The Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting, a Systematic Review—Gut Permeability as Potential Treatment Target
Abstract
1. Introduction
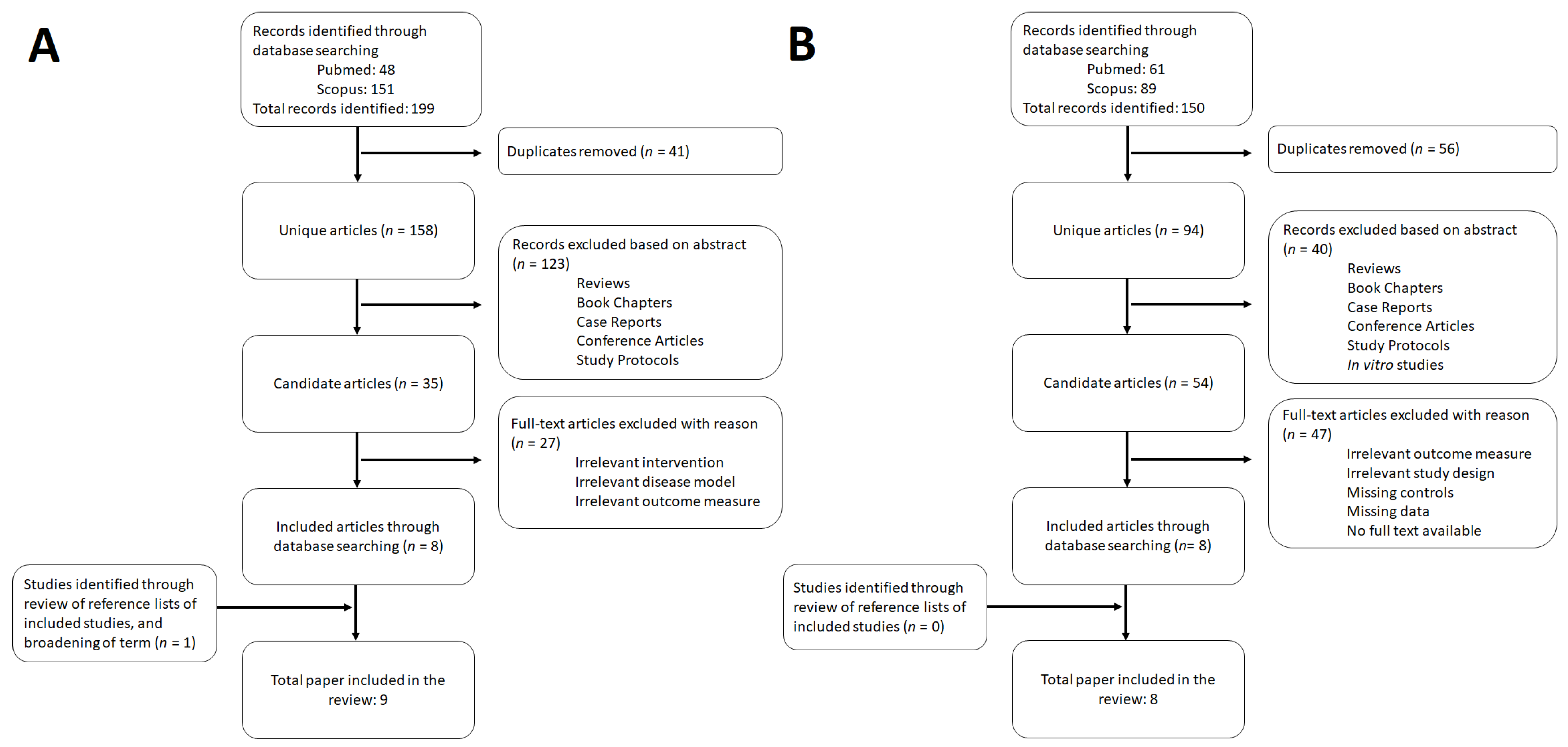
2. Methods
3. Results
3.1. Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting
3.2. Gut Permeability and Muscle Wasting
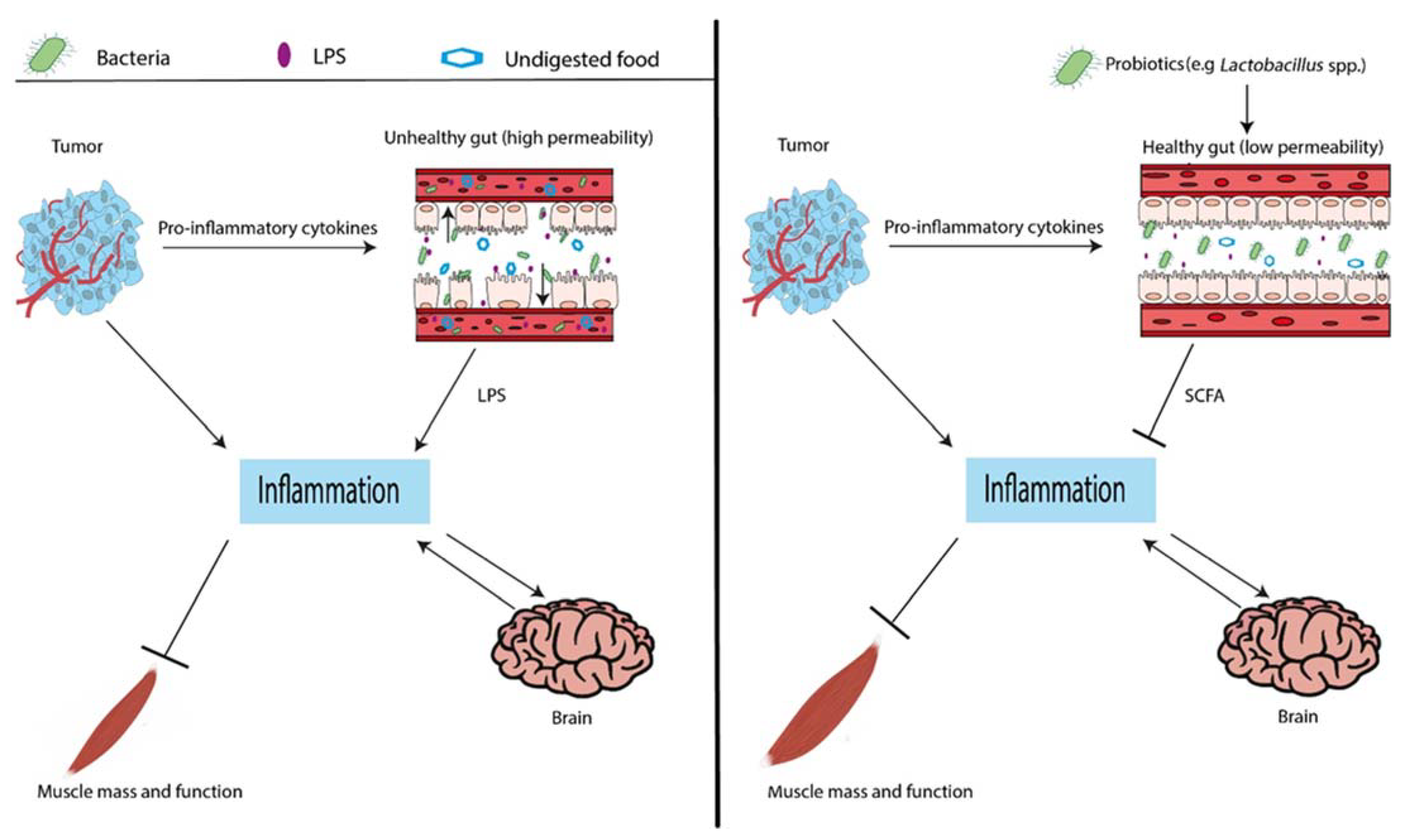
4. Discussion
4.1. Lactobacillus spp., Microbiome Composition, and Gut Permeability
4.2. Lactobacillus spp. and Metabolites
4.3. Lactobacillus spp., Inflammation, and Organ Crosstalk
4.4. Translatability of Mouse Models
4.5. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef] [PubMed]
- Lenk, K.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2010, 1, 9–21. [Google Scholar] [CrossRef]
- Cole, C.L.; Kleckner, I.R.; Jatoi, A.; Schwarz, E.M.; Dunne, R.F. The role of systemic inflammation in cancer-associated muscle wasting and rationale for exercise as a therapeutic intervention. JCSM Clin. Rep. 2018, 3, e00065. [Google Scholar] [CrossRef]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2018, 15, 9–20. [Google Scholar] [CrossRef]
- Witkamp, R.F.; van Norren, K. Let thy food be thy medicine…when possible. Eur. J. Pharmacol. 2018, 836, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.; Sovran, B.; Hugenholtz, F.; Meijer, B.; Hoogerland, J.; Mihailova, V.; van der Ploeg, C.; Belzer, C.; Boekschoten, M.; Hoeijmakers, J.; et al. Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1−/Δ7 Mice. Front. Immunol. 2016, 7, 408. [Google Scholar] [CrossRef] [PubMed]
- Varian, B.J.; Goureshetti, S.; Poutahidis, T.; Lakrits, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Suzuki, H.; Kirisako, T.; Sugihara, Y.; Fujiwara, D. Long-term administration of pDC-Stimulative Lactococcus lactis strain decelerates senescence and prolongs the lifespan of mice. Int. Immunopharmacol. 2018, 58, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, S.; Huan, K.; Hsu, C.; Yang, K.; Li, L.; Chan, C.; Huang, H. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging 2019, 11, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Obermüller, B.; Singer, G.; Kienesberger, B.; Klymiuk, I.; Sperl, D.; Stadlbauer, V.; Horvath, A.; Miekisch, W.; Gierschner, P.; Grabherr, R.; et al. The Effects of Prebiotic Supplementation with OMNi-LOGiC® FIBRE on Fecal Microbiome, Fecal Volatile Organic Compounds, and Gut Permeability in Murine Neuroblastoma-Induced Tumor-Associated Cachexia. Nutrients 2020, 12, 2029. [Google Scholar] [CrossRef]
- Bindels, L.B.; Nayrinck, A.; Salazar, N.; Taminiau, B.; Druart, C.; Muccioli, G.; Francois, E.; Blecker, C.; Richel, A.; Daube, G.; et al. Non Digestible Oligosaccharides Modulate the Gut Microbiota to Control the Development of Leukemia and Associated Cachexia in Mice. PLoS ONE 2015, 10, e0131009. [Google Scholar] [CrossRef]
- Buigues, C.; Gernandez-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martinez, R.; Martinez-Martinez, M.; Verdejo, Y.; Mascaros, M.C.; Peris, C.; Cauli, O. Effect of a prebiotic formulation on frailty syndrome: A randomized, double-blind clinical trial. Int. J. Mol. Sci. 2016, 17, 932. [Google Scholar] [CrossRef]
- An, J.M.; Kang, E.A.; Han, Y.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Kim, D.H.; Hahm, K.B. Dietary intake of probiotic kimchi ameliorated IL-6-driven cancer cachexia. J. Clin. Biochem. Nutr. 2019, 65, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, C.I.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachiklan, B.D.; Neyrinck, A.M.; Thissen, J.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, 37971. [Google Scholar] [CrossRef]
- Cuoco, L.; Vescovo, G.; Castaman, R.; Ravara, B.; Cammarota, G.; Angelini, A.; Salvaginini, M.; Dalla Libera, L. Skeletal muscle wastage in Crohn’s disease: A pathway shared with heart failure? Int. J. Cardiol. 2008, 127, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Neyrinck, A.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Wakino, S.; Irie, J.; Miyamoto, J.; Matsui, A.; Tajima, T.; Itoh, T.; Oshima, Y.; Ysohifuji, A.; Kimura, I.; et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol. Dial. Transplant. 2020, 35, 501–1517. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Goel, R.; Kim, S.; Richards, E.; Carter, C.; Pepine, C.; Raizada, M.; Buford, T. Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, e1–e810. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, B.S.; Deutz, N.E.P.; Rodriguez, R.E.; Engelen, M.K.P.J. Early Signs of Impaired Gut Function Affect Daily Functioning in Patients With Advanced Cancer Undergoing Chemotherapy. J. Parenter. Enter. Nutr. 2020. [Google Scholar] [CrossRef]
- Pötgens, S.A.; Brossel, H.; Sboarina, M.; Catry, E.; Cani, P.; Neyrinck, A.; Delzenne, N.; Bindels, L. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Ko, J.S.; Yang, H.R.; Chang, J.Y.; Seo, J.K. Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-α. World J.Gastroenterol. 2007, 13, 1962–1965. [Google Scholar] [CrossRef]
- Rao, R.K.; Polk, D.B.; Seth, A.; Yan, F. Probiotics the good neighbor: Guarding the gut mucosal barrier. Am. J. Infect. Dis. 2009, 5, 195–199. [Google Scholar] [CrossRef]
- Parassol, N.; Freita, M.; Thoreaux, K.; Dalmasso, G.; Bourdet-Sicard, R.; Rampal, P. Lactobacillus casei DN-114001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res. Microbiol. 2005, 156, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.; Wong, J.; Pahl, M.; Piceno, Y.; Yuan, J.; Desantis, T.; Ni, Z.; Nguyen, T.; Andersen, G. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Haran, J.P.; Bucci, V.; Dutta, P.; Ward, D.; McCormick, B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J. Med. Microbiol. 2018, 67, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.; De La Fuente, M.; Landskron, G.; Gonzalez, M.; Quera, R.; Dijkstra, G.; Harmsen, H.; Faber, K.; Hermoso, M. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Tarashi, S.; Siadat, S.; Badi, S.; Zali, M.; Biassoni, R.; Ponzoni, M.; Moshiri, A. Gut Bacteria and their Metabolites: Which One Is the Defendant for Colorectal Cancer? Microorganisms 2019, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Physiol. Liver Physiol. 2009, 297, G506–G513. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 2011, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.; Hoyles, L.; McCartney, A.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Gullón, B.; Remoroza, C.; Schols, H.A.; Parajó, J.C.; Alonso, J.L. Purification, Characterization, and Prebiotic Properties of Pectic Oligosaccharides from Orange Peel Wastes. J. Agric. Food Chem. 2014, 62, 9769–9782. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.; Liu, W.; Li, T.; Liu, C.; Wu, S.; Wang, Z. Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Verschaeren, A.; De Bruycker, F.; De Preter, V.; Verbeke, K.; Leroy, F.; De Vuyst, L. In vitro kinetics of prebiotic inulin-type fructan fermentation by butyrate-producing colon bacteria: Implementation of online gas chromatography for quantitative analysis of carbon dioxide and hydrogen gas production. Appl. Environ. Microbiol. 2009, 75, 5884–5892. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.; Wells, J.; Flint, H.; Duncan, S. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef] [PubMed]
- Narsale, A.A.; Carson, J.A. Role of interleukin-6 in cachexia: Therapeutic Implications. Curr. Opin. Support Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Szumowski, M.; Levasseur, P.; Grossberg, A.; Zhu, X.; Agarwal, A.; Marks, D. Muscle Atrophy in Response to Cytotoxic Chemotherapy Is Dependent on Intact Glucocorticoid Signaling in Skeletal Muscle. PLoS ONE 2014, 9, e106489. [Google Scholar] [CrossRef]
- Gareau, M.; Jury, J.; MacQueen, G.; Sherman, P.; Perdue, M. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Dwarkasing, T.; Witkamp, R.; Boekschoten, M.; Ter Laak, M.; Heins, M.; van Norren, K. Increased hypothalamic serotonin turnover in inflammation-induced anorexia. BMC Neurosci. 2016, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.; Stehle, P.; Pacini, G.; et al. Intake of lactobacillus reuteri improves incretin and insulin secretion in Glucose-Tolerant humans: A proof of concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.; Lloyd, J.; Walter, P.; Rane, S. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Ayala, J.E.; Bracy, D.P.; James, F.D.; Julien, B.M.; Wasserman, D.H.; Drucker, D.J. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 2009, 150, 1155–1164. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; van Norren, K.; Grefte, S.; Koppelman, S.J.; Lenaerts, K.; Keijer, J.; Witkamp, R.F.; Wichers, H.J. Endurance Exercise Increases Intestinal Uptake of the Peanut Allergen Ara h 6 after Peanut Consumption in Humans. Nutrients 2017, 9, 84. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Henning, J.-W.; Baydoun, M.; Piedalue, K.-A.; McLennan, A.; Carlson, L.E. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; Study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef]
- Smiljanec, K.; Lennon, S.L. Sodium, hypertension, and the gut: Does the gut microbiota go salty? Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1173–h1182. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, M.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.L.; Beck, C.A.; Robinson, D.; Ye, J.; Mills, B.; Gerber, S.A.; Schwarz, E.M.; Linehan, D. Dual Energy X-ray Absorptiometry (DEXA) as a longitudinal outcome measure of cancer-related muscle wasting in mice. PLoS ONE 2020, 15, e0230695. [Google Scholar] [CrossRef] [PubMed]
| Probiotics | |||||||
| Family | Source | Model | Condition | Intervention | Muscle Outcome | Secondary Outcome | Reference |
| Lactobacillus | reuteri | C57BL/6 Apcmin/+ mice | Spontaneous intestinal adenoma | 3.5 × 105 CFU/day, 20 weeks | Muscle-to-BW ratio *, fiber size * | Intestinal polyps * and blood neutrophils * | [12] |
| Lactobacillus | reuteri + gasseri | BALB/c mice (female) | BaF acute leukemia | 2 × 108 CFU/mL drinking water, from disease induction onwards | Muscle (mg) | Lactobacillus spp. *, food intake (-), body weight change (-), and IL-6 * | [21] |
| Lactobacillus | lactis | SAMP6 mice (female) | Aging (senescence-accelerated) | 1 mg/day from 7 to 12 weeks of age | Muscle-to-BW ratio * | Survival *, senescence score *, and IL1beta * | [13] |
| Lactobacillus | paracasei | SAMP8 mice (female) | Aging (senescence-accelerated) | 1 × 109 CFU/day from 16 to 28 weeks of age | Muscle (% of body) *, muscle strength * | Food intake (-), protein intake (-), TNFalfa *, and IL-6 * | [14] |
| Lactobacillus | reuteri | CD-1 mice | Aging | 3.5 × 105 CFU/day from 2 to 12 months of age | Muscle-to-BW ratio *, fiber size * | Survival *, blood neutrophils * | [12] |
| Lactobacillus | casei | C57BL/6 mice (male) | Aging | 2 × 109 CFU/day for 12 weeks from 10 months of age | Muscle-to-BW ratio *, forelimb grip strength * | Food intake (-), fatigue *, gut barrier proteins mRNA *, Lactobacillus spp. *, Bifidobacterium spp. | [15] |
| Bifidobacterium | longum | C57BL/6 mice (male) | Aging | 2 × 109 CFU/day for 12 weeks from 10 months of age | Muscle-to-BW ratio *, forelimb grip strength | Food intake (-), fatigue *, gut barrier proteins mRNA *, Bifidobacterium spp. | [15] |
| Prebiotics | |||||||
| Type | Model | Condition | Intervention | Muscle Outcome | Secondary Outcome | Reference | |
| POS | BALB/c Rj:ATHYM-Foxn1nu/numice (male) | Neuroblastoma | 200 mg/day | Muscle/mm (-) (no cachexia developed) | Lactobacillus spp. (-), gut permeability (-), food consumption (-) | [16] | |
| POS | BALB/c mice (male) | BaF acute leukemia | 5% POS for 2 weeks | Muscle (mg) (-) (no cachexia developed) | Lactobacillus spp. (-), anorexia * and propionate * | [17] | |
| Inulin | BALB/c mice (male) | BaF acute leukemia | 5% inulin for 2 weeks | Muscle (mg) (-) (no cachexia developed) | Lactobacillus spp. (-), anorexia *, propionate and butyrate * | [17] | |
| Inulin + FOS | Elderly (aged 65 and over) | Frailty syndrome | 3375 mg inulin + 3488 mg FOS/day for 13 weeks | Hand grip strength * | Energy intake (-), exhaustion * | [18] | |
| Synbiotics | |||||||
| Probiotic | Prebiotic | Model | Condition | Intervention | Muscle Outcome | Secondary Outcome | Reference |
| Leuconostoc mesenteroides + Lactobacillus plantarum | Kimchi | BALB/c mice (male) | C26 colon carcinoma | Normal diet and cpKimchi diet for 3 weeks | Muscle mass *, ubiquitin *, AMPK *, PGC1-a * | Cachexia-induced lipolysis *, lipogenesis *, NF-κB *, AKT *, mTOR *, PI3K * and IL-6 * | [19] |
| Lactobacillus reuteri | OF | BALB/c mice (female) | BaF acute leukemia | 2 × 108 CFU/mL probiotic + 0.2 g/day prebiotic from disease induction onwards | Muscle (% BW) * | Energy intake (-), survival (-), and gut barrier proteins mRNA * | [20] |
| Model | Condition | Type of Intervention | Gut Permeability | Muscle Mass | Reference |
|---|---|---|---|---|---|
| BALB/c Rj:ATHYM-Foxn1nu/nu male mice | NB cells | Prebiotics: 200 mg/day oligosaccharides | Gut permeability in NB *, no difference after intervention (-) | Muscle mass in NB (-) (no cachexia developed), no difference after intervention (-) | [16] |
| Female Balb/c mice | Leukemia (BaF cells) | Synbiotic: inulin-type fructans (0.2 g/day) and Lactobacillus reuteri (average: 5.8 × 108 CFU/day) | mRNA expression tight junction genes after BaF injection * mRNA expression tight junction genes after intervention * | Muscle mass after BaF injection *, muscle mass after intervention | [20] |
| ICR-specific pathogen-free male mice | CKD | FMT | Expression tight junction protein in CKD *, expression tight junction protein after intervention | Muscle mass in CKD *, muscle mass in after intervention * | [24] |
| Male CD2F1 mice | C26 cells, cancer | N.A. | Gut permeability after C26 injection * | Muscle mass after C26 injection * | [23] |
| CD1 mice | Aging | Probiotics: Lactobacillus casei or Bifidobacterium longum (3.5 × 105 CFU/day) from 2 to 12 months of age | mRNA expression tight junction genes in old mice *, mRNA expression tight junction genes after intervention * | Muscle-to-BW ratio in old mice *, muscle-to-BW ratio after intervention * Forelimb grip strength in old mice *, forelimb strength after intervention | [15] |
| Patients with solid tumors undergoing chemotherapy (n = 16) | Cancer | N.A. | Small-intestinal membrane permeability (-) | Muscle strength * | [26] |
| Newly diagnosed patients (n = 13) 17–49 years | Crohn’s disease | N.A. | Gut permeability * | Muscle mass * | [22] |
| Healthy elderly (n = 18) >70 years | Aging | N.A. | Gut permeability * | Muscle strength * | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Krimpen, S.J.; Jansen, F.A.C.; Ottenheim, V.L.; Belzer, C.; van der Ende, M.; van Norren, K. The Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting, a Systematic Review—Gut Permeability as Potential Treatment Target. Nutrients 2021, 13, 1115. https://doi.org/10.3390/nu13041115
van Krimpen SJ, Jansen FAC, Ottenheim VL, Belzer C, van der Ende M, van Norren K. The Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting, a Systematic Review—Gut Permeability as Potential Treatment Target. Nutrients. 2021; 13(4):1115. https://doi.org/10.3390/nu13041115
Chicago/Turabian Stylevan Krimpen, Sandra J., Fleur A. C. Jansen, Veerle L. Ottenheim, Clara Belzer, Miranda van der Ende, and Klaske van Norren. 2021. "The Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting, a Systematic Review—Gut Permeability as Potential Treatment Target" Nutrients 13, no. 4: 1115. https://doi.org/10.3390/nu13041115
APA Stylevan Krimpen, S. J., Jansen, F. A. C., Ottenheim, V. L., Belzer, C., van der Ende, M., & van Norren, K. (2021). The Effects of Pro-, Pre-, and Synbiotics on Muscle Wasting, a Systematic Review—Gut Permeability as Potential Treatment Target. Nutrients, 13(4), 1115. https://doi.org/10.3390/nu13041115








