Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Ethics
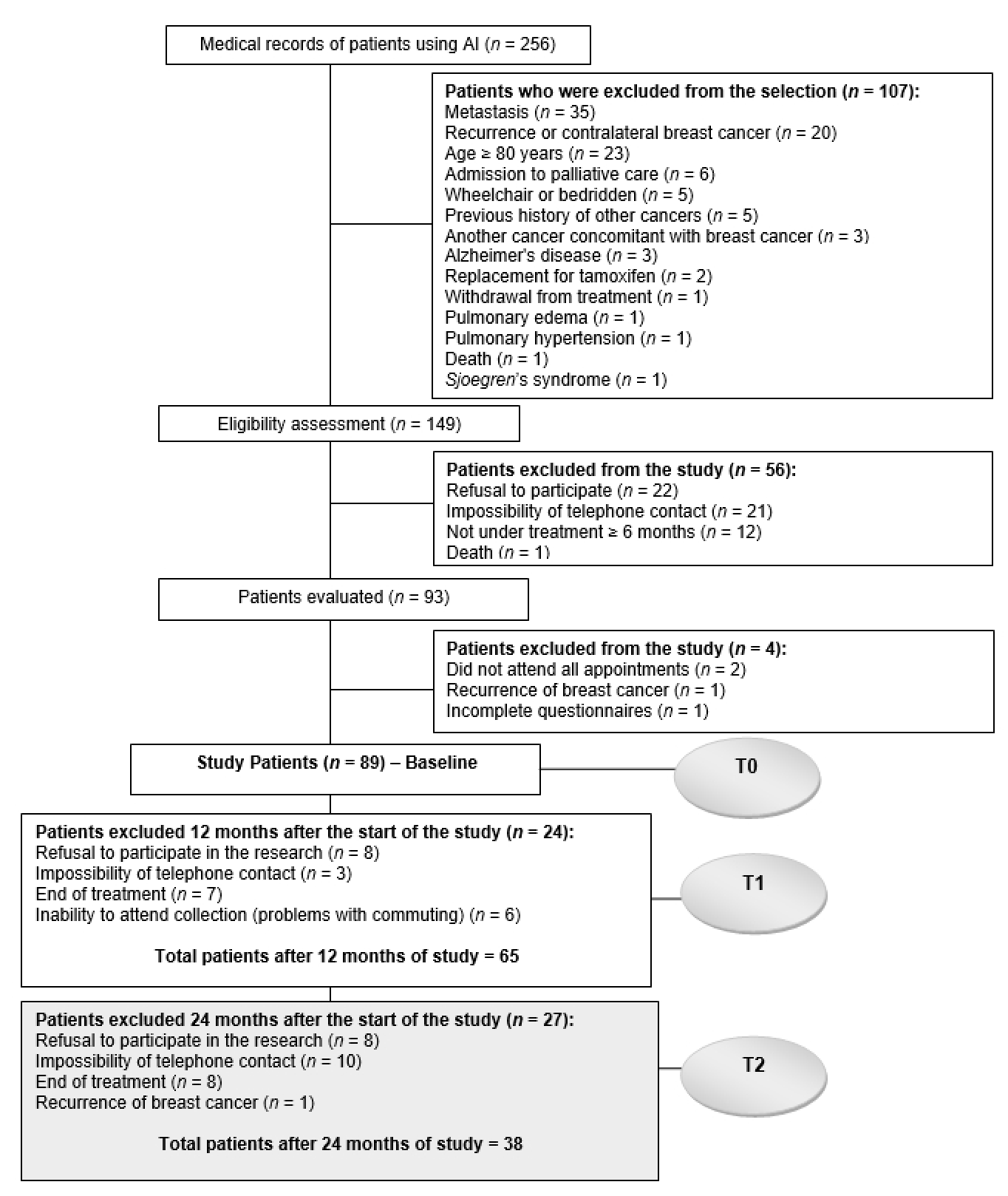
2.2. Sample Size and Eligibility Criteria
2.3. Clinical, Sociodemographic and Lifestyle Data
2.4. Anthropometric Measurement
2.5. Biochemical Data
2.6. Dietary Data
Brazilian Healthy Eating Index—Revised
2.7. Cardiovascular Risk Factors
2.8. Statistical Analysis
3. Results
3.1. Sample Characterization
3.2. Analysis of Changes in Risk Factors for CVDs over Time
3.3. Percentage of Inadequacy and Number of Risk Factors for CVDs
3.4. Number of Risk Factors, C-Reactive Protein and Phase Angle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breast Cancer Now Most Common Form of Cancer: WHO Taking Action. Available online: https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common-form-of-cancer-who-taking-action (accessed on 13 March 2021).
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.T.; Stevens, J.; Khankari, N.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology 2016, 27, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Buttros, D.d.A.; Branco, M.; Orsatti, C.; Almeida-Filho, B.D.; Nahas-Neto, J.; Nahas, E. High Risk for Cardiovascular Disease in Postmenopausal Breast Cancer Survivors. Menopause 2019, 26, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef]
- Lee Chuy, K.; Yu, A.F. Cardiotoxicity of Contemporary Breast Cancer Treatments. Curr. Treat. Options Oncol. 2019, 20, 51. [Google Scholar] [CrossRef]
- Sharma, A.V.; Reddin, G.; Forrestal, B.; Barac, A. Cardiovascular Disease Risk in Survivors of Breast Cancer. Curr. Treat. Options Cardio. Med. 2019, 21, 79. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Reinbolt, R.E.; Mangini, N.; Hill, J.L.; Levine, L.B.; Dempsey, J.L.; Singaravelu, J.; Koehler, K.A.; Talley, A.; Lustberg, M.B. Endocrine Therapy in Breast Cancer: The Neoadjuvant, Adjuvant, and Metastatic Approach. Semin. Oncol. Nurs. 2015, 31, 146–155. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F. Effect of Anastrozole and Tamoxifen as Adjuvant Treatment for Early-Stage Breast Cancer: 10-Year Analysis of the ATAC Trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase Inhibitors versus Tamoxifen in Early Breast Cancer: Patient-Level Meta-Analysis of the Randomised Trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Matthews, A.; Stanway, S.; Farmer, R.E.; Strongman, H.; Thomas, S.; Lyon, A.R.; Smeeth, L.; Bhaskaran, K. Long Term Adjuvant Endocrine Therapy and Risk of Cardiovascular Disease in Female Breast Cancer Survivors: Systematic Review. BMJ 2018, 363. [Google Scholar] [CrossRef]
- Cheung, Y.-M.; Ramchand, S.K.; Yeo, B.; Grossmann, M. Cardiometabolic Effects of Endocrine Treatment of Estrogen Receptor-Positive Early Breast Cancer. J. Endocr. Soc. 2019, 3, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Arieas, E.T.; Zhang, Z.; DeFilippis, A.; Tarpinian, K.; Jeter, S.; Nguyen, A.; Henry, N.L.; Flockhart, D.A.; Hayes, D.F.; et al. Comparison of Breast Cancer Recurrence Risk and Cardiovascular Disease Incidence Risk among Postmenopausal Women with Breast Cancer. Breast Cancer Res. Treat. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation 2015, 132, 2212–2219. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Mattei, J.; Fung, T.T.; Li, Y.; Pan, A.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. N. Engl. J. Med. 2017, 377, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Kwan, M.L.; Kushi, L.; Weltzien, E.K.; Castillo, A.; Caan, B.J. Adiposity, Post-Diagnosis Weight Change and Risk of Cardiovascular Events among Early-Stage Breast Cancer Survivors. Breast Cancer Res. Treat. 2017, 162, 549–557. [Google Scholar] [CrossRef]
- Kirkham, A.A.; Bland, K.A.; Sayyari, S.; Campbell, K.L.; Davis, M.K. Clinically Relevant Physical Benefits of Exercise Interventions in Breast Cancer Survivors. Curr. Oncol. Rep. 2016, 18, 12. [Google Scholar] [CrossRef]
- Xavier, H.T.; Izar, M.C.; Faria Neto, J.R.; Assad, M.H.; Rocha, V.Z.; Sposito, A.C.; Fonseca, F.A.; Santos, J.E.d.; Santos, R.D.; Bertolami, M.C.; et al. V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq. Bras. Cardiol. 2013, 101, 1–22. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Lee, K.; Kiwata, J.L. Reducing the Risk of Breast Cancer Recurrence: An Evaluation of the Effects and Mechanisms of Diet and Exercise. Curr. Breast Cancer Rep. 2016, 8, 139–150. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- O’Keefe, E.L.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog. Cardiovasc. Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Kampman, E.; Trentham-Dietz, A.; Egan, K.M.; Titus, L.J.; Baron, J.A.; Hampton, J.M.; Passarelli, M.N.; Willett, W.C. Alcohol Consumption before and after Breast Cancer Diagnosis: Associations with Survival from Breast Cancer, Cardiovascular Disease, and Other Causes. J. Clin. Oncol. 2013, 31, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Banks, E.; Joshy, G.; Korda, R.J.; Stavreski, B.; Soga, K.; Egger, S.; Day, C.; Clarke, N.E.; Lewington, S.; Lopez, A.D. Tobacco Smoking and Risk of 36 Cardiovascular Disease Subtypes: Fatal and Non-Fatal Outcomes in a Large Prospective Australian Study. BMC Med. 2019, 17, 128. [Google Scholar] [CrossRef]
- Kianoush, S.; Yakoob, M.Y.; Al-Rifai, M.; DeFilippis, A.P.; Bittencourt, M.S.; Duncan, B.B.; Bensenor, I.M.; Bhatnagar, A.; Lotufo, P.A.; Blaha, M.J. Associations of Cigarette Smoking With Subclinical Inflammation and Atherosclerosis: ELSA-Brasil (The Brazilian Longitudinal Study of Adult Health). J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-Reactive Protein in the Prediction of Cardiovascular Diseases: Overview of the Latest Clinical Studies and Public Health Practice. J. Cell. Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical Phase Angle and Impedance Vector Analysis—Clinical Relevance and Applicability of Impedance Parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.A.; Jorge, A.J.; de Andrade Martins, W.; Cardoso, G.P.; Dos Santos, M.M.; Rosa, M.L.; Lima, G.A.; de Moraes, R.Q.; da Cruz Filho, R.A. Phase Angle Measured by Electrical Bioimpedance and Global Cardiovascular Risk in Older Adults. Geriatr. Gerontol. Int. 2018, 18, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical Impedance Phase Angle as a Prognostic Indicator in Breast Cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated Biomarkers of Inflammation Are Associated With Reduced Survival Among Breast Cancer Patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion. What Is Excessive Alcohol Use? Available online: https://www.cdc.gov/alcohol/onlinemedia/infographics/excessive-alcohol-use.html (accessed on 10 September 2020).
- Brasil. Ministério da Saúde. Vigitel Brasil 2019: Vigilância de Fatores de Risco e Proteção Para Doenças Crônicas Por Inquérito Telefônico: Estimativas Sobre Frequência e Distribuição Sociodemográfica de Fatores de Risco e Proteção Para Doenças Crônicas Nas Capitais Dos 26 Estados Brasileiros e No Distrito Federal Em 2019. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco.pdf (accessed on 27 August 2020).
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; ISBN 978-0-87322-121-4. [Google Scholar]
- WHO. Obesity: Preventing and Managing the Global Epidemic. Available online: http://www.who.int/entity/nutrition/publications/obesity/WHO_TRS_894/en/index.html (accessed on 6 January 2020).
- Lipschitz, D.A. Screening for Nutritional Status in the Elderly. Prim. Care 1994, 21, 55–67. [Google Scholar] [PubMed]
- Ashwell, M.; Hsieh, S.D. Six Reasons Why the Waist-to-Height Ratio Is a Rapid and Effective Global Indicator for Health Risks of Obesity and How Its Use Could Simplify the International Public Health Message on Obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Rato, Q. Índice de conicidade: Uma medida antropométrica a avaliar. Rev. Port. Cardiol. 2017, 36, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Cômodo, A.R.O.; Dias, A.C.F.; Tomaz, B.A.; Silva Filho, A.A.; Werustsky, C.A.; Ribas, D.F.; Spolidoro, J.; Marchini, J.S. Utilização da bioimpedância para avaliação da massa corpórea. Available online: https://diretrizes.amb.org.br/_BibliotecaAntiga/utilizacao-da-bioimpedancia-para-avaliacao-da-massa-corporea.pdf (accessed on 6 January 2020).
- Faria, E.R. Critérios diagnósticos e fatores de risco para síndrome metabólica, em adolescentes que já apresentaram a menarca, de escolas públicas de Viçosa-MG. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2007. [Google Scholar]
- Faludi, A.; Izar, M.; Saraiva, J.; Chacra, A.; Bianco, H.; Afiune Neto, A.; Bertolami, A.; Pereira, A.; Lottenberg, A.; Sposito, A.; et al. Atualização da diretriz brasileira de dislipidemias e prevenção da aterosclerose—2017. Arq. Bras. Cardiol. 2017, 109. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Kahn, H.S. The “Lipid Accumulation Product” Performs Better than the Body Mass Index for Recognizing Cardiovascular Risk: A Population-Based Comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef]
- Santos, R.; Gagliardi, A.; Xavier, H.; Magnoni, C.; Cassani, R.; Lottenberg, A.; Casella Filho, A.; Araújo, D.; Cesena, F.; Alves, R.; et al. I Diretriz sobre o consumo de Gorduras e Saúde Cardiovascular. Arq. Bras. Cardiol. 2013, 100, 1–40. [Google Scholar] [CrossRef]
- INSTITUTE OF MEDICINE (IOM) Dietary Reference Intakes Tables and Application: Health and Medicine Division. Available online: http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx (accessed on 4 February 2020).
- Nusser, S.M.; Carriquiry, A.L.; Dodd, K.W.; Fuller, W.A. A Semiparametric Transformation Approach to Estimating Usual Daily Intake Distributions. J. Am. Stat. Assoc. 1996, 91, 1440–1449. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Fisberg, R.M.; Slater, B.; Barros, R.R.; Lima, F.D.d.; Cesar, C.L.G.; Carandina, L.; Barros, M.B.D.A.; Goldbaum, M. Índice de Qualidade da Dieta: Avaliação da adaptação e aplicabilidade. Rev. Nutr. 2004, 17, 301–318. [Google Scholar] [CrossRef]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and Applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Previdelli, Á.N.; Andrade, S.C.D.; Pires, M.M.; Ferreira, S.R.G.; Fisberg, R.M.; Marchioni, D.M. Índice de Qualidade da Dieta Revisado para população brasileira. Rev. Saúde Pública 2011, 45, 794–798. [Google Scholar] [CrossRef]
- Pinheiro, A.B.V.; Lacerda, E.M.D.A.; Benzecry, E.H.; Gomes, M.C.D.S.; Costa, V.M.D. Tabela Para Avaliação de Consumo Alimentar Em Medidas Caseiras, 4th ed.; Atheneu: Rio de Janeiro, Brazil, 2001. [Google Scholar]
- BRASIL. MINISTÉRIO DA SAÚDE Guia Alimentar Para a População Brasileira: Promovendo a Alimentação Saudável. Available online: http://189.28.128.100/nutricao/docs/geral/guia_alimentar_conteudo.pdf (accessed on 6 January 2020).
- Lima, M.T.M.; Maruyama, T.C.; Custódio, I.D.D.; Marinho, E.D.C.; Ferreira, I.B.; Crispim, C.A.; Paiva, C.E.; Maia, Y.C.D.P. The Impact Of A Higher Eating Frequency On The Diet Quality And Nutritional Status Of Women With Breast Cancer Undergoing Chemotherapy. Br. J. Nutr. 2019, 1–24. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline Cardiotoxicity: An Update on Mechanisms, Monitoring and Prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Deiner, C.; Shagdarsuren, E.; Schwimmbeck, P.L.; Rosenthal, P.; Loddenkemper, C.; Rauch, U.; Pauschinger, M.; Dietz, R.; Schultheiss, H.-P.; Dechend, R.; et al. Nf-Kappab and AP-1 Activation Is Associated with Late Lumen Loss after Porcine Coronary Angioplasty and Antiproliferative Beta-Irradiation. Cardiovasc. Res. 2007, 75, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Milliron, B.-J.; Vitolins, M.Z.; Tooze, J.A. Usual Dietary Intake among Female Breast Cancer Survivors Is Not Significantly Different from Women with No Cancer History: Results of the National Health and Nutrition Examination Survey, 2003–2006. J. Acad. Nutr. Diet. 2014, 114, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Onvani, S.; Haghighatdoost, F.; Surkan, P.J.; Larijani, B.; Azadbakht, L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index Dietary Patterns and Mortality from All Causes, Cardiovascular Disease and Cancer: A Meta-Analysis of Observational Studies. J. Hum. Nutr. Diet. 2017, 30, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary Fat and Cardiometabolic Health: Evidence, Controversies, and Consensus for Guidance. BMJ 2018, k2139. [Google Scholar] [CrossRef]
- Nestel, P.J.; Beilin, L.J.; Clifton, P.M.; Watts, G.F.; Mori, T.A. Practical Guidance for Food Consumption to Prevent Cardiovascular Disease. Heart Lung Circ. 2021, 30, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Ceccatto, V.; Cesa, C.; Kunradi Vieira, F.G.; Altenburg de Assis, M.A.; Crippa, C.G.; Faria Di Pietro, P. Characteristics of Newly Diagnosed Women with Breast Cancer: A Comparison with the Recommendations of the WCRF/AICR Second Report. Nutr. Hosp. 2012, 27, 1973–1980. [Google Scholar] [CrossRef]
- Boyle, T.; Vallance, J.K.; Ransom, E.K.; Lynch, B.M. How Sedentary and Physically Active Are Breast Cancer Survivors, and Which Population Subgroups Have Higher or Lower Levels of These Behaviors? Support Care Cancer 2016, 24, 2181–2190. [Google Scholar] [CrossRef]
- Huneidi, S.A.; Wright, N.C.; Atkinson, A.; Bhatia, S.; Singh, P. Factors Associated with Physical Inactivity in Adult Breast Cancer Survivors-A Population-Based Study. Cancer Med. 2018, 7, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Lanier, J.B.; Bury, D.C.; Richardson, S.W. Diet and Physical Activity for Cardiovascular Disease Prevention. Am. Fam. Physician 2016, 93, 919–924. [Google Scholar] [PubMed]
- Lei, Y.-Y.; Ho, S.C.; Cheng, A.; Kwok, C.; Lee, C.-K.I.; Cheung, K.L.; Lee, R.; Loong, H.H.F.; He, Y.-Q.; Yeo, W. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Guideline Is Associated With Better Health-Related Quality of Life Among Chinese Patients With Breast Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.K.; Wiggins, C.L.; Nibbe, A.M.; Storlie, C.B.; Prossnitz, E.R.; Royce, M.; Lomo, L.C.; Hill, D.A. Obesity and Survival among a Cohort of Breast Cancer Patients Is Partially Mediated by Tumor Characteristics. NPJ Breast Cancer 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cercato, C.; Fonseca, F.A. Cardiovascular Risk and Obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Bradshaw, P.T.; Prado, C.M.; Alexeeff, S.; Albers, K.B.; Castillo, A.L.; Caan, B.J. Adipose Tissue Distribution and Cardiovascular Disease Risk Among Breast Cancer Survivors. JCO 2019, 37, 2528–2536. [Google Scholar] [CrossRef]
- Chen, G.-C.; Arthur, R.; Iyengar, N.M.; Kamensky, V.; Xue, X.; Wassertheil-Smoller, S.; Allison, M.A.; Shadyab, A.H.; Wild, R.A.; Sun, Y.; et al. Association between Regional Body Fat and Cardiovascular Disease Risk among Postmenopausal Women with Normal Body Mass Index. Eur. Heart J. 2019, 40, 2849–2855. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Kushner, R.F.; Sorensen, K.W. Lifestyle Medicine: The Future of Chronic Disease Management. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. American Cancer Society’s SCS-II Cancer Survivors’ Adherence to Lifestyle Behavior Recommendations and Associations with Health-Related Quality of Life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, C.J.; Tsaroucha, A.K.; Gogas, H.J. Effect of Aromatase Inhibitors on the Lipid Profile of Postmenopausal Breast Cancer Patients. Clin. Lipidol. 2010, 5, 245–254. [Google Scholar] [CrossRef]
- Lee, I.; Kim, S.; Kang, H. Lifestyle Risk Factors and All-Cause and Cardiovascular Disease Mortality: Data from the Korean Longitudinal Study of Aging. Int. J. Environ. Res. Public Health 2019, 16, 3040. [Google Scholar] [CrossRef]
- Guarino, A.; Polini, C.; Forte, G.; Favieri, F.; Boncompagni, I.; Casagrande, M. The Effectiveness of Psychological Treatments in Women with Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 209. [Google Scholar] [CrossRef] [PubMed]

| Category | Factor | Criteria for Cardiovascular Risk | Analysis 1 (NRF = 20) | Analysis 2 (NRF = 25) |
|---|---|---|---|---|
| Treatment | Potentially Cardiotoxic Chemotherapy | Underwent | X | X |
| Radiotherapy | Underwent | X | X | |
| Comorbidities | Diabetes Mellitus | Presence | X | X |
| Arterial Hypertension | Presence | X | X | |
| Lifestyle | Smoking | Presence | X | X |
| Alcohol Consumption | ≥8 Drinks per Week | X | X | |
| Physical Activity | Physical Inactivity | X | X | |
| Anthropometry | Overweight | Presence | X | X |
| Abdominal Adiposity | WHtR > 0.5 | X | X | |
| Quantitative Food Consumption | Total Fat | >30% TCV | X | X |
| Saturated Fat | >7% TCV | X | X | |
| Polyunsaturated Fat | <6% e > 10% TCV | X | X | |
| Monounsaturated Fat | <15% e > 20% TCV | X | X | |
| Trans Fat | >1% TCV | X | X | |
| Omega 3 Fatty Acid | <1 g/Day | X | X | |
| Omega 6/Omega 3 Ratio | >5:1 | X | X | |
| Cholesterol | >300 mg/Day | X | X | |
| Fiber | Total < 25 g/Day and Soluble < 6 g/Day | X | X | |
| Sodium | <2300 mg/Day | X | X | |
| Qualitative Food Consumption | Total BHEI-R | <64.38 | X | X |
| Lipid Profile | Total Cholesterol | ≥240 mg/dL | * | X |
| Non-HDL Cholesterol | >160 mg/dL | * | X | |
| LDL Cholesterol | >160 mg/dL | * | X | |
| HDL Cholesterol | <40 mg/dL | * | X | |
| Triglycerides | >200 mg/dL | * | X |
| Variable | Median (p25–p75) and n (%) |
|---|---|
| Age (years) | 65 (58.5–69.5) |
| Education level | |
| Below high school | 61 (68.5) |
| High school or higher education | 28 (31.5) |
| Income (minimum wage) | |
| <3 | 53 (59.6) |
| ≥3 | 36 (40.4) |
| Surgery | |
| Breast-conserving surgery | 51 (57.3) |
| Mastectomy | 38 (42.7) |
| Radiotherapy | 75 (84.3) |
| Chemotherapy | |
| Adjuvant | 53 (59.6) |
| Neoadjuvant | 15 (16.9) |
| Chemotherapy regimen | |
| Potentially cardiotoxic | 53 (59.6) |
| Non-cardiotoxic | 34 (38.2) |
| NR | 2 (2.2) |
| Tumoral subtype | |
| Ductal | 86 (96.6) |
| Lobular | 3 (3.4) |
| Clinical stage | |
| I | 26 (29.2) |
| II | 48 (53.9) |
| III | 13 (14.6) |
| NR | 2 (2.2) |
| Tumor grade | |
| G1 | 14 (15.7) |
| G2 | 66 (74.2) |
| G3 | 5 (5.6) |
| NR | 4 (4.5) |
| Positive estrogen receptor | 85 (95.5) |
| Positive progesterone receptor | 76 (85.4) |
| HER-2 negative | 71 (79.8) |
| Molecular subtype | |
| Luminal A | 30 (33.7) |
| Luminal B | 54 (60.7) |
| NR | 5 (5.6) |
| Median AI usage time (in months) | 29.5 (18.1–41.8) |
| Component BHEI-R | Punctuation Minimum–Maximum | T0 | T1 | T2 | p-Value |
|---|---|---|---|---|---|
| Mean ± SE | Mean ± SD | Mean ± SD | |||
| Total Fruit | 0–5 | 2.81 ± 0.32 | 3.20 ± 0.26 | 3.04 ± 0.33 | 0.502 |
| Whole Fruit | 0–5 | 3.15 ± 0.36 | 3.42 ± 0.27 | 3.06 ± 0.39 | 0.410 |
| Total Vegetables | 0–5 | 3.46 ± 0.20 | 3.68 ± 0.17 | 3.82 ± 0.22 | 0.534 |
| Dark Green and Orange Vegetables and Legumes | 0–5 | 2.38 ± 0.27 | 2.87 ± 0.23 | 2.87 ± 0.28 | 0.318 |
| Total Grains | 0–5 | 4.43 ± 0.12 | 4.39 ± 0.10 | 4.31 ± 0.12 | 0.774 |
| Whole Grains | 0–5 | 0.70 ± 0.21 | 0.61 ± 0.17 | 0.89 ± 0.22 | 0.434 |
| Milk and Dairy Products | 0–10 | 4.73 ± 0.73 | 3.97 ± 0.64 | 4.08 ± 0.74 | 0.316 |
| Meat, Eggs and Legumes | 0–10 | 7.59 ± 0.35 a | 8.61 ± 0.24 b | 7.85 ± 0.32 a.b | 0.008 |
| Oils | 0–10 | 9.57 ± 0.18 a.b | 9.51 ± 0.18 a | 10.03 ± 0.13 b | 0.022 |
| Saturated Fat | 0–10 | 5.55 ± 0.50 | 5.73 ± 0.42 | 5.89 ± 0.52 | 0.913 |
| Sodium | 0–10 | 3.81 ± 0.42 | 3.26 ± 0.34 | 3.20 ± 0.44 | 0.444 |
| Calories from SoFAAS | 0–20 | 11.90 ± 1.02 | 12.22 ± 0.81 | 12.63 ± 1.06 | 0.884 |
| Total BHEI-R | 0–100 | 60.31 ± 2.15 | 61.33 ± 1.48 | 61.22 ± 1.88 | 0.888 |
| Nutrients | T0 | T1 | T2 | p-Value |
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | ||
| Energy (kcal) | 1345.86 ± 46.31 a | 1161.59 ± 24.35 b | 1182.90 ± 22.30 b | 0.002 |
| Protein (g) | 60.21 ± 1.56 a | 54.54 ± 0.49 b | 51.50 ± 0.78 c | <0.001 |
| Carbohydrates (g) | 173.90 ± 3.79 a | 150.44 ± 1.96 b | 153.60 ± 1.86 b | <0.001 |
| Sugars (g) | 62.12 ± 3.44 a | 50.89 ± 1.97 b | 47.50 ± 2.34 b | 0.004 |
| Total Fiber (g) | 14.67 ± 0.53 | 14.07 ± 0.45 | 15.01 ± 0.55 | 0.212 |
| Soluble Fiber (g) | 3.83 ± 0.15 a | 3.59 ± 0.07 a | 3.35 ± 0.10 b | 0.015 |
| Total Fat (g) | 47.57 ± 1.01 a | 43.78 ± 0.48 b | 43.01 ± 0.71 b | 0.001 |
| Saturated Fat (g) | 15.45 ± 0.46 a | 15.34 ± 0.26 a | 13.52 ± 0.39 b | <0.001 |
| Polyunsaturated Fat (g) | 11.40 ± 0.30 a | 10.40 ± 0.19 b | 10.56 ± 0.24 a.b | 0.010 |
| Monounsaturated Fat (g) | 16.38 ± 0.45 a | 14.27 ± 0.20 b | 14.32 ± 0.31 b | <0.001 |
| Trans Fat (g) | 1.63 ± 0.07 a | 1.26 ± 0.04 b | 1.08 ± 0.05 c | <0.001 |
| Cholesterol (g) | 185.53 ± 6.97 a | 175.91 ± 3.75 a | 151.19 ± 9.24 b | 0.019 |
| Omega 3 Fatty Acid (g) | 1.47 ± 0.03 a.b | 1.42 ± 0.03 a | 1.56 ± 0.03 b | 0.006 |
| Omega 6 Fatty Acid (g) | 9.82 ± 0.27 a | 8.90 ± 0.19 b | 8.87 ± 0.21 b | 0.011 |
| Omega 6/Omega 3 Fatty Acid Ratio | 6.77 ± 0.16 a | 6.30 ± 0.13 b | 5.70 ± 0.12 c | <0.001 |
| Sodium (mg) | 2088.41 ± 53.65 a.b | 2060.03 ± 34.73 a | 2217.48 ± 45.00 b | 0.002 |
| Variables | T0 Mean ± SE; (n = X) | T1 Mean ± SE; (n = X) | T2 Mean ± SE; (n = X) | p-Value |
|---|---|---|---|---|
| BMI (Kg/m2) | 28.56 ± 1.10 (n = 38) | 29.29 ± 0.93 (n = 38) | 29.45 ± 1.12 (n = 38) | 0.308 |
| WC (cm) | 91.91 ± 2.95 (n = 38) | 94.42 ± 2.32 (n = 38) | 94.30 ± 3.07 (n = 38) | 0.279 |
| WHR | 0.90 ± 0.02 (n = 38) | 0.89 ± 0.02 (n = 38) | 0.088 ± 0.20 (n = 38) | 0.827 |
| WHtR | 0.59 ± 0.02 (n = 38) | 0.61 ± 0.01 (n = 38) | 0.61 ± 0.02 (n = 38) | 0.284 |
| FFM (Kg) | 42.38 ± 1.08 (n = 29) | 43.38 ± 0.87 (n = 28) | 43.07 ± 1.15 (n = 27) | 0.109 |
| Body Fat (%) | 40.55 ± 1.25 (n = 29) | 39.36 ± 1.02 (n = 28) | 39.78 ± 1.34 (n = 27) | 0.367 |
| PhA | 5.40 ± 0.20 (n = 38) a | 6.27 ± 0.11 (n = 36) b | 6.11 ± 0.15 (n = 36) b | <0.001 |
| CI | 1.27 ± 0.02 (n = 38) | 1.29 ± 0.02 (n = 38) | 1.28 ± 0.02 (n = 38) | 0.560 |
| LAP | - | 71.34 ± 8.48 (n = 30) | 47.40 ± 7.59 (n = 35) | 0.007 |
| VAI | - | 2.70 ± 0.40 (n = 30) | 2.12 ± 0.34 (n = 32) | 0.102 |
| Total Cholesterol (mg/dL) | - | 199.20 ± 6.96 (n = 34) | 179.37 ± 6.87 (n = 35) | 0.011 |
| Non-HDL Cholesterol (mg/dL) | - | 152.00 ± 10.83 (n = 34) | 129.35 ± 10.39 (n = 32) | 0.002 |
| LDL (mg/dL) | - | 114.16 ± 5.83 (n = 33) | 98.26 ± 5.27 (n = 33) | 0.020 |
| HDL (mg/dL) | - | 52.76 ± 3.99 (n = 34) | 54.69 ± 3.96 (n = 32) | 0.565 |
| VLDL (mg/dL) | - | 32.13 ± 3.78 (n = 31) | 26.80 ± 3.17 (n = 34) | 0.038 |
| Triglycerides (mg/dL) | - | 164.30 ± 18.79 (n = 30) | 133.65 ± 15.87 (n = 35) | 0.014 |
| CRP (mg/dL) | - | 0.71 ± 0.14 (n = 33) | 0.73 ± 0.15 (n = 34) | 0.827 |
| Cardiovascular Risk Factor | Criteria | T0 (n = 89) | T1 (n = 65) | T2 (n = 38) |
|---|---|---|---|---|
| Total Fat | >30% TCV | 77.5 | 98.5 | 92.1 |
| Saturated Fat | >7% TCV | 96.6 | 100 | 100 |
| Polyunsaturated Fat | <6% ou >10% TCV | 11.2 | 3.1 | 2.6 |
| Monounsaturated Fat | <15% ou >20% TCV | 100 | 100 | 100 |
| Trans Fat | >1% TCV | 47.2 | 49.2 | 34.2 |
| Cholesterol | >300 mg/Day | 2.2 | 0 | 0 |
| Fiber | <25 g Total Fiber <6 g Soluble Fiber | 88.8 | 98.5 | 100 |
| Sodium | >2300 mg/Day | 28.1 | 24.6 | 34.2 |
| Omega 3 Fatty Acid | <1 g/Day | 2.2 | 0 | 5.3 |
| Omega 6/Omega 3 Ratio | >5:1 | 95.5 | 98.5 | 92.1 |
| Total BHEI-R | <64.38 | 66.3 | 58.5 | 73.7 |
| Diabetes | Presence | 21.3 | 29.2 | 23.7 |
| Arterial Hypertension | Presence | 56.2 | 58.5 | 55.3 |
| Physical activity | Physical Inactivity | 59.6 | 47.7 | 57.9 |
| Smoking | Presence | 10.1 | 10.8 | 7.9 |
| Alcohol Consumption | ≥8 Drinks per Week | 0 | 0 | 0 |
| % (n = X) | % (n = X) | |||
| BMI | Overweight | 60.7 | 65.6 (n = 63) | 65.8 (n = 38) |
| WC | >80 cm | 85.4 | 93.7 (n = 64) | 86.8 (n = 38) |
| WHtR | ≥0.5 | 93.3 | 93.7 (n = 64) | 94.7 (n = 38) |
| WHR | >0.85 | 68.5 | 71.4 (n = 64) | 68.4 (n = 38) |
| Total Cholesterol | ≥240 mg/dL | * | 17.9 (n = 56) | 5.7 (n = 35) |
| LDL Cholesterol | >160 mg/dL | * | 7.3 (n = 55) | 0 (n = 33) |
| HDL Cholesterol | <40 mg/dL | * | 18.5 (n = 54) | 12.5 (n = 32) |
| Non-HDL Cholesterol | >160 mg/dL | * | 43.4 (n = 53) | 25 (n = 32) |
| Triglycerides | >150 mg/dL | * | 15.4 (n = 52) | 17.1 (n = 35) |
| NRF | Model Effects Tests | |||||
|---|---|---|---|---|---|---|
| Study Time | < 11 | ≥ 11 | Fixed Effects | Df | p-Value | |
| Mean ± SE | Mean ± SE | Mean ± SE | ||||
| C-Reactive Protein | 0.37 ± 0.13 | 0.69 ± 0.11 | NRF | 1 | 0.033 | |
| T1 | 0.51 ± 0.11 | 0.37 ± 0.16 | 0.65 ± 0.13 | Time | 1 | 0.690 |
| T2 | 0.56 ± 0.11 | 0.38 ± 0.16 | 0.74 ± 0.12 | NRF × Time | 1 | 0.708 |
| Phase Angle | 6.03 ± 0.14 | 5.85 ± 0.13 | ||||
| T0 | 5.40 ± 0.20 a | 5.62 ± 0.26 | 5.18 ± 0.24 | NRF | 1 | 0.256 |
| T1 | 6.26 ± 0.11 b | 6.22 ± 0.16 | 6.31 ± 0.14 | Time | 2 | <0.001 |
| T2 | 6.15 ± 0.15 b | 6.24 ± 0.20 | 6.06 ± 0.16 | NRF × Time | 2 | 0.234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzutti, F.S.; Custódio, I.D.D.; Lima, M.T.M.; Carvalho, K.P.d.; Pereira, T.S.S.; Molina, M.d.C.B.; Canto, P.P.L.; Paiva, C.E.; Maia, Y.C.d.P. Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases. Nutrients 2021, 13, 1114. https://doi.org/10.3390/nu13041114
Mazzutti FS, Custódio IDD, Lima MTM, Carvalho KPd, Pereira TSS, Molina MdCB, Canto PPL, Paiva CE, Maia YCdP. Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases. Nutrients. 2021; 13(4):1114. https://doi.org/10.3390/nu13041114
Chicago/Turabian StyleMazzutti, Fernanda S., Isis D. D. Custódio, Mariana T. M. Lima, Kamila P. de Carvalho, Taísa S. S. Pereira, Maria del C. B. Molina, Paula P. L. Canto, Carlos E. Paiva, and Yara C. de P. Maia. 2021. "Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases" Nutrients 13, no. 4: 1114. https://doi.org/10.3390/nu13041114
APA StyleMazzutti, F. S., Custódio, I. D. D., Lima, M. T. M., Carvalho, K. P. d., Pereira, T. S. S., Molina, M. d. C. B., Canto, P. P. L., Paiva, C. E., & Maia, Y. C. d. P. (2021). Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases. Nutrients, 13(4), 1114. https://doi.org/10.3390/nu13041114







