Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions
Abstract
1. Introduction
2. Profile of Milk Fat
Polyunsaturated Fatty Acids (PUFAs)
3. Milk Fatty Acid Composition in Different Animal Species
3.1. Ruminants Milk
3.2. Non-Ruminants (Monogastric Animals) Milk
3.3. Human Milk
4. Factors Affecting Fatty Acid Profiles in Milk from Different Animal Species
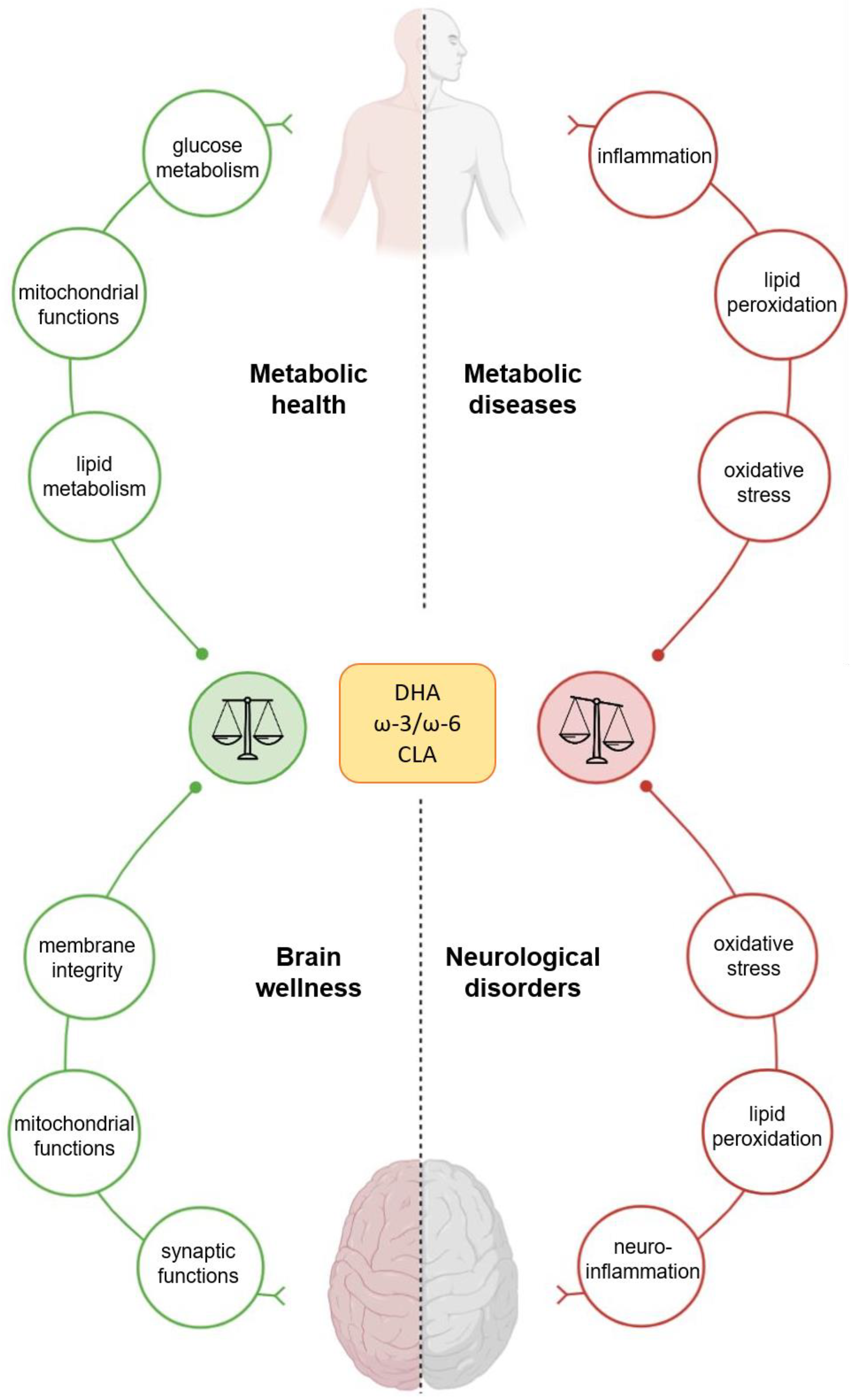
5. Polyunsaturated Fatty Acids Effects on Human Health
5.1. Polyunsaturated Fatty Acids Effects on Metabolism
5.2. Polyunsaturated Fatty Acids Effects on Nervous System
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FAs | Fatty acids |
| LA | Linoleic Acid |
| CLA | Conjugated linoleic acids |
| PUFAs | Polyunsaturated fatty acids |
| SFAs | Saturated fatty acids |
| CNS | Central nervous system |
| AA | Arachidonic acid |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| RA | Rumenic acid |
| ALA | α-linolenic acid |
| tVA | Trans-vaccenic acid |
| OEA | Oleoylethanolamide |
| PEA | Palmitoylethanolamide |
| PPAR | Peroxisome proliferators-activated receptors |
| BCFA | Branched chain fatty acids |
| OCFAs | Odd-chain fatty acids |
| OBCFAs | Odd-branched-chain fatty acids |
| BDNF | Brain derived neurotropic factor |
| Nrf | Nuclear factor erythroid 2-related factor 2 |
References
- Mollet, B.; Rowland, I. Functional foods: At the frontier between food and pharma. Curr. Opin. Biotechnol. 2002, 13, 483–485. [Google Scholar] [CrossRef]
- Menrad, K. Market and marketing of functional food in Europe. J. Food Eng. 2003, 56, 181–188. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Burlingame, B.; Nishida, C.; Uauy, R.; Weisell, R. Fats and fatty acids in human nutrition: Introduction. Ann. Nutr. Metab. 2009, 55, 5–7. [Google Scholar] [CrossRef]
- Kliem, K.E.a.S.K.J. Manipulation of milk fatty acid composition in lactating cows: Opportunities and challenges. Eur. J. Lipid Sci. Technol. 2016, 118, 1661–1683. [Google Scholar] [CrossRef]
- Astrup, A.; Geiker, N.R.W.; Magkos, F. Effects of Full-Fat and Fermented Dairy Products on Cardiometabolic Disease: Food Is More than the Sum of Its Parts. Adv. Nutr. 2019, 10, 924S–930S. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.J.; Ahvenjärvi, S.; Toivonen, V.; Vanhatalo, A.; Huhtanen, P.; Griinari, J.M. Effect of incremental levels of sunflower-seed oil in the diet on ruminal lipid metabolism in lactating cows. Br. J. Nutr. 2008, 99, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Saturated Fats and Health; SACN Report: London, UK, 2019.
- Timon, C.M.; O’Connor, A.; Bhargava, N.; Gibney, E.R.; Feeney, E.L. Dairy Consumption and Metabolic Health. Nutrients 2020, 12, 3040. [Google Scholar] [CrossRef] [PubMed]
- Hirahatake, K.M.; Astrup, A.; Hill, J.O.; Slavin, J.L.; Allison, D.B.; Maki, K.C. Potential Cardiometabolic Health Benefits of Full-Fat Dairy: The Evidence Base. Adv. Nutr. 2020, 11, 533–547. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Robbins, M.A. Relation between dairy food intake and cognitive function: The Maine-Syracuse Longitudinal Study. Int. Dairy J. 2012, 22, 15–23. [Google Scholar] [CrossRef]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fu, Z.; Chung, M.; Jang, D.J.; Lee, H.J. Role of milk and dairy intake in cognitive function in older adults: A systematic review and meta-analysis. Nutr. J. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Triana, F.; Verdejo-Bravo, C.; Fernández-Pérez, C.; Martín-Sánchez, F.J. Effect of Milk and Other Dairy Products on the Risk of Frailty, Sarcopenia, and Cognitive Performance Decline in the Elderly: A Systematic Review. Adv. Nutr. 2019, 10, S105–S119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 1–18. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk--A Nutrient System of Mammalian Evolution Promoting mTORC1-Dependent Translation. Int. J. Mol. Sci. 2015, 16, 17048–17087. [Google Scholar] [CrossRef]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef]
- Medicine, I.O. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358. [Google Scholar]
- López, P.M.; Ortega, R.M. Omega-3 fatty acids in the prevention and control of cardiovascular disease. Eur. J. Clin. Nutr. 2003, 57, S22–S25. [Google Scholar] [CrossRef]
- Haag, M. Essential fatty acids and the brain. Can. J. Psychiatry 2003, 48, 195–203. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Butler, G.; Latif, M.A.; Leifert, C.; Davis, D.R. Organic production enhances milk nutritional quality by shifting fatty acid composition: A United States-wide, 18-month study. PLoS ONE 2013, 8, e82429. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Kepler, C.R.; Tove, S.B. Biohydrogenation of unsaturated fatty acids. 3. Purification and properties of a linoleate delta-12-cis, delta-11-trans-isomerase from Butyrivibrio fibrisolvens. J Biol. Chem. 1967, 242, 5686–5692. [Google Scholar] [CrossRef]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Nurmela, K.V.; Bauman, D.E. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta(9)-desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Collomb, M.; Schmid, A.; Sieber, R.; Wechsler, D.; Ryhänen, E.-L. Conjugated linoleic acids in milk fat: Variation and physiological effects. Int. Dairy J. 2006, 16, 1347–1361. [Google Scholar] [CrossRef]
- Butler, G.; Collomb, M.; Rehberger, B.; Sanderson, R.; Eyre, M.; Leifert, C. Conjugated linoleic acid isomer concentrations in milk from high- and low-input management dairy systems. J. Sci. Food Agric. 2009, 89, 697–705. [Google Scholar] [CrossRef]
- Chin, S.; Liu, W.; Storkson, J.; Ha, Y.; Pariza, M. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J. Food Compos. Anal. 1992, 5, 185–197. [Google Scholar] [CrossRef]
- Kepler, C.R.; Hirons, K.P.; McNeill, J.J.; Tove, S.B. Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibrio fibrisolvens. J. Biol. Chem. 1966, 241, 1350–1354. [Google Scholar] [CrossRef]
- Corl, B.A.; Barbano, D.M.; Bauman, D.E.; Ip, C. cis-9, trans-11 CLA derived endogenously from trans-11 18:1 reduces cancer risk in rats. J. Nutr. 2003, 133, 2893–2900. [Google Scholar] [CrossRef]
- Gerosa, S.; Skoet, J. Milk Availability Trends in Production and Demand and Medium-Term Outlook; Food and Agriculture Organization of the United Nations FAO: Rome, Italy, 2012. [Google Scholar]
- Shingfield, K.J.; Chilliard, Y.; Toivonen, V.; Kairenius, P.; Givens, D.I. Trans Fatty Acids and Bioactive Lipids in Ruminant Milk. Adv. Exp. Med. Biol. 2008, 606, 3–65. [Google Scholar] [CrossRef]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugère, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Chilliard, Y.; Rouel, J.; Ferlay, A.; Bernard, L.; Gaborit, P.; Raynal-Ljutovac, K.; Lauret, A.; Leroux, C. Optimising Goat’s Milk and Cheese Fatty Acid Composition; Elsevier: Amsterdam, The Netherlands, 2006; pp. 281–312. [Google Scholar]
- Cavaliere, G.; Trinchese, G.; Musco, N.; Infascelli, F.; De Filippo, C.; Mastellone, V.; Morittu, V.M.; Lombardi, P.; Tudisco, R.; Grossi, M.; et al. Milk from cows fed a diet with a high forage: Concentrate ratio improves inflammatory state, oxidative stress, and mitochondrial function in rats. J. Dairy Sci. 2018, 101, 1843–1851. [Google Scholar] [CrossRef]
- Correddu, F.; Serdino, J.; Manca, M.G.; Cosenza, G.; Pauciullo, A.; Ramunno, L.; Macciotta, N.P. Use of multivariate factor analysis to characterize the fatty acid profile of buffalo milk. J. Food Compos. Anal. 2017, 60, 25–31. [Google Scholar] [CrossRef]
- D’Urso, S.; Cutrignelli, M.I.; Calabrò, S.; Bovera, F.; Tudisco, R.; Piccolo, V.; Infascelli, F. Influence of pasture on fatty acid profile of goat milk. J. Anim. Physiol. Anim. Nutr. 2008, 92, 405–410. [Google Scholar] [CrossRef]
- Gantner, V.; Mijić, P.; Baban, M.; Škrtić, Z.; Turalija, A. The overall and fat composition of milk of various species. Mljekarstvo 2015, 65, 223–231. [Google Scholar] [CrossRef]
- Tudisco, R.; Chiofalo, B.; Presti, V.L.; Morittu, V.M.; Moniello, G.; Grossi, M.; Musco, N.; Grazioli, R.; Mastellone, V.; Lombardi, P.; et al. Influence of Feeding Linseed on SCD Activity in Grazing Goat Mammary Glands. Animals 2019, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Tudisco, R.; Morittu, V.M.; Addi, L.; Moniello, G.; Grossi, M.; Musco, N.; Grazioli, R.; Mastellone, V.; Pero, M.E.; Lombardi, P.; et al. Influence of Pasture on Stearoyl-CoA Desaturase and miRNA 103 Expression in Goat Milk: Preliminary Results. Animals 2019, 9, 606. [Google Scholar] [CrossRef]
- Muehlhoff, E.; Bennet, A.; McMahon, D.; Food and Agriculture Organisation of the United Nations (FAO). Milk and Dairy Products in Human Nutrition (2013). Dairy Technol. 2014, 67, 303–304. [Google Scholar]
- Jenness, R. Composition and Characteristics of Goat Milk: Review 1968−1979. J. Dairy Sci. 1980, 63, 1605–1630. [Google Scholar] [CrossRef]
- Boza, J.; Sanz Sampelayo, M.R. Aspectos nutricionales de la leche de cabra (Nutritional aspects of goat milk). ACVAO 1997, 10, 109–139. [Google Scholar]
- Goudjil, H.; Fontecha, J.; Luna, P.; De La Fuente, M.A.; Alonso, L.; Juárez, M. Quantitative characterization of unsaturated and trans fatty acids in ewe’s milk fat. Le Lait 2004, 84, 473–482. [Google Scholar] [CrossRef]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar] [CrossRef]
- Salimei, E.; Chiofalo, B. Asses: Milk yield and composition. In Nutrition and Feeding of the Broodmare; Miraglia, N., Martin-Rosset, W., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 117–131, EAAP Publication No. 120. [Google Scholar]
- Salimei, E.; Fantuz, F. Equid milk for human consumption. Int. Dairy J. 2012, 24, 130–142. [Google Scholar] [CrossRef]
- Young, W.P.; George, F.W.H. Milk and Dairy Products in Human Nutrition: Production, Composition and Health, 1st ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2013. [Google Scholar]
- Innis, S.M. Palmitic Acid in Early Human Development. Crit. Rev. Food Sci. Nutr. 2016, 56, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Canani, R.B.; Matamoros, S.; Bergamo, P.; De Filippo, C.; Aceto, S.; Gaita, M.; Cerino, P.; Negri, R.; et al. Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota. J. Nutr. Biochem. 2015, 26, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Lisai, S.; Sirigu, A.; Piras, A.; Collu, M.; Batetta, B.; Gambelli, L.; Banni, S. Dietary triacylglycerols with palmitic acid in the sn-2 position modulate levels of N-acylethanolamides in rat tissues. PLoS ONE 2015, 10, e0120424. [Google Scholar] [CrossRef]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef]
- Pontis, S.; Ribeiro, A.; Sasso, O.; Piomelli, D. Macrophage-derived lipid agonists of PPAR-α as intrinsic controllers of inflammation. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as bioactive human milk protective component against food allergy. Allergy 2020. [Google Scholar] [CrossRef]
- Wagner, C.L.; Anderson, D.M.; Pittard, W.B. Special Properties of Human Milk. Clin. Pediatr. 1996, 35, 283–293. [Google Scholar] [CrossRef]
- Schanler, R.J.; Atkinson, S.A. Effects of nutrients in human milk on the recipient premature infant. J. Mammary Gland. Biol. Neoplasia 1999, 4, 297–307. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef]
- Verruck, S.; Dantas, A.; Prudencio, E.S. Functionality of the components from goat’s milk, recent advances for functional dairy products development and its implications on human health. J. Funct. Foods 2019, 52, 243–257. [Google Scholar] [CrossRef]
- Palupi, E.; Jayanegara, A.; Ploeger, A.; Kahl, J. Comparison of nutritional quality between conventional and organic dairy products: A meta-analysis. J. Sci. Food Agric. 2012, 92, 2774–2781. [Google Scholar] [CrossRef]
- Schwendel, B.H.; Morel, P.C.; Wester, T.J.; Tavendale, M.H.; Deadman, C.; Fong, B.; Shadbolt, N.M.; Thatcher, A.; Otter, D.E. Fatty acid profile differs between organic and conventionally produced cow milk independent of season or milking time. J. Dairy Sci. 2015, 98, 1411–1425. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Seal, C.J.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M.; et al. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta- and redundancy analyses. Br. J. Nutr. 2016, 115, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Kotrotsios, V.; Hadjigeorgiou, I.; Zervas, G. Differences in sheep and goats milk fatty acid profile between conventional and organic farming systems. J. Dairy Res. 2010, 77, 343–349. [Google Scholar] [CrossRef]
- Tudisco, R.; Cutrignelli, M.I.; Calabrò, S.; Piccolo, G.; Bovera, F.; Guglielmelli, A.; Moniello, G.; Infascelli, F. Influence of organic systems on milk fatty acid profile and CLA in goats. Small Rumin. Res. 2010, 88, 151–155. [Google Scholar] [CrossRef]
- Cabiddu, A.; Addis, M.; Spada, S.; Sitzia, M.; Molle, G.; Piredda, G. The Effect of Different Legume-Based Pastures on the Fatty Acid Composition of Sheep Milk with Focus on CLA; Zürich: Zürich, Switzerland, 2004; pp. 1133–1135. [Google Scholar]
- Griinari, J.M.; Baumann, D.E. Biosynthesis of Conjugated Linoleic Acid and Its Incorporation into Meat and Milk in Ruminant; Yurawecz, M.P., Mossoba, M.M., Kramer, J.K.G., Pariza, M.W., Nelson, G.J., Eds.; Advances in Conjugated Linoleic Acid Research: Champaign, IL, USA, 1999; pp. 180–200. [Google Scholar]
- Banni, S.; Carta, G.; Contini, M.S.; Angioni, E.; Deiana, M.; Dessì, M.A.; Melis, M.P.; Corongiu, F.P. Characterization of conjugated diene fatty acids in milk, dairy products, and lamb tissues. J. Nutr. Biochem. 1996, 7, 150–155. [Google Scholar] [CrossRef]
- Dhiman, T.R.; Anand, G.R.; Satter, L.D.; Pariza, M.W. Conjugated linoleic acid content of milk from cows fed different diets. J. Dairy Sci. 1999, 82, 2146–2156. [Google Scholar] [CrossRef]
- Bergamo, P.; Fedele, E.; Iannibelli, L.; Marzillo, G. Fat-soluble vitamin contents and fatty acid composition in organic and conventional Italian dairy products. Food Chem. 2003, 82, 625–631. [Google Scholar] [CrossRef]
- Glover, K.E.; Budge, S.; Rose, M.; Rupasinghe, H.P.; Maclaren, L.; Green-Johnson, J.; Fredeen, A.H. Effect of feeding fresh forage and marine algae on the fatty acid composition and oxidation of milk and butter. J. Dairy Sci. 2012, 95, 2797–2809. [Google Scholar] [CrossRef]
- Tudisco, R.; Calabrò, S.; Cutrignelli, M.; Moniello, G.; Grossi, M.; Gonzalez, O.; Piccolo, V.; Infascelli, F. Influence of organic systems on Stearoyl-CoA desaturase gene expression in goat milk. Small Rumin. Res. 2012, 106, S37–S42. [Google Scholar] [CrossRef]
- Tudisco, R.; Grossi, M.; Calabrò, S.; Cutrignelli, M.I.; Musco, N.; Addi, L.; Infascelli, F. Influence of pasture on goat milk fatty acids and Stearoyl-CoA desaturase expression in milk somatic cells. Small Rumin. Res. 2014, 122, 38–43. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mountzouris, K.; Zervas, G. Concentration of conjugated linoleic acid in grazing sheep and goat milk fat. Livest. Sci. 2006, 103, 74–84. [Google Scholar] [CrossRef]
- Nudda, A.; Cannas, A.; Correddu, F.; Atzori, A.S.; Lunesu, M.F.; Battacone, G.; Pulina, G. Sheep and Goats Respond Differently to Feeding Strategies Directed to Improve the Fatty Acid Profile of Milk Fat. Animals 2020, 10, 1290. [Google Scholar] [CrossRef]
- Marcello, M.; Arianna, B.; Francesco, P.; Andrea, S.; Sebastiano, B.; Mauro, A.; Pierlorenzo, S. Effect of forage/concentrate ratio and soybean oil supplementation on milk yield, and composition from Sarda ewes. Anim. Res. 2006, 55, 273–285. [Google Scholar]
- Kim, Y.J.; Liu, R.H.; Bond, D.R.; Russell, J.B. Effect of linoleic acid concentration on conjugated linoleic acid production by Butyrivibrio fibrisolvens A38. Appl. Environ. Microbiol. 2000, 66, 5226–5230. [Google Scholar] [CrossRef]
- Aii, T.; Takahashi, S.; Kurihara, M.; Kune, S. The effects of Italian rye grass hay, haylage and fresh Italian Rye grass on the fatty acid composition of cow’s milk. Jpn. J. Zootech. Sci. 1988, 59, 718–724. [Google Scholar]
- Dewhurst, R.; Shingfield, K.; Lee, M.; Scollan, N. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed. Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Davis, D.R.; Heins, B.J.; Latif, M.A.; Leifert, C.; Peterman, L.; Butler, G.; Faergeman, O.; Abel-Caines, S.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018, 6, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Chatzidimitriou, E.; Leifert, C.; Butler, G. Evidence That Forage-Fed Cows Can Enhance Milk Quality. Sustainability 2020, 12, 3688. [Google Scholar] [CrossRef]
- Bisig, W.; Eberhard, P.; Collomb, M.; Rehberger, B. Influence of processing on the fatty acid composition and the content of conjugated linoleic acid in organic and conventional dairy products—A review. Le Lait 2007, 87, 1–19. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.; Piva, G. A comparative study of fatty acid composition and CLA concentration in commercial cheeses. J. Food Compos. Anal. 2011, 24, 55–61. [Google Scholar] [CrossRef]
- Ellis, K.A.; Innocent, G.; Grove-White, D.; Cripps, P.; McLean, W.G.; Howard, C.V.; Mihm, M. Comparing the fatty acid composition of organic and conventional milk. J. Dairy Sci. 2006, 89, 1938–1950. [Google Scholar] [CrossRef]
- Lourenço, M.; Van Ranst, G.; Vlaeminck, B.; De Smet, S.; Fievez, V. Influence of different dietary forages on the fatty acid composition of rumen digesta as well as ruminant meat and milk. Anim. Feed. Sci. Technol. 2008, 145, 418–437. [Google Scholar] [CrossRef]
- Uzun, P.; Masucci, F.; Serrapica, F.; Napolitano, F.; Braghieri, A.; Romano, R.; Manzo, N.; Esposito, G.; Di Francia, A. The inclusion of fresh forage in the lactating buffalo diet affects fatty acid and sensory profile of mozzarella cheese. J. Dairy Sci. 2018, 101, 6752–6761. [Google Scholar] [CrossRef]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol. 2004, 50, 137–143. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.; Jetton, T.; Satish, B.; Gupta, D. Dairy-derived bioactive fatty acids improve pancreatic ß-cell function. FASEB J. 2015, 29, 608–625. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.; Campos, H.; Hu, F.B. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am. J. Clin. Nutr. 2007, 86, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.; Fonseca, A.; Dewhurst, R. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed. Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Massart-Leën, A.; Roets, E.; Peeters, G.; Verbeke, R. Propionate for Fatty Acid Synthesis by the Mammary Gland of the Lactating Goat. J. Dairy Sci. 1983, 66, 1445–1454. [Google Scholar] [CrossRef]
- Alonso, L.; Fontecha, J.; Lozada, L.; Fraga, M.J.; Juárez, M. Fatty acid composition of caprine milk: Major, branched-chain, and trans fatty acids. J. Dairy Sci. 1999, 82, 878–884. [Google Scholar] [CrossRef]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.D.; Kraft, J. Content and Composition of Branched-Chain Fatty Acids in Bovine Milk Are Affected by Lactation Stage and Breed of Dairy Cow. PLoS ONE 2016, 11, e0150386. [Google Scholar] [CrossRef] [PubMed]
- Craninx, M.; Steen, A.; Van Laar, H.; Van Nespen, T.; Martín-Tereso, J.; De Baets, B.; Fievez, V. Effect of lactation stage on the odd- and branched-chain milk fatty acids of dairy cattle under grazing and indoor conditions. J. Dairy Sci. 2008, 91, 2662–2677. [Google Scholar] [CrossRef]
- Lopez, A.; Vasconi, M.; Moretti, V.M.; Bellagamba, F. Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems. Animals 2019, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Serra, A.; Buccioni, A.; Conte, G.; Pollicardo, A.; Secchiari, P. Effect of soybean oil supplementation on milk fatty acid composition from Saanen goats fed diets with different forage:concentrate ratios. Ital. J. Anim. Sci. 2008, 7, 297–311. [Google Scholar] [CrossRef]
- Serment, A.; Schmidely, P.; Giger-Reverdin, S.; Chapoutot, P.; Sauvant, D. Effects of the percentage of concentrate on rumen fermentation, nutrient digestibility, plasma metabolites, and milk composition in mid-lactation goats. J. Dairy Sci. 2011, 94, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Martínez Marín, A.L.; Gómez-Cortés, P.; Gómez Castro, A.G.; Juárez, M.; Pérez Alba, L.M.; Pérez Hernández, M.; de la Fuente, M.A. Animal performance and milk fatty acid profile of dairy goats fed diets with different unsaturated plant oils. J. Dairy Sci. 2011, 94, 5359–5368. [Google Scholar] [CrossRef] [PubMed]
- Falchero, L.; Lombardi, G.; Gorlier, A.; Lonati, M.; Odoardi, M.; Cavallero, A. Variation in fatty acid composition of milk and cheese from cows grazed on two alpine pastures. Dairy Sci. Technol. 2010, 90, 657–672. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Gleason, K.; Griffin, B.; Miller, G.J. Influence of an algal triacylglycerol containing docosahexaenoic acid (22: 6n-3) and docosapentaenoic acid (22: 5n-6) on cardiovascular risk factors in healthy men and women. Br. J. Nutr. 2006, 95, 525–531. [Google Scholar] [CrossRef]
- Hardman, W.E. Omega-3 fatty acids to augment cancer therapy. J. Nutr. 2002, 132, 3508S–3512S. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, Z.; Wang, X.; Shi, H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS ONE 2012, 7, e45990. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Mollica, M.P.; Donizzetti, I.; Gifuni, G.; Sica, R.; Pignalosa, A.; Cavaliere, G.; Gaita, M.; De Filippo, C.; Zorzano, A.; et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS ONE 2014, 9, e92753. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Bergamo, P.; De Filippo, C.; Mattace Raso, G.; Gifuni, G.; Putti, R.; Moni, B.H.; Canani, R.B.; Meli, R.; et al. Polyunsaturated Fatty Acids Attenuate Diet Induced Obesity and Insulin Resistance, Modulating Mitochondrial Respiratory Uncoupling in Rat Skeletal Muscle. PLoS ONE 2016, 11, e0149033. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated Linoleic Acid: Potential Health Benefits as a Functional Food Ingredient. Annu. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, W. Review of the roles of conjugated linoleic acid in health and disease. J. Funct. Foods 2015, 15, 314–325. [Google Scholar] [CrossRef]
- Risérus, U.; Basu, S.; Jovinge, S.; Fredrikson, G.N.; Arnlöv, J.; Vessby, B. Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: A potential link to fatty acid-induced insulin resistance. Circulation 2002, 106, 1925–1929. [Google Scholar] [CrossRef]
- Moloney, F.; Toomey, S.; Noone, E.; Nugent, A.; Allan, B.; Loscher, C.E.; Roche, H.M. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes 2007, 56, 574–582. [Google Scholar] [CrossRef]
- Choi, J.S.; Koh, I.U.; Jung, M.H.; Song, J. Effects of three different conjugated linoleic acid preparations on insulin signalling, fat oxidation and mitochondrial function in rats fed a high-fat diet. Br. J. Nutr. 2007, 98, 264–275. [Google Scholar] [CrossRef]
- Castro-Webb, N.; Ruiz-Narváez, E.A.; Campos, H. Cross-sectional study of conjugated linoleic acid in adipose tissue and risk of diabetes. Am. J. Clin. Nutr. 2012, 96, 175–181. [Google Scholar] [CrossRef]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Trinchese, G.; Cavaliere, G.; De Filippo, C.; Cocca, E.; Gaita, M.; Della-Gatta, A.; Marano, A.; Mazzarella, G.; Bergamo, P. c9,t11-Conjugated linoleic acid ameliorates steatosis by modulating mitochondrial uncoupling and Nrf2 pathway. J. Lipid Res. 2014, 55, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, P.; Palmieri, G.; Cocca, E.; Ferrandino, I.; Gogliettino, M.; Monaco, A.; Maurano, F.; Rossi, M. Adaptive response activated by dietary cis9, trans11 conjugated linoleic acid prevents distinct signs of gliadin-induced enteropathy in mice. Eur. J. Nutr. 2016, 55, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Carta, G.; Murru, E.; Lopes, P.A.; Martins, S.V.; Prates, J.A.; Banni, S. Effects of dietary CLA on n-3 HUFA score and N-acylethanolamides biosynthesis in the liver of obese Zucker rats. Prostaglandins Leukot. Essent. Fat. Acids 2015, 98, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Carta, G.; Cordeddu, L.; Melis, M.P.; Desogus, E.; Ansar, H.; Chilliard, Y.; Ferlay, A.; Stanton, C.; Coakley, M.; et al. Dietary Conjugated Linoleic Acid-Enriched Cheeses Influence the Levels of Circulating n-3 Highly Unsaturated Fatty Acids in Humans. Int. J. Mol. Sci. 2018, 19, 1730. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; Penna, E.; De Filippo, C.; Cimmino, F.; Catapano, A.; Musco, N.; Tudisco, R.; Lombardi, P.; Infascelli, F.; et al. Milk From Cow Fed With High Forage/Concentrate Ratio Diet: Beneficial Effect on Rat Skeletal Muscle Inflammatory State and Oxidative Stress Through Modulation of Mitochondrial Functions and AMPK Activity. Front. Physiol. 2019, 9. [Google Scholar] [CrossRef]
- Mollica, M.P.; Lionetti, L.; Moreno, M.; Lombardi, A.; De Lange, P.; Antonelli, A.; Lanni, A.; Cavaliere, G.; Barletta, A.; Goglia, F. 3,5-diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J. Hepatol. 2009, 51, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Cimmino, F.; Catapano, A.; Carta, G.; Pirozzi, C.; Murru, E.; Lama, A.; Meli, R.; Bergamo, P.; et al. Decreased Metabolic Flexibility in Skeletal Muscle of Rat Fed with a High-Fat Diet Is Recovered by Individual CLA Isomer Supplementation via Converging Protective Mechanisms. Cells 2020, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Benoit, B.; Plaisancié, P.; Géloën, A.; Estienne, M.; Debard, C.; Meugnier, E.; Loizon, E.; Daira, P.; Bodennec, J.; Cousin, O.; et al. Pasture v. standard dairy cream in high-fat diet-fed mice: Improved metabolic outcomes and stronger intestinal barrier. Br. J. Nutr. 2014, 112, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, P.; Maurano, F.; Rossi, M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic. Biol. Med. 2007, 43, 71–79. [Google Scholar] [CrossRef]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Pintus, S.; Murru, E.; Carta, G.; Cordeddu, L.; Batetta, B.; Accossu, S.; Pistis, D.; Uda, S.; Elena Ghiani, M.; Mele, M.; et al. Sheep cheese naturally enriched in α-linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br. J. Nutr. 2013, 109, 1453–1462. [Google Scholar] [CrossRef]
- Santurino, C.; López-Plaza, B.; Fontecha, J.; Calvo, M.V.; Bermejo, L.M.; Gómez-Andrés, D.; Gómez-Candela, C. Consumption of Goat Cheese Naturally Rich in Omega-3 and Conjugated Linoleic Acid Improves the Cardiovascular and Inflammatory Biomarkers of Overweight and Obese Subjects: A Randomized Controlled Trial. Nutrients 2020, 12, 1315. [Google Scholar] [CrossRef]
- Costa, A.; Lopez-Villalobos, N.; Sneddon, N.; Shalloo, L.; Franzoi, M.; De Marchi, M.; Penasa, M. Invited review: Milk lactose—Current status and future challenges in dairy cattle. J. Dairy Sci. 2019, 102, 5883–5898. [Google Scholar] [CrossRef]
- Lock, A.L.; Bauman, D.E. Modifying milk fat composition of dairy cows to enhance fatty acids beneficial to human health. Lipids 2004, 39, 1197–1206. [Google Scholar] [CrossRef]
- Mele, M.C.; Cannelli, G.; Carta, G.; Cordeddu, L.; Melis, M.P.; Murru, E.; Stanton, C.; Banni, S. Metabolism of c9,t11-conjugated linoleic acid (CLA) in humans. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 115–119. [Google Scholar] [CrossRef]
- Rist, L.; Mueller, A.; Barthel, C.; Snijders, B.; Jansen, M.; Simões-Wüst, A.P.; Huber, M.; Kummeling, I.; von Mandach, U.; Steinhart, H.; et al. Influence of organic diet on the amount of conjugated linoleic acids in breast milk of lactating women in the Netherlands. Br. J. Nutr. 2007, 97, 735–743. [Google Scholar] [CrossRef][Green Version]
- Aro, A.; Männistö, S.; Salminen, I.; Ovaskainen, M.L.; Kataja, V.; Uusitupa, M. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr. Cancer. 2000, 38, 151–157. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Ip, C.; Ip, M.M.; McGuire, M.K.; Muti, P.; Edge, S.B.; Trevisan, M.; Freudenheim, J.L. Dietary intake of conjugated linoleic acids and risk of premenopausal and postmenopausal breast cancer, Western New York Exposures and Breast Cancer Study (WEB Study). Cancer Epidemiol. Biomark. Prev. 2004, 13, 1480–1484. [Google Scholar]
- Haug, A.; Sjøgren, P.; Hølland, N.; Müller, H.; Kjos, N.P.; Taugbøl, O.; Fjerdingby, N.; Biong, A.S.; Selmer-Olsen, E.; Harstad, O.M. Effects of butter naturally enriched with conjugated linoleic acid and vaccenic acid on blood lipids and LDL particle size in growing pigs. Lipids Health Dis. 2008, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, K.; Kanwal, H.K.; Tyagi, A.K.; Stanton, C.; Ross, P. High conjugated linoleic acid enriched ghee (clarified butter) increases the antioxidant and antiatherogenic potency in female Wistar rats. Lipids Health Dis. 2013, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.M.; Luquetti, S.C.P.D.; Sabarense, C.M.; Corrêa, J.O.D.A.; Dos Reis, L.G.; Da Conceição, E.P.S.; Lisboa, P.C.; De Moura, E.G.; Gameiro, J.; Da Gama, M.A.S.; et al. Butter naturally enriched in cis-9, trans-11 CLA prevents hyperinsulinemia and increases both serum HDL cholesterol and triacylglycerol levels in rats. Lipids Health Dis. 2014, 13, 200. [Google Scholar] [CrossRef]
- Gama, M.A.S.; Raposo, N.R.B.; Mury, F.B.; Lopes, F.C.F.; Dias-Neto, E.; Talib, L.L.; Gattaz, W.F. Conjugated linoleic acid-enriched butter improved memory and up-regulated phospholipase A2 encoding-genes in rat brain tissue. J. Neural Transm. 2015, 122, 1371–1380. [Google Scholar] [CrossRef]
- Bergamo, P.; Cocca, E.; Monaco, A.; Cozzolino, V.; Boscaino, F.; Ferrandino, I.; Maurano, F.; Rossi, M. Protective effect of Rumenic acid rich cow’s milk against colitis is associated with the activation of Nrf2 pathway in a murine model. Prostaglandins Leukot. Essent. Fatty Acids 2017, 125, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Tricon, S.; Burdge, G.C.; Kew, S.; Banerjee, T.; Russell, J.J.; Jones, E.L.; Grimble, R.F.; Williams, C.M.; Yaqoob, P.; Calder, P.C. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am. J. Clin. Nutr. 2004, 80, 614–620. [Google Scholar] [CrossRef]
- Sofi, F.; Buccioni, A.; Cesari, F.; Gori, A.M.; Minieri, S.; Mannini, L.; Casini, A.; Gensini, G.F.; Abbate, R.; Antongiovanni, M. Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: A dietary intervention study. Nutr Metab Cardiovasc. Dis. 2010, 20, 117–124. [Google Scholar] [CrossRef]
- Penedo, L.A.; Nunes, J.C.; Gama, M.A.; Leite, P.E.; Quirico-Santos, T.F.; Torres, A.G. Intake of butter naturally enriched with cis9,trans11 conjugated linoleic acid reduces systemic inflammatory mediators in healthy young adults. J. Nutr. Biochem. 2013, 24, 2144–2151. [Google Scholar] [CrossRef]
- Derakhshande-Rishehri, S.M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of foods enriched in conjugated linoleic acid (CLA) and CLA supplements with lipid profile in human studies: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P. Brain Fuel Utilization in the Developing Brain. Ann. Nutr. Metab. 2019, 75, 8–18. [Google Scholar] [CrossRef]
- Goyal, M.S.; Iannotti, L.L.; Raichle, M.E. Brain Nutrition: A Life Span Approach. Annu. Rev. Nutr. 2018, 38, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.J.; Marko, D.M.; Fenech, R.K.; Yang, A.J.T.; MacPherson, R.E.K. Healthy brain, healthy life: A review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl. Physiol. Nutr. Metab. 2020, 45, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, K.; Kusumoto, Y.; Kanchi, N.; Kinoshita, H.; Kanegae, S.; Yamaguchi, N.; Ozawa, H. Recent trends in mental illness and omega-3 fatty acids. J. Neural Transm. 2020, 127, 1491–1499. [Google Scholar] [CrossRef]
- Matura, S.; Prvulovic, D.; Mohadjer, N.; Fusser, F.; Oertel, V.; Reif, A.; Pantel, J.; Karakaya, T. Association of dietary fat composition with cognitive performance and brain morphology in cognitively healthy individuals. Acta Neuropsychiatr. 2021, 1–7. [Google Scholar] [CrossRef]
- Alessandri, J.M.; Guesnet, P.; Vancassel, S.; Astorg, P.; Denis, I.; Langelier, B.; Aïd, S.; Poumès-Ballihaut, C.; Champeil-Potokar, G.; Lavialle, M. Polyunsaturated fatty acids in the central nervous system: Evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004, 44, 509–538. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Eckert, G.P.; Lipka, U.; Muller, W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 105–114. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, E.; Agrawal, R.; Zhuang, Y.; Abad, C.; Waschek, J.A.; Gomez-Pinilla, F. Vulnerability imposed by diet and brain trauma for anxiety-like phenotype: Implications for post-traumatic stress disorders. PLoS ONE 2013, 8, e57945. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Lai, H.C.; Yang, H.T.; Su, W.P.; Peng, C.Y.; Chang, J.P.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatry 2014, 76, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin. Neurosci. 2010, 64, 341–357. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef]
- Blondeau, N.; Nguemeni, C.; Debruyne, D.N.; Piens, M.; Wu, X.; Pan, H.; Hu, X.; Gandin, C.; Lipsky, R.H.; Plumier, J.C.; et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: A versatile potential therapy for stroke. Neuropsychopharmacology 2009, 34, 2548–2559. [Google Scholar] [CrossRef]
- Venna, V.R.; Deplanque, D.; Allet, C.; Belarbi, K.; Hamdane, M.; Bordet, R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology 2009, 34, 199–211. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Tyagi, E. Diet and cognition: Interplay between cell metabolism and neuronal plasticity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 726–733. [Google Scholar] [CrossRef]
- Di Miceli, M.; Bosch-Bouju, C.; Layé, S. PUFA and their derivatives in neurotransmission and synapses: A new hallmark of synaptopathies. Proc. Nutr Soc. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cefaliello, C.; Penna, E.; Barbato, C.; Di Ruberto, G.; Mollica, M.P.; Trinchese, G.; Cigliano, L.; Borsello, T.; Chun, J.T.; Giuditta, A.; et al. Deregulated Local Protein Synthesis in the Brain Synaptosomes of a Mouse Model for Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 1529–1541. [Google Scholar] [CrossRef]
- Eyman, M.; Cefaliello, C.; Mandile, P.; Piscopo, S.; Crispino, M.; Giuditta, A. Training old rats selectively modulates synaptosomal protein synthesis. J. Neurosci Res. 2013, 91, 20–29. [Google Scholar] [CrossRef]
- Crispino, M.; Chun, J.T.; Cefaliello, C.; Perrone Capano, C.; Giuditta, A. Local gene expression in nerve endings. Dev. Neurobiol 2014, 74, 279–291. [Google Scholar] [CrossRef]
- Crispino, M.; Cefaliello, C.; Kaplan, B.; Giuditta, A. Protein synthesis in nerve terminals and the glia-neuron unit. Results Probl. Cell. Differ. 2009, 48, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Chao, O.Y.; Marron Fernandez de Velasco, E.; Pathak, S.S.; Maitra, S.; Zhang, H.; Duvick, L.; Wickman, K.; Orr, H.T.; Hirai, H.; Yang, Y.M. Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism. Neuropsychopharmacology 2020, 45, 1159–1170. [Google Scholar] [CrossRef]
- Noseworthy, M.D.; Bray, T.M. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radic. Biol. Med. 1998, 24, 942–951. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol. Neurobiol. 1998, 18, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Manabe, S.; Wada, O.; Crawford, M.A. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int. J. Vitam. Nutr. Res. 1997, 67, 272–278. [Google Scholar] [PubMed]
- Brenna, J.T.; Diau, G.Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between Peripheral and Central Inflammation in Obesity-Promoted Disorders: The Impact on Synaptic Mitochondrial Functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef] [PubMed]
- De Mello, A.H.; Schraiber, R.D.B.; Goldim, M.P.D.S.; Garcez, M.L.; Gomes, M.L.; Silveira, G.D.B.; Zaccaron, R.P.; Schuck, P.F.; Budni, J.; Silveira, P.C.L.; et al. Omega-3 Fatty Acids Attenuate Brain Alterations in High-Fat Diet-Induced Obesity Model. Mol. Neurobiol. 2019, 56, 513–524. [Google Scholar] [CrossRef]
- Fà, M.; Diana, A.; Carta, G.; Cordeddu, L.; Melis, M.P.; Murru, E.; Sogos, V.; Banni, S. Incorporation and metabolism of c9,t11 and t10,c12 conjugated linoleic acid (CLA) isomers in rat brain. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2005, 1736, 61–66. [Google Scholar] [CrossRef]
- Soares, J.K.; Rocha-De-Melo, A.P.; Medeiros, M.C.; Queiroga, R.C.; Bomfim, M.A.; De Souza, A.F.; Nascimento, A.L.; Guedes, R.C. Conjugated linoleic acid in the maternal diet differentially enhances growth and cortical spreading depression in the rat progeny. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1490–1495. [Google Scholar] [CrossRef]
- Murru, E.; Carta, G.; Manca, C.; Sogos, V.; Pistis, M.; Melis, M.; Banni, S. Conjugated Linoleic Acid and Brain Metabolism: A Possible Anti-Neuroinflammatory Role Mediated by PPARα Activation. Front. Pharmacol. 2021, 11, 587140. [Google Scholar] [CrossRef]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Im, D.S. GPR119 and GPR55 as Receptors for Fatty Acid Ethanolamides, Oleoylethanolamide and Palmitoylethanolamide. Int. J. Mol. Sci. 2021, 22, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef]
- Saba, F.; Sirigu, A.; Pillai, R.; Caria, P.; Cordeddu, L.; Carta, G.; Murru, E.; Sogos, V.; Banni, S. Downregulation of inflammatory markers by conjugated linoleic acid isomers in human cultured astrocytes. Nutr. Neurosci. 2019, 22, 207–214. [Google Scholar] [CrossRef]
- Nakanishi, T.; Koutoku, T.; Kawahara, S.; Murai, A.; Furuse, M. Dietary conjugated linoleic acid reduces cerebral prostaglandin E(2) in mice. Neurosci. Lett. 2003, 341, 135–138. [Google Scholar] [CrossRef]
- Sikorski, A.M.; Hebert, N.; Swain, R.A. Conjugated Linoleic Acid (CLA) inhibits new vessel growth in the mammalian brain. Brain Res. 2008, 1213, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, S.; Oikawa, D.; Tsuji, Y.; Akimoto, Y.; Jikuya, H.; Furuse, M. Dietary conjugated linoleic acid modifies the brain endocannabinoid system in mice. Nutr. Neurosci. 2009, 12, 155–159. [Google Scholar] [CrossRef]
- Monaco, A.; Ferrandino, I.; Boscaino, F.; Cocca, E.; Cigliano, L.; Maurano, F.; Luongo, D.; Spagnuolo, M.S.; Rossi, M.; Bergamo, P. Conjugated linoleic acid prevents age-dependent neurodegeneration in a mouse model of neuropsychiatric lupus via the activation of an adaptive response. J. Lipid Res. 2018, 59, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Cigliano, L.; Spagnuolo, M.S.; Boscaino, F.; Ferrandino, I.; Monaco, A.; Capriello, T.; Cocca, E.; Iannotta, L.; Treppiccione, L.; Luongo, D.; et al. Dietary Supplementation with Fish Oil or Conjugated Linoleic Acid Relieves Depression Markers in Mice by Modulation of the Nrf2 Pathway. Mol. Nutr. Food Res. 2019, 63, e1900243. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.T.; Kamboj, A.; Anderson, H.D.; Anderson, C.M. Protection of cortical neurons from excitotoxicity by conjugated linoleic acid. J. Neurochem 2010, 115, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.P.; Lima, M.D.S.; Barbosa, M.Q.; De Melo, M.F.F.T.; Bertozzo, C.C.D.M.S.; De Oliveira, M.E.G.; Bessa, R.J.B.; Alves, S.P.A.; Souza, M.I.A.; Queiroga, R.D.C.R.D.E.; et al. Effect of Conjugated Linoleic Acid on Memory and Reflex Maturation in Rats Treated During Early Life. Front. Neurosci. 2019, 13, 370. [Google Scholar] [CrossRef] [PubMed]

| Cow | Buffalo | Sheep | Goat | Horse | Donkey | |
|---|---|---|---|---|---|---|
| Fat (g/100 g) | 3.3.–6.4 | 5.3–15.0 | 4.0–9.0 | 3.0–7.2 | 0.4–7.2 | 0.3–1.8 |
| % of total FAs | ||||||
| SFAs | 55.0–73.0 | 62.0–74.0 | 57.0–75.0 | 59.0–74.0 | 37.0–55.0 | 46.0–68.0 |
| MUFAs | 2.0–30.0 | 24.0–29.0 | 23.0–39.0 | 19.0–36.0 | 18.0–36.0 | 15.0–35.0 |
| PUFAs | 2.4–6.3 | 2.3–3.9 | 2.6–7.3 | 2.6–5.6 | 13.0–51.0 | 14.0–30.0 |
| CLA | 0.2–2.4 | 0.4–1.0 | 0.6–1.1 | 0.3–1.2 | 0.02–0.1 | Trace |
| ω-6 | 1.2–3.0 | 1.74–2.0 | 1.6–3.6 | 1.9–4.3 | 3.6–20.3 | 6.0–15.2 |
| ω-3 | 0.3–1.8 | 0.2–1.4 | 0.5–2.3 | 0.3–1.48 | 2.2 -31.2 | 4.0–16.3 |
| Study | Treatment | Outcome | |
|---|---|---|---|
| Animal models | |||
| Female growing pigs | RA-enriched butter | Undetectable effects on blood lipoproteins [142] | |
| Wistar rats | RA-enriched clarified butter | ↑ Antioxidants [143] | |
| Wistar rats | RA-enriched butter | ↑ HDL, Triacylglycerol [144] | |
| Mice fed high fat diet | Pasture dairy cream | ↓ Inflammation, Triacylglycerol ↑ Protective cells in the gut [131] | |
| Wistar rats | RA-enriched butter | ↑ PLA2 [145] | |
| Mouse model of chemically -induced (DSS) colitis | RA-enriched butter | ↑ Nrf-2- mediated defenses ↓ Colitis signs [146] | |
| Wistar rats | RA-enriched butter | ↑ Mitochondrial function [37] | |
| Wistar rats | RA-enriched butter | ↓ Muscle inflammation, oxidative stress ↑ Mitochondrial function [128] | |
| Clinical studies | |||
| Healthy middle-age subjects | Naturally enriched dairy products | Undetectable effects on blood lipoproteins [147] | |
| Healthy normal-weight and over-weight subjects | Naturally enriched cheese | ↓ IL-6, Tnf-α [148] | |
| Hypercholesterolemic subjects | Naturally enriched cheese | ↓ LDL [134] | |
| Healthy young subjects | Naturally enriched cheese | ↑ IL-10 ↓ NFkB, Tnf-α, IL-2, IL-8 [149] | |
| Meta-analyses | CLA enriched food | ↓ LDL, cholesterol [150] | |
| Healthy middle-age subjects | RA enriched cheese | ↑ Highly unsaturated fatty acids in blood plasma [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollica, M.P.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. https://doi.org/10.3390/nu13041111
Mollica MP, Trinchese G, Cimmino F, Penna E, Cavaliere G, Tudisco R, Musco N, Manca C, Catapano A, Monda M, et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients. 2021; 13(4):1111. https://doi.org/10.3390/nu13041111
Chicago/Turabian StyleMollica, Maria P., Giovanna Trinchese, Fabiano Cimmino, Eduardo Penna, Gina Cavaliere, Raffaella Tudisco, Nadia Musco, Claudia Manca, Angela Catapano, Marcellino Monda, and et al. 2021. "Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions" Nutrients 13, no. 4: 1111. https://doi.org/10.3390/nu13041111
APA StyleMollica, M. P., Trinchese, G., Cimmino, F., Penna, E., Cavaliere, G., Tudisco, R., Musco, N., Manca, C., Catapano, A., Monda, M., Bergamo, P., Banni, S., Infascelli, F., Lombardi, P., & Crispino, M. (2021). Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients, 13(4), 1111. https://doi.org/10.3390/nu13041111















