusions within 2–3 weeks | 55.8% received anti-infective drug | 4-point Likert scale | Fatigue improved in 78.2% of the patients; Impaired concentration improved in 81.8% of the patients |
| [27] | Multi-center, prospective observational trial; n = 71; patients with respiratory and cutaneous allergies | 7.5 g; 2–3 times/week for 2–3 weeks in acute and 11–12 weeks in chronic states | 35 % received anti-allergic drugs | 4-point Likert scale | Sum score (0–12) of the 4 symptoms: fatigue, sleep disorders, depression, and lack of mental concentration decreased from 5.93 to 1.09 (* p < 0.0001) Fatigue improved in 93.5% of patients Sleep disorders improved in 92.5%, depression in 95.5%, and impaired concentration in 91.7% |
| Others | |||||
| [28] | Single-center, randomized, double-blind, controlled clinical trial; n = 97; patients under-going laparoscopic colectomy | 50 mg/ kg bw; Single application after induction of anesthesia | Analgesics | NRS (0–10) | No significant differences in fatigue score 2, 6, and 24 h post operation Pain ↓ (** p < 0.05) |
| [29] | Multi-center, randomized, double-blind, controlled clinical trial; n = 147; apparently healthy full-time worker | 10 g, single application | None | NRS (0–10) | Fatigue (mean ± SD) Verum: Pre: 5.64 ± 2.02, after 2 h: 5.10 ± 2.04, after 24 h: 4.97 ± 2.33 Control: Pre: 5.54 ± 2.07, after 2 h: 5.31 ± 2.00, after 24 h: 5.66 ± 2.16 (** p = 0.004) Plasma vitamin C increased after 2 h, marker for oxidative stress decreased in the verum group (** p < 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollbracht, C.; Kraft, K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients 2021, 13, 1154. https://doi.org/10.3390/nu13041154
Vollbracht C, Kraft K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients. 2021; 13(4):1154. https://doi.org/10.3390/nu13041154
Chicago/Turabian StyleVollbracht, Claudia, and Karin Kraft. 2021. "Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue" Nutrients 13, no. 4: 1154. https://doi.org/10.3390/nu13041154
APA StyleVollbracht, C., & Kraft, K. (2021). Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients, 13(4), 1154. https://doi.org/10.3390/nu13041154