The Relationship between Resistance Exercise Performance and Ventilatory Efficiency after Beetroot Juice Intake in Well-Trained Athletes
Abstract
1. Introduction
2. Materials and Methods
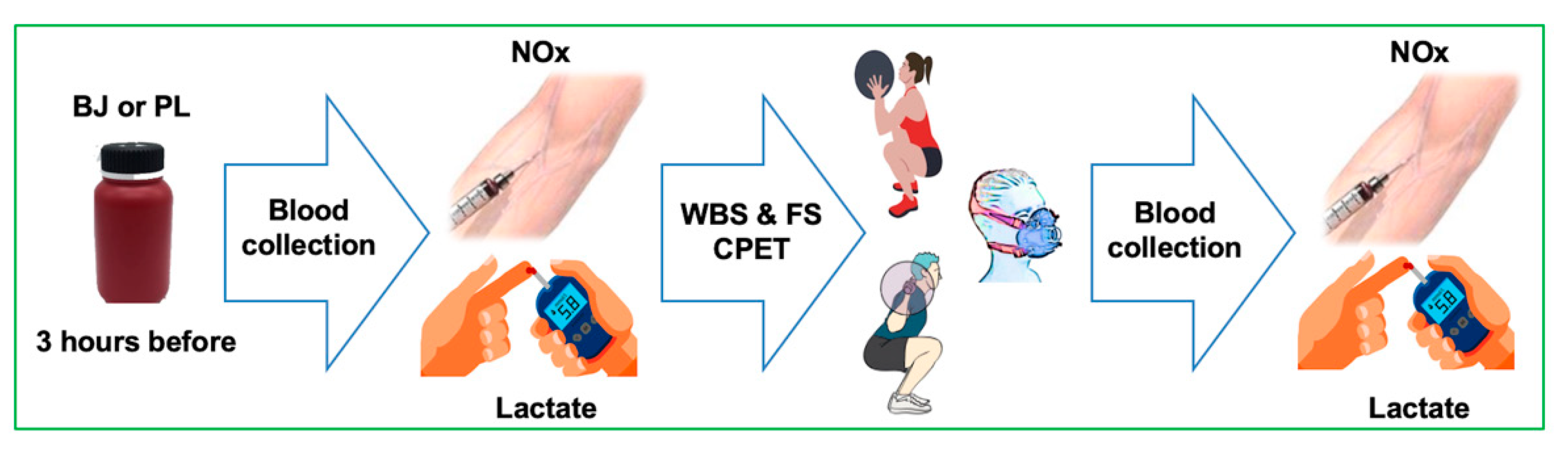
2.1. Study Design
2.2. Participants
2.3. Diet Control and Beetroot Juice Intake
2.4. Resistance Exercise Tests
2.5. Plasma NOx Concentrations
2.6. Pulmonary Gas Exchange
2.7. Blood Lactate
2.8. Statistical Analysis
3. Results
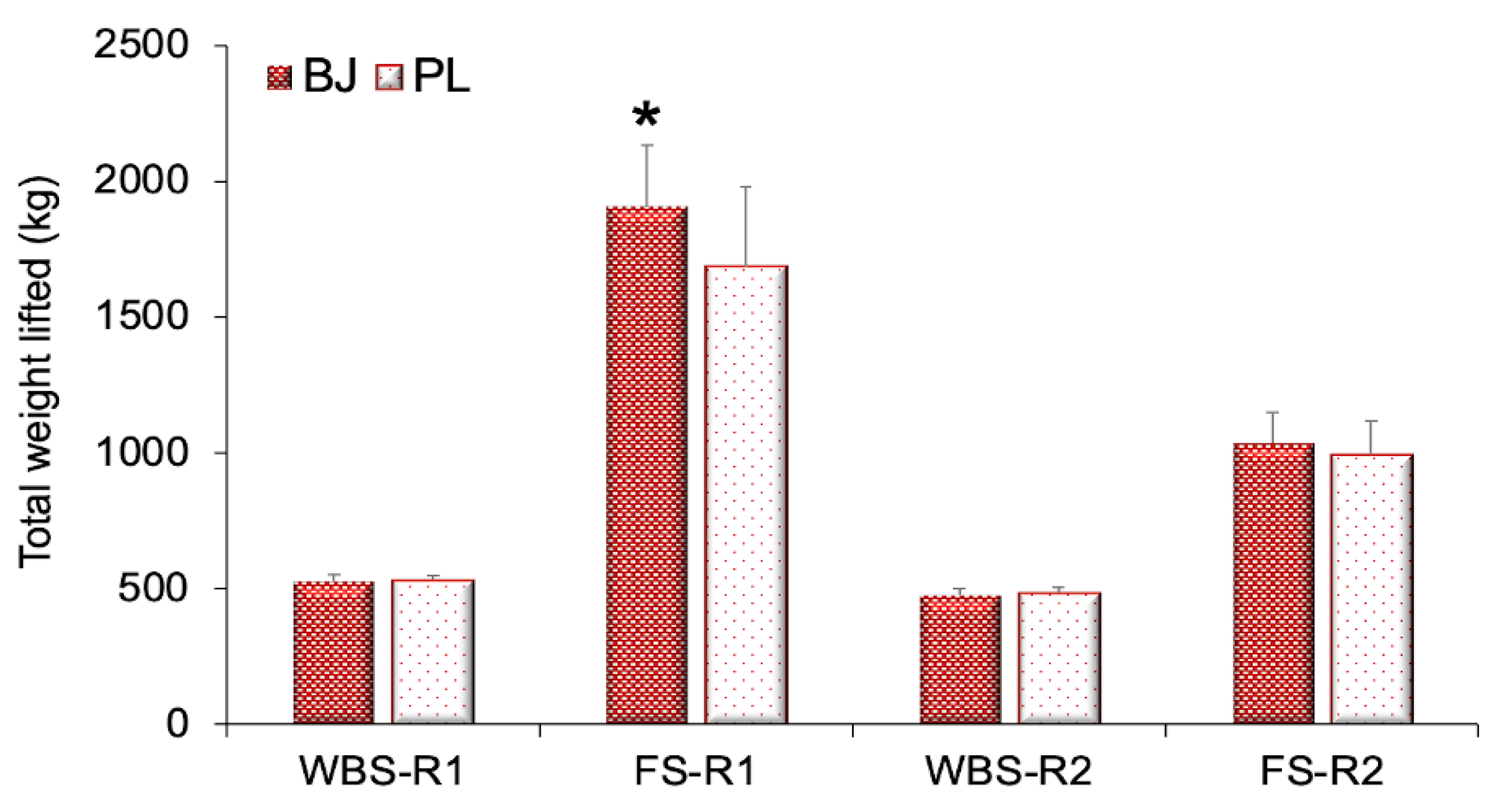
3.1. Resistance Exercise Performance
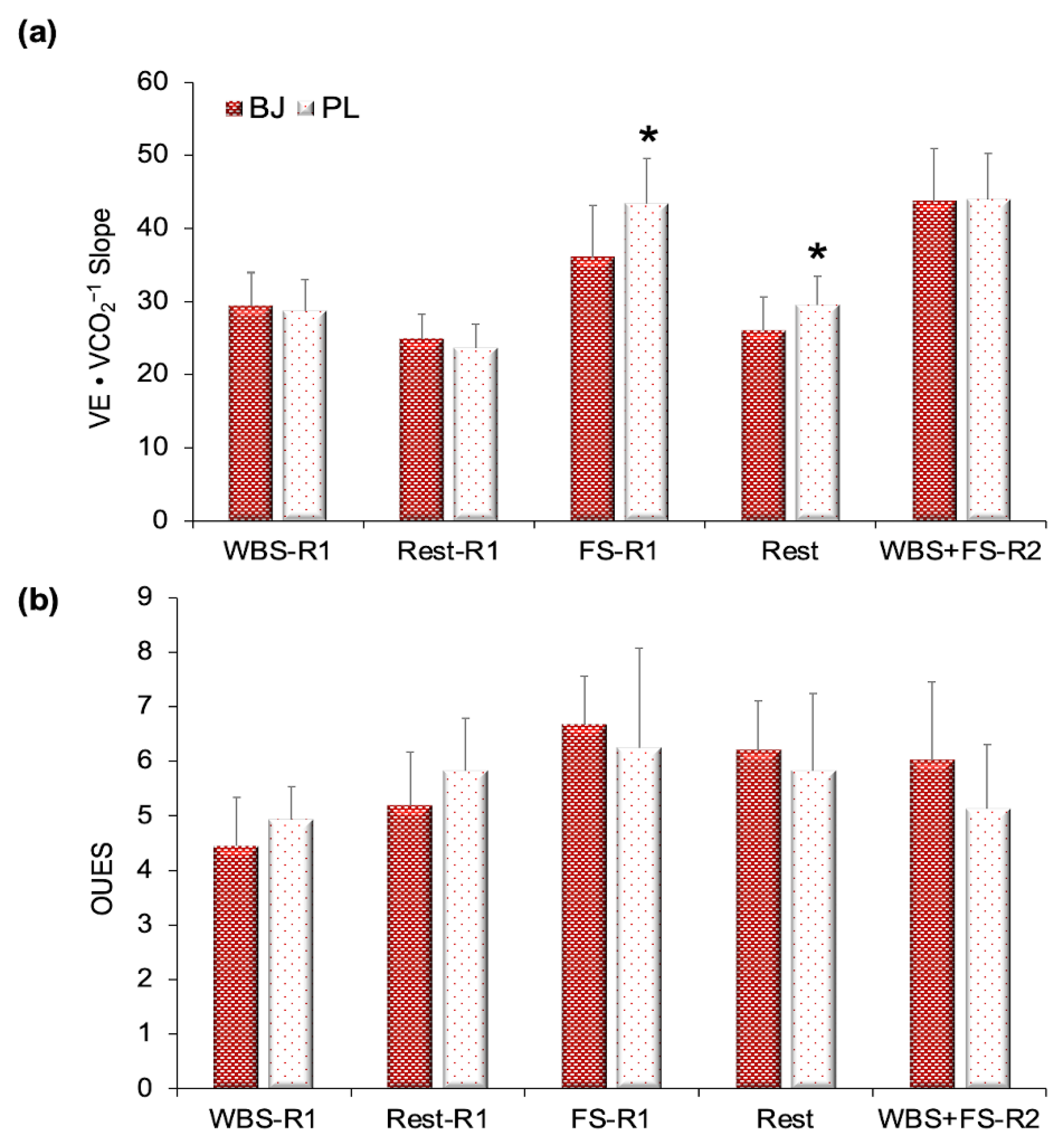
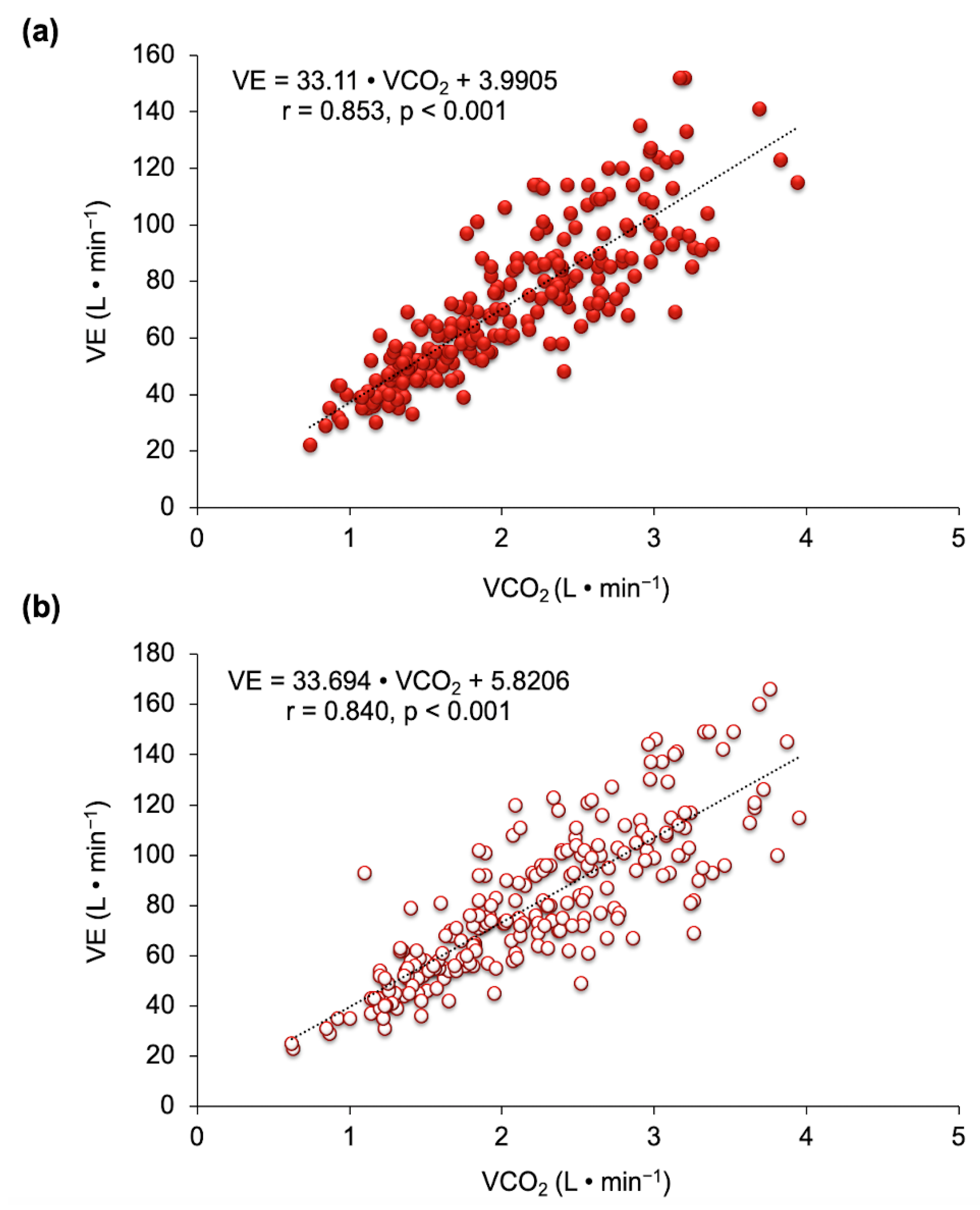
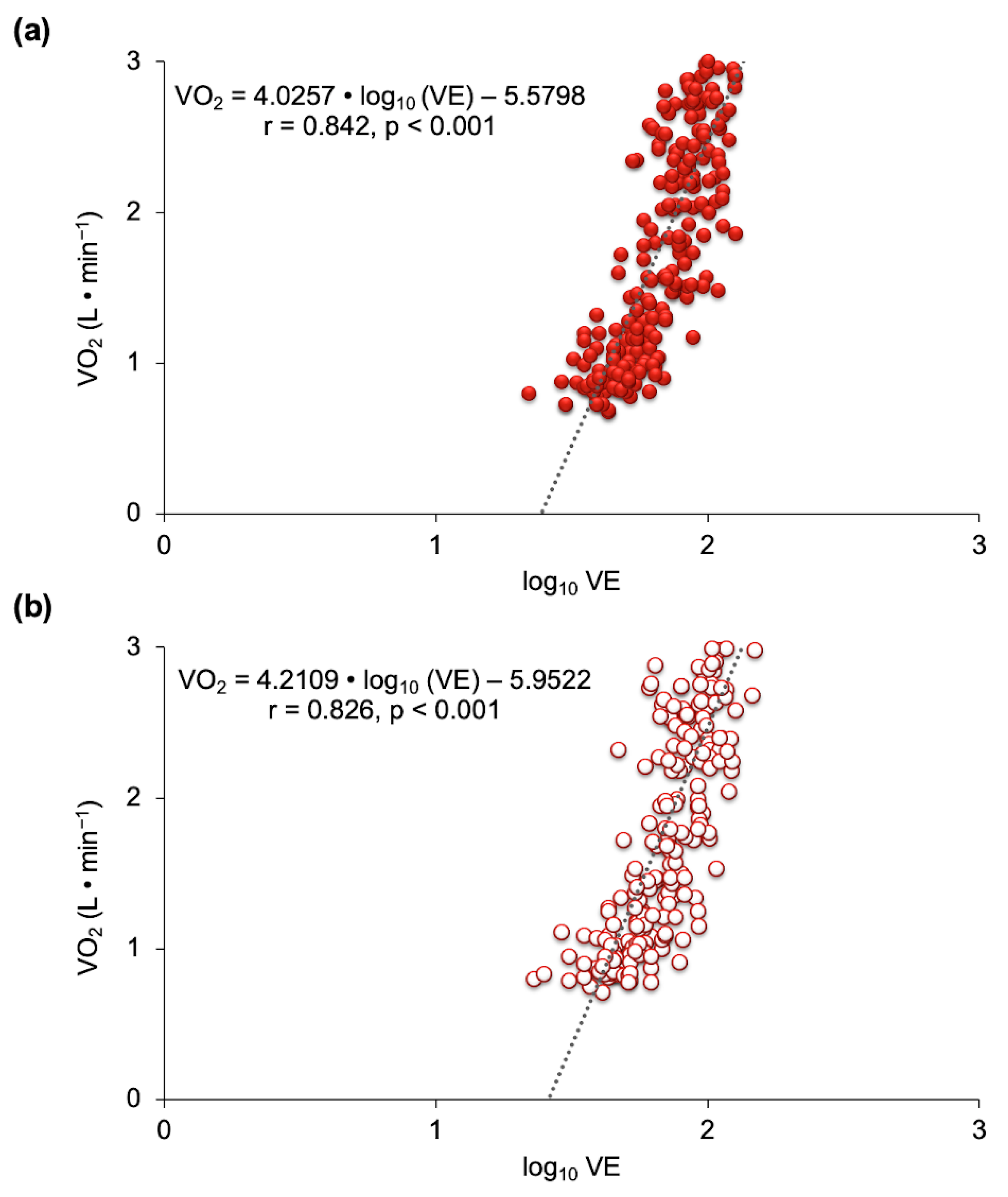
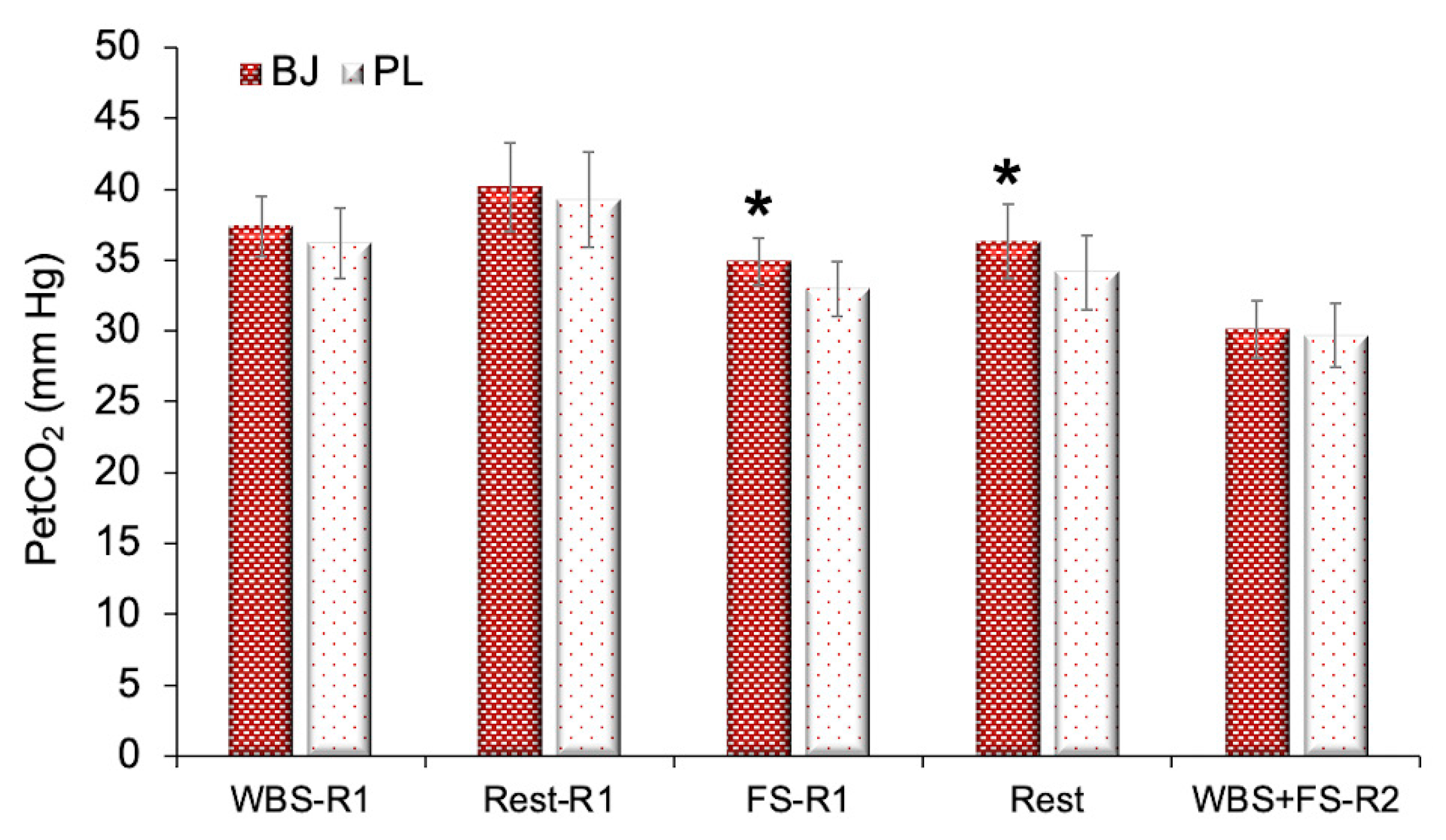
3.2. Ventilatory Efficiency
3.3. Blood Lactate Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reindl, I.; Kleber, R.X. Exertional hyperpnea in patients with chronic heart failure is a reversible cause of exercise intolerance. Basic Res. Cardiol. 1996, 91, 37–43. [Google Scholar] [PubMed]
- Brown, S.J.; Raman, A.; Schlader, Z.; Stannard, S.R. Ventilatory efficiency in juvenile elite cyclists. J. Sci. Med. Sport 2013, 16, 266–270. [Google Scholar] [CrossRef]
- Romer, L.M.; Polkey, M.I. Exercise-induced respiratory muscle fatigue: Implications for performance. J. Appl. Physiol. 2008, 104, 879–888. [Google Scholar] [CrossRef]
- Whipp, B.J. The bioenergetic and gas exchange basis of exercise testing. Clin. Chest Med. 1994, 15, 173–192. [Google Scholar]
- Hammond, M.D.; Gale, G.E.; Kapitan, K.S.; Ries, A.; Wagner, P.D. Pulmonary gas exchange in humans during exercise at sea level. J. Appl. Physiol. 1986, 60, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Schaffartzik, W.; Poole, D.C.; Derion, T.; Tsukimoto, K.; Hogan, M.C.; Arcos, J.P.; Bebout, D.E.; Wagner, P.D. V̇A/Q̇ distribution during heavy exercise and recovery in humans: Implications for pulmonary edema. J. Appl. Physiol. 1992, 72, 1657–1667. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the American heart association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory efficiency during exercise in healthy subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Gavotto, A.; Huguet, H.; Picot, M.C.; Guillaumont, S.; Matecki, S.; Amedro, P. The V̇e/V̇co2 slope: A useful tool to evaluate the physiological status of children with congenital heart disease. J. Appl. Physiol. 2020, 129, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Guazzi, M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: An evidence-based review. Heart Fail. Rev. 2008, 13, 245–269. [Google Scholar] [CrossRef]
- Chlif, M.; Chaouachi, A.; Ahmaidi, S. Effect of aerobic exercise training on ventilatory efficiency and respiratory drive in obese subjects. Respir. Care 2017, 62, 936–946. [Google Scholar] [CrossRef]
- Grinstein, J.; Sawalha, Y.; Hofmeyer, M.; Sheikh, F.; Rodrigo, M.; Kadakkal, A.; Barnett, C.; Kalantari, S.; Talati, I.; Zaghlol, R.; et al. VE/VCO2 Predicts RV Dysfunction and Mortality after Left Ventricular Assist Device: A Fresh Look at Cardiopulmonary Stress Testing for Prognostication. J. Heart Lung Transplant. 2019, 38, S106–S107. [Google Scholar] [CrossRef]
- Fung, E.; Ting Lui, L.; Gustafsson, F.; Yau, F.C.F.; Leung, J.C.S.; Wiklund, P.; Järvelin, M.R.; Macdonald, P.S.; Woo, J. Predicting 10-year mortality in older adults using VO2max, oxygen uptake efficiency slope and frailty class. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Da Luz Goulart, C.; dos Santos, P.B.; Caruso, F.R.; Arêas, G.P.T.; Marinho, R.S.; De Faria Camargo, P.; Da Silva Alexandre, T.; Oliveira, C.R.; da Silva, A.L.G.; Mendes, R.G.; et al. The Value of cardiopulmonary exercise testing in Determining Severity in patients with both Systolic Heart failure and COPD open. Sci. Rep. 2020, 10, 4309. [Google Scholar] [CrossRef]
- Laveneziana, P.; Agostoni, P.; Mignatti, A.; Mushtaq, S.; Colombo, P.; Sims, D.; Uriel, N.; Jorde, U.P. Effect of Acute β-blocker Withholding on Ventilatory Efficiency in Patients With Advanced Chronic Heart Failure. J. Card. Fail. 2010, 16, 548–555. [Google Scholar] [CrossRef]
- Tanabe, Y.; Hosaka, Y.; Ito, M.; Ito, E.; Suzuki, K. Significance of end-tidal PCO2 response to exercise and its relation to functional capacity in patients with chronic heart failure. Chest 2001, 119, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Ulubay, G.; Chow, B.F.; Sun, X.G.; Wasserman, K. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest 2007, 132, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Oudiz, R.J.; Roveran, G.; Hansen, J.E.; Sun, X.G.; Wasserman, K. Effect of sildenafil on ventilatory efficiency and exercise tolerance in pulmonary hypertension. Eur. J. Heart Fail. 2007, 9, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martínez, E.; Terrados, N.; Burtscher, M.; Santalla, A.; Naranjo Orellana, J. Ventilatory efficiency and breathing pattern in world-class cyclists: A three-year observational study. Respir. Physiol. Neurobiol. 2016, 229, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Sorrentino, K.M.; Ninness, E.M.; Pham, P.H.; Dorado, S.; Costello, K.B. Test-retest reliability for two indices of ventilatory efficiency measured during cardiopulmonary exercise testing in healthy men and women. Clin. Physiol. Funct. Imaging 2006, 26, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Albesa-Albiol, L.; Serra-Payá, N.; Garnacho-Castaño, M.A.; Cano, L.G.; Cobo, E.P.; Maté-Muñoz, J.L.; Garnacho-Castaño, M.V. Ventilatory efficiency during constant-load test at lactate threshold intensity: Endurance versus resistance exercises. PLoS ONE 2019, 14, e0216824. [Google Scholar] [CrossRef]
- Zapol, W.M.; Rimar, S.; Gillis, N.; Marletta, M.; Bosken, C.H. Nitric oxide and the lung. Am. J. Respir. Crit. Care Med. 1994, 149, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.P.; Prendergast, B. Intravenous L-arginine reduces VE/VCO2 slope acutely in patients with severe chronic heart failure. Eur. J. Heart Fail. 1999, 1, 187–190. [Google Scholar] [CrossRef]
- Lewis, G.D.; Lachmann, J.; Camuso, J.; Lepore, J.J.; Shin, J.; Martinovic, M.E.; Systrom, D.M.; Bloch, K.D.; Semigran, M.J. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 2007, 115, 59–66. [Google Scholar] [CrossRef]
- Curtis, K.J. Spiral: Augmenting Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease: Studies of ACE-Inhibition and Nitrate Supplementation. Ph.D. Thesis, Imperial College London, London, UK, 2017. [Google Scholar]
- Hwang, I.-C.; Kim, Y.-J.; Park, J.-B.; Yoon, Y.E.; Lee, S.-P.; Kim, H.-K.; Cho, G.-Y.; Sohn, D.-W. Pulmonary hemodynamics and effects of phosphodiesterase type 5 inhibition in heart failure: A meta-analysis of randomized trials. BMC Cardiovasc. Disord. 2017, 17, 150. [Google Scholar] [CrossRef]
- Michelakis, E.; Tymchak, W.; Lien, D.; Webster, L.; Hashimoto, K.; Archer, S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: Comparison with inhaled nitric oxide. Circulation 2002, 105, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Lepore, J.J.; Maroo, A.; Pereira, N.L.; Ginns, L.C.; Dec, G.W.; Zapol, W.M.; Bloch, K.D.; Semigran, M.J. Effect of Sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am. J. Cardiol. 2002, 90, 677–680. [Google Scholar] [CrossRef]
- Hoon, M.W.; Jones, A.M.; Johnson, N.A.; Blackwell, J.R.; Broad, E.M.; Lundy, B.; Rice, A.J.; Burke, L.M. The effect of variable doses of inorganic nitrate-rich beetroot juice on simulated 2000-m rowing performance in trained athletes. Int. J. Sports Physiol. Perform. 2014, 9, 615–620. [Google Scholar] [CrossRef]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Palau-Salvà, G.; Cuenca, E.; Muñoz-González, A.; García-Fernández, P.; del Carmen Lozano-Estevan, M.; Veiga-Herreros, P.; Maté-Muñoz, J.L.; Domínguez, R. Effects of a single dose of beetroot juice on cycling time trial performance at ventilatory thresholds intensity in male triathletes. J. Int. Soc. Sports Nutr. 2018, 15, 49. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Palau-Salvà, G.; Serra-Payá, N.; Ruiz-Hermosel, M.; Berbell, M.; Viñals, X.; Gomis Bataller, M.; Carbonell, T.; Vilches-Saez, S.; Pleguezuelos Cobo, E.; et al. Understanding the effects of beetroot juice intake on CrossFit performance by assessing hormonal, metabolic and mechanical response: A randomized, double-blind, crossover design. J. Int. Soc. Sports Nutr. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. High-carbohydrate versus high-fat diets in endurance sports Übersichtsartikel High-carbohydrate versus high-fat diets in endurance sports. Schweizerische Zeitschrift Sport. Sport. 2003, 51, 17–23. [Google Scholar]
- Govoni, M.; Jansson, E.Å.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Albesa-Albiol, L.; Serra-Payá, N.; Gomis Bataller, M.; Pleguezuelos Cobo, E.; Guirao Cano, L.; Guodemar-Pérez, J.; Carbonell, T.; Domínguez, R.; Maté-Muñoz, J.L. Oxygen Uptake Slow Component and the Efficiency of Resistance Exercises. J. Strength Cond. Res. 2018. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Albesa-Albiol, L.; Serra-Payá, N.; Gomis Bataller, M.; Felíu-Ruano, R.; Guirao Cano, L.; Pleguezuelos Cobo, E.; Maté-Muñoz, J.L. The Slow Component of Oxygen Uptake and Efficiency in Resistance Exercises: A Comparison With Endurance Exercises. Front. Physiol. 2019, 10, 357. [Google Scholar] [CrossRef]
- Bonaventura, J.M.; Sharpe, K.; Knight, E.; Fuller, K.L.; Tanner, R.K.; Gore, C.J. Reliability and accuracy of six hand-held blood lactate analysers. J. Sports Sci. Med. 2014, 14, 203–214. [Google Scholar]
- Cohen, J. Quantitative methods in psychology: A power primer. Psychol. Bull. 1992, 112, 1155–1159. [Google Scholar] [CrossRef]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.R.; Milsom, A.B.; Gunaruwan, P.; Abozguia, K.; Ahmed, I.; Weaver, R.A.; Thomas, P.; Ashrafian, H.; Born, G.V.R.; James, P.E.; et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 2008, 117, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C.; Ghosh, S.; Janocha, A.J.; Xu, W.; Bauer, S.; Bryan, N.S.; Tejero, J.; Hemann, C.; Hille, R.; Stuehr, D.J.; et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc. Natl. Acad. Sci. USA 2007, 104, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Lühker, O.; Berger, M.M.; Pohlmann, A.; Hotz, L.; Gruhlke, T.; Hochreiter, M. Changes in acid–base and ion balance during exercise in normoxia and normobaric hypoxia. Eur. J. Appl. Physiol. 2017, 117, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, M.I.; McKelvie, R.S.; Heigenhauser, G.J.F. K+ and Lac- distribution in humans during and after high-intensity exercise: Role in muscle fatigue attenuation? J. Appl. Physiol. 1995, 78, 765–777. [Google Scholar] [CrossRef]
- Stickland, M.K.; Lindinger, M.I.; Olfert, I.M.; Heigenhauser, G.J.F.; Hopkins, S.R. Pulmonary gas exchange and acid-base balance during exercise. Compr. Physiol. 2013, 3, 693–739. [Google Scholar] [PubMed]
- Gladden, L.B. Lactate metabolism: A new paradigm for the third millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood lactate measurements and analysis during exercise: A guide for clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef]
- Vohwinkel, C.U.; Lecuona, E.; Sun, H.; Sommer, N.; Vadász, I.; Chandel, N.S.; Sznajder, J.I. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011, 286, 37067–37076. [Google Scholar] [CrossRef]
- Phillips, D.B.; Collins, S.É.; Stickland, M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020, 11, 659. [Google Scholar] [CrossRef]
- Brown, S.J.; Brown, J.A. Heart rate variability and ventilatory efficiency. Int. J. Sports Med. 2009, 30, 496–502. [Google Scholar] [CrossRef]
- Sun, X.G.; Hansen, J.E.; Stringer, W.W. Oxygen uptake efficiency plateau: Physiology and reference values. Eur. J. Appl. Physiol. 2012, 112, 919–928. [Google Scholar] [CrossRef]
- Arena, R.; Guazzi, M.; Myers, J. Prognostic value of end-tidal carbon dioxide during exercise testing in heart failure. Int. J. Cardiol. 2007, 117, 103–108. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Hsu, L.; Peberdy, M.A.; Pinkstaff, S.; Bensimhon, D.; Chase, P.; Vicenzi, M.; Guazzi, M. The Minute Ventilation/Carbon Dioxide Production Slope is Prognostically Superior to the Oxygen Uptake Efficiency Slope. J. Card. Fail. 2007, 13, 462–469. [Google Scholar] [CrossRef]
- Hopkins, S.R.; McKenzie, D.C.; Schoene, R.B.; Glenny, R.W.; Robertson, H.T. Pulmonary gas exchange during exercise in athletes I. Ventilation-perfusion mismatch and diffusion limitation. J. Appl. Physiol. 1994, 77, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.R.; Gavin, T.P.; Siafakas, N.M.; Haseler, L.J.; Olfert, I.M.; Wagner, H.; Wagner, P.D. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J. Appl. Physiol. 1998, 85, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Frostell, C.; Fratacci, M.D.; Wain, J.C.; Jones, R.; Zapol, W.M. Inhaled nitric oxide: A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991, 83, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Frostell, C.G.; Blomqvist, H.; Hedenstierna, G.; Lundberg, J.; Zapol, W.M. Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology 1993, 78, 427–435. [Google Scholar] [CrossRef]
- Behnia, M.; Wheatley, C.M.; Avolio, A.; Johnson, B.D. Influence of dietary nitrate supplementation on lung function and exercise gas exchange in COPD patients. Nitric Oxide 2018, 76, 53–61. [Google Scholar] [CrossRef]
- Coggan, A.R.; Broadstreet, S.R.; Mahmood, K.; Mikhalkova, D.; Madigan, M.; Bole, I.; Park, S.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; et al. Dietary Nitrate Increases VO2peak and Performance but Does Not Alter Ventilation or Efficiency in Patients With Heart Failure With Reduced Ejection Fraction. J. Card. Fail. 2018, 24, 65–73. [Google Scholar] [CrossRef]
- Ellis, L.A.; Ainslie, P.N.; Armstrong, V.A.; Morris, L.E.; Simair, R.G.; Sletten, N.R.; Tallon, C.M.; McManus, A.M. Anterior cerebral blood velocity and end-tidal CO2 responses to exercise differ in children and adults. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1195–H1202. [Google Scholar] [CrossRef]
- Komiyama, T.; Tanoue, Y.; Sudo, M.; Costello, J.T.; Uehara, Y.; Higaki, Y.; Ando, S. Cognitive Impairment during High-Intensity Exercise: Influence of Cerebral Blood Flow. Med. Sci. Sports Exerc. 2020, 52, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Gujja, P.; Neelagaru, S.; Hsu, L.; Vittorio, T.; Jackson-Nelson, T.; Burkhoff, D. End-tidal CO2 pressure and cardiac performance during exercise in heart failure. Med. Sci. Sports Exerc. 2009, 41, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, N.; Fan, J.L.; Uva, B.; Müller, H.; Meyer, P.; Kayser, B. Effect of oral nitrate supplementation on pulmonary hemodynamics during exercise and time trial performance in normoxia and hypoxia: A randomized controlled trial. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra-Payá, N.; Garnacho-Castaño, M.V.; Sánchez-Nuño, S.; Albesa-Albiol, L.; Girabent-Farrés, M.; Moizé Arcone, L.; Fernández, A.P.; García-Fresneda, A.; Castizo-Olier, J.; Viñals, X.; et al. The Relationship between Resistance Exercise Performance and Ventilatory Efficiency after Beetroot Juice Intake in Well-Trained Athletes. Nutrients 2021, 13, 1094. https://doi.org/10.3390/nu13041094
Serra-Payá N, Garnacho-Castaño MV, Sánchez-Nuño S, Albesa-Albiol L, Girabent-Farrés M, Moizé Arcone L, Fernández AP, García-Fresneda A, Castizo-Olier J, Viñals X, et al. The Relationship between Resistance Exercise Performance and Ventilatory Efficiency after Beetroot Juice Intake in Well-Trained Athletes. Nutrients. 2021; 13(4):1094. https://doi.org/10.3390/nu13041094
Chicago/Turabian StyleSerra-Payá, Noemí, Manuel Vicente Garnacho-Castaño, Sergio Sánchez-Nuño, Lluís Albesa-Albiol, Montserrat Girabent-Farrés, Luciana Moizé Arcone, Alba Pardo Fernández, Adrián García-Fresneda, Jorge Castizo-Olier, Xavier Viñals, and et al. 2021. "The Relationship between Resistance Exercise Performance and Ventilatory Efficiency after Beetroot Juice Intake in Well-Trained Athletes" Nutrients 13, no. 4: 1094. https://doi.org/10.3390/nu13041094
APA StyleSerra-Payá, N., Garnacho-Castaño, M. V., Sánchez-Nuño, S., Albesa-Albiol, L., Girabent-Farrés, M., Moizé Arcone, L., Fernández, A. P., García-Fresneda, A., Castizo-Olier, J., Viñals, X., Molina-Raya, L., & Gomis Bataller, M. (2021). The Relationship between Resistance Exercise Performance and Ventilatory Efficiency after Beetroot Juice Intake in Well-Trained Athletes. Nutrients, 13(4), 1094. https://doi.org/10.3390/nu13041094







