Effects of n-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Eligibility
2.2. Data Extraction
2.3. Assessment of Risk of Bias
2.4. Data Synthesis and Analysis
3. Results
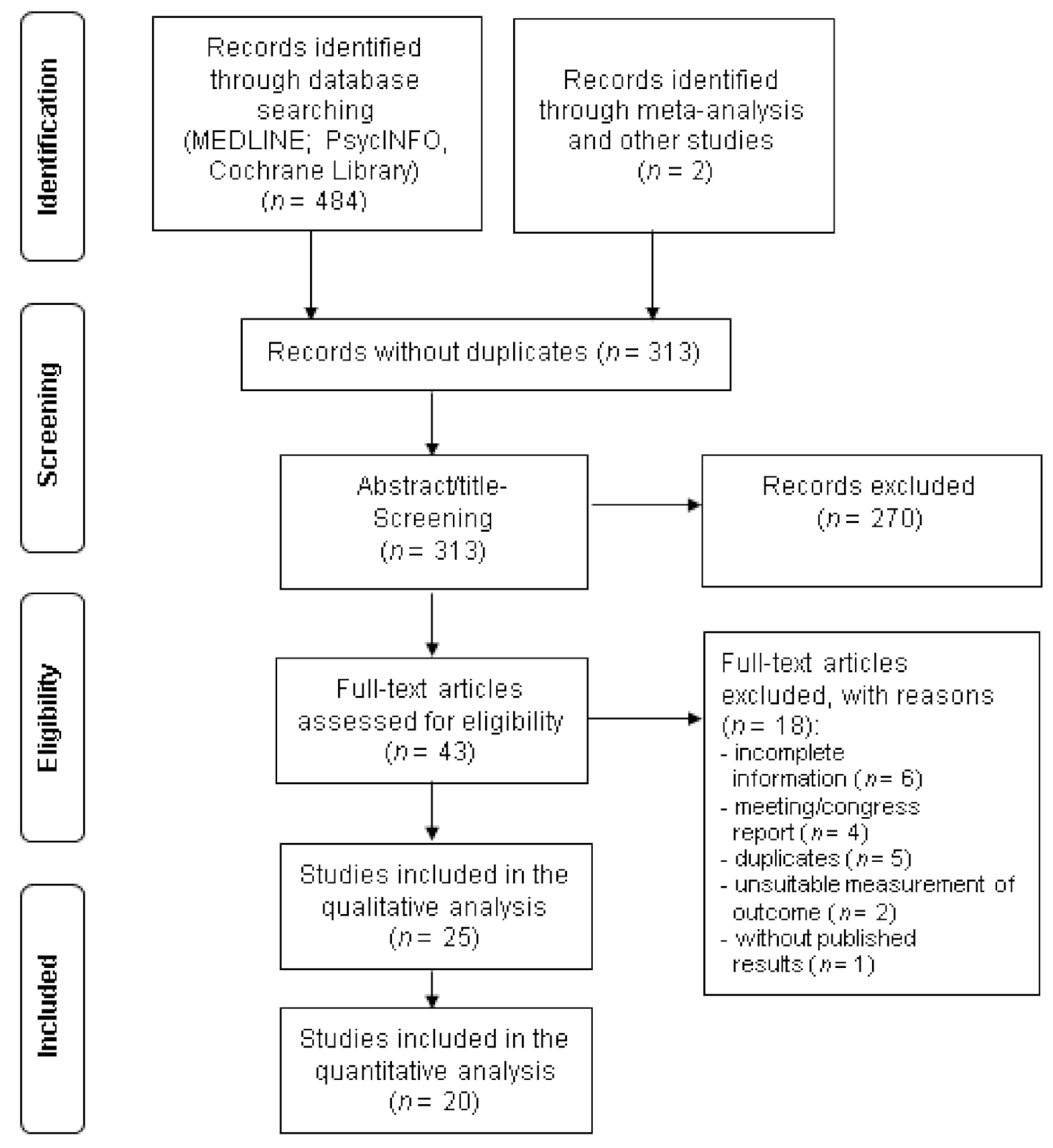
3.1. Search Results and Characteristics of the Studies
3.2. Risk of Bias
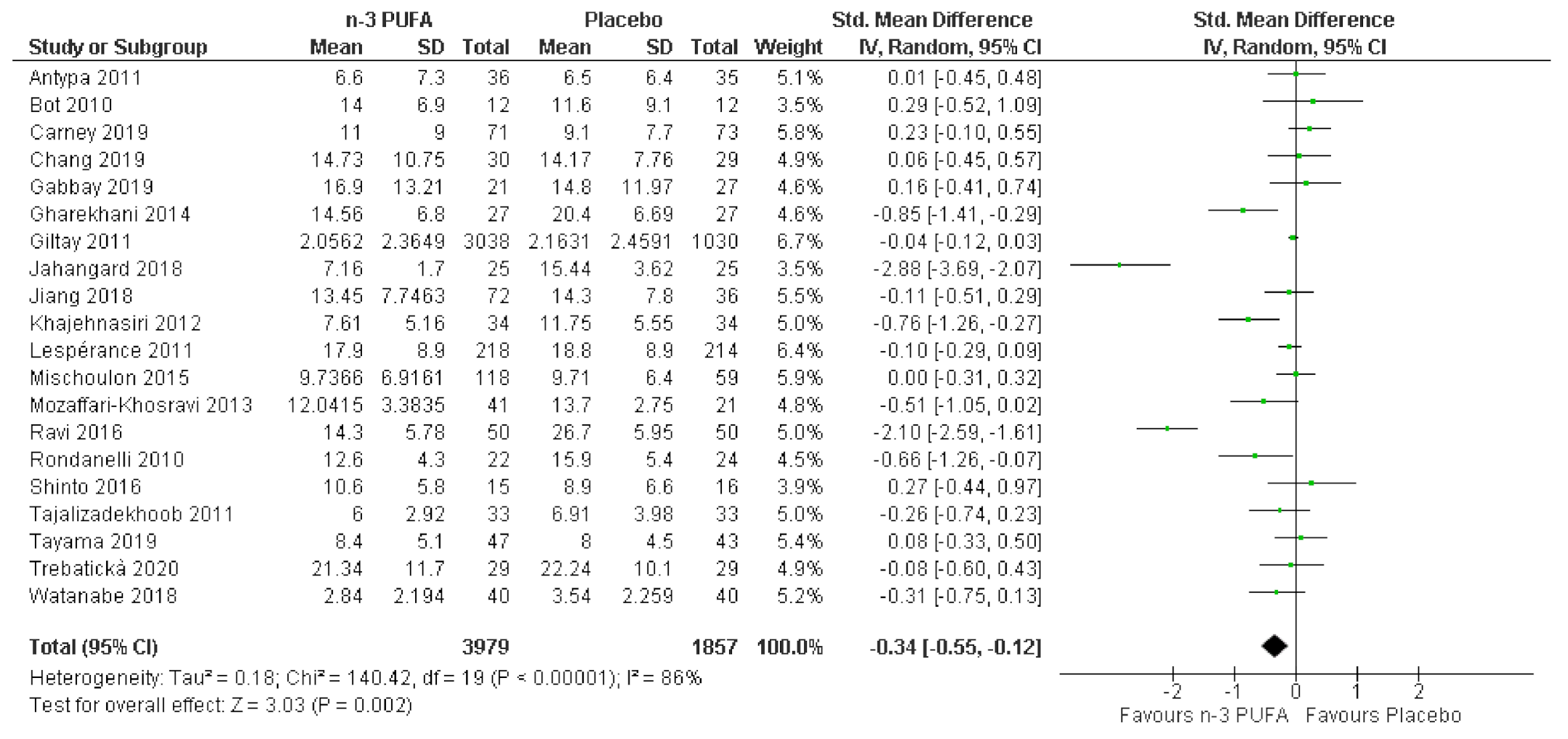
3.3. Effects of n-3 PUFA in Meta-Analysis
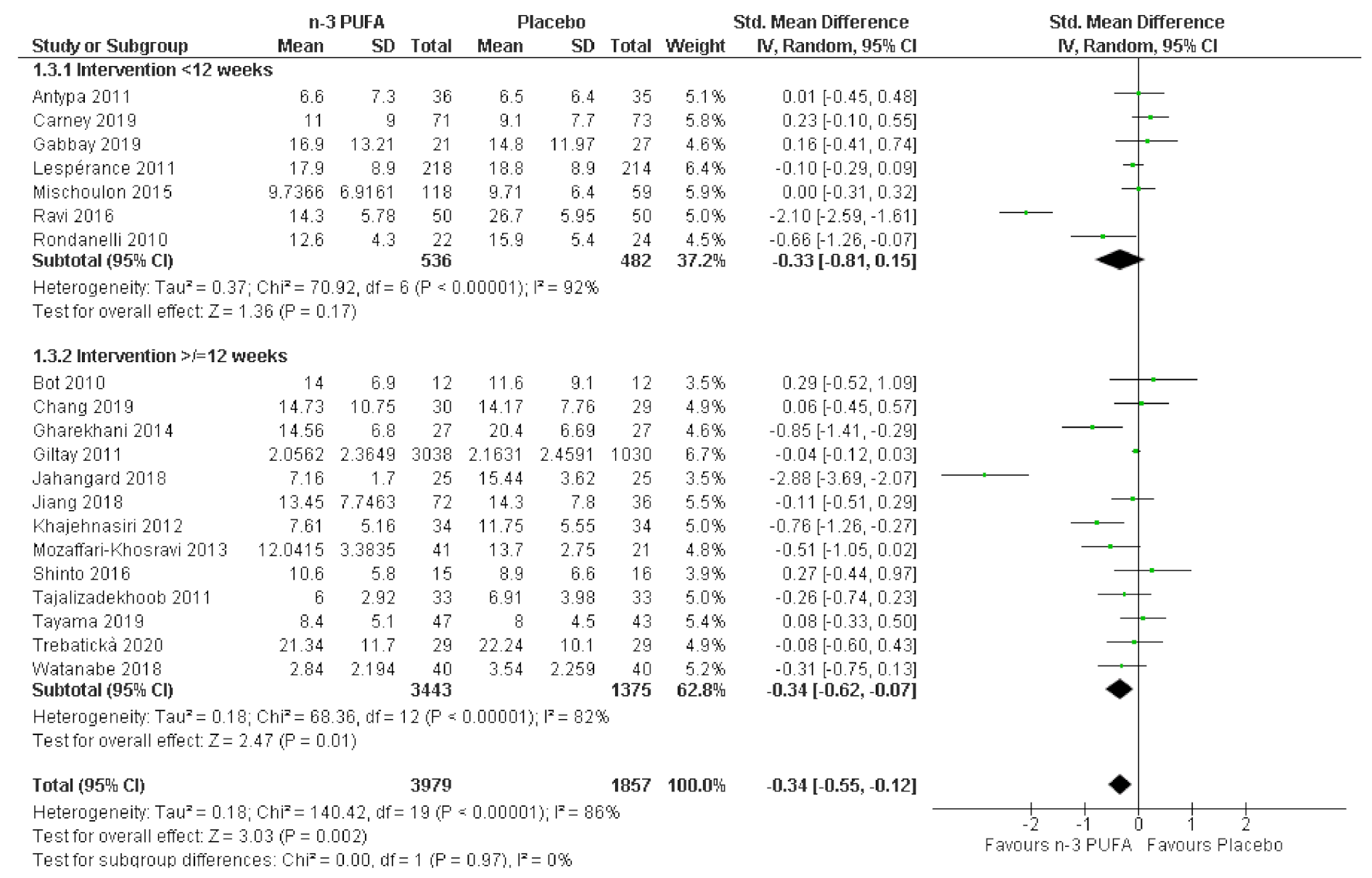
3.4. Subgroup Analyses
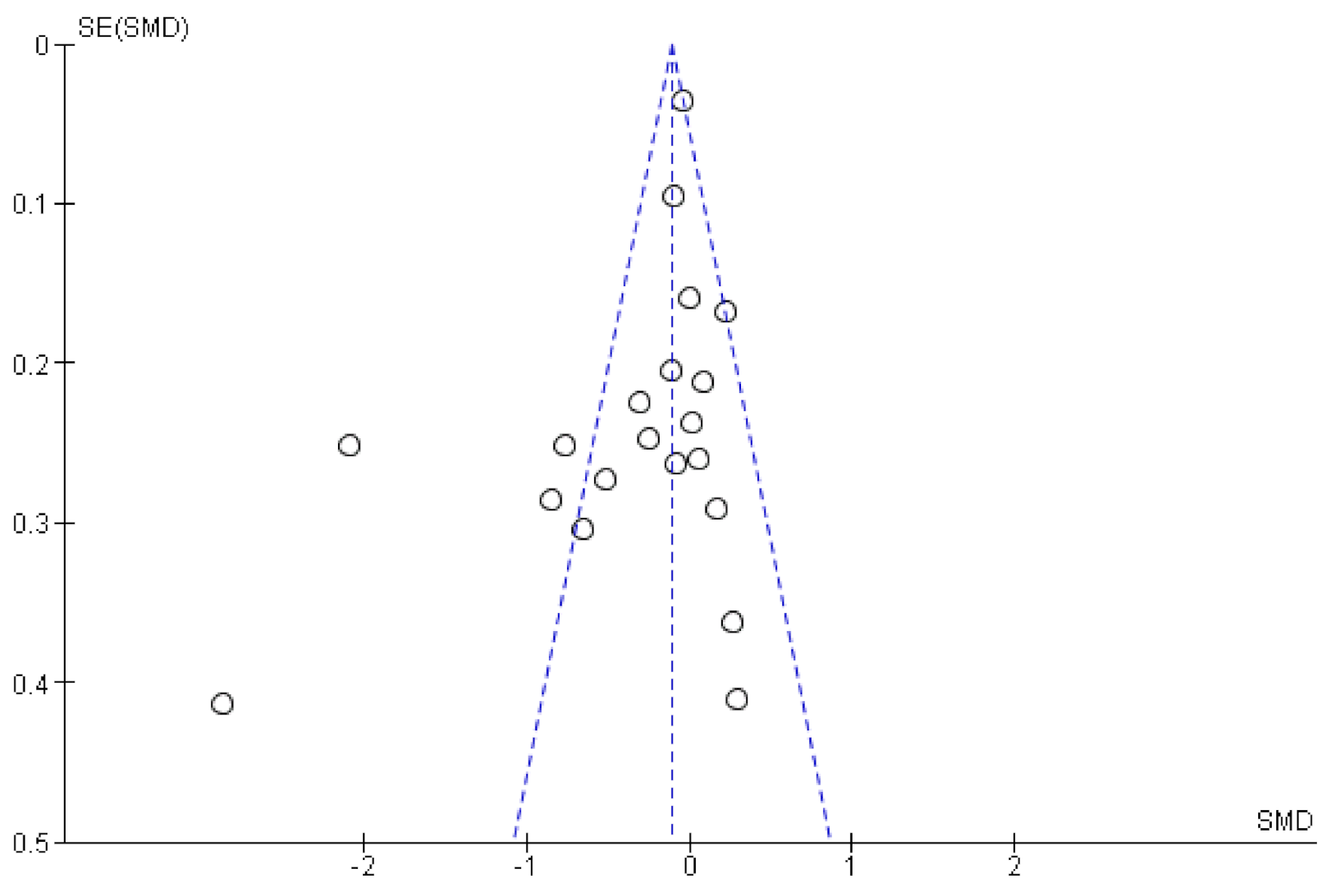
3.5. Sensitivity Analyses
3.6. Descriptive Synthesis of Studies Not Included in the Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 26 February 2020).
- Institute for Quality and Efficiency in Health Care (IQWiG). Depression: How Effective Are Antidepressants? Available online: https://www.ncbi.nlm.nih.gov/books/NBK361016/ (accessed on 28 January 2020).
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Perica, M.M.; Delaš, I. Essential Fatty Acids and Psychiatric Disorders. Nutr. Clin. Pr. 2011, 26, 409–425. [Google Scholar] [CrossRef]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 Fatty Acids and Depression: Scientific Evidence and Biological Mechanisms. Oxidative Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef]
- Song, C.; Shieh, C.-H.; Wu, Y.-S.; Kalueff, A.; Gaikwad, S.; Su, K.-P. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.L.; Cardoos, A.; Walker, R.S.W.; Mischoulon, D. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: A proof-of-concept study. Mol. Psychiatry 2016, 21, 71–79. [Google Scholar] [CrossRef]
- Fernandes, M.F.; Mutch, D.M.; Leri, F. The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants. Nutrients 2017, 9, 298. [Google Scholar] [CrossRef]
- Colin, A.; Reggers, J.; Castronovo, V.; Ansseau, M. Lipids, depression and suicide. L’Encéphale 2003, 29, 49–58. [Google Scholar]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Baghai, T.C.; Varallo-Bedarida, G.; Born, C.; Häfner, S.; Schüle, C.; Eser, D.; Rupprecht, R.; Bondy, B.; Von Schacky, C. Major Depressive Disorder Is Associated with Cardiovascular Risk Factors and Low Omega-3 Index. J. Clin. Psychiatry 2010, 72, 1242–1247. [Google Scholar] [CrossRef]
- McNamara, R.K.; Jandacek, R.; Rider, T.; Tso, P.; Dwivedi, Y.; Pandey, G.N. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J. Affect. Disord. 2010, 126, 303–311. [Google Scholar] [CrossRef]
- Messamore, E.; Almeida, D.M.; Jandacek, R.J.; McNamara, R.K. Polyunsaturated fatty acids and recurrent mood disorders: Phenomenology, mechanisms, and clinical application. Prog. Lipid Res. 2017, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.H.O.; Jimoh, O.F.; Biswas, P.; O’Brien, A.; Hanson, S.; Abdelhamid, A.S.; Fox, C.; Hooper, L. Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: Systematic review and meta-analysis of randomised trials. Br. J. Psychiatry 2021, 218, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, B.; Ryan, T.; Hibbeln, J.R.; Murray, I.T.; Glynn, S.; Ramsden, C.E.; SanGiovanni, J.P.; Davis, J.M. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatry 2016, 209, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.; Hempel, S.; Booth, M.; Ewing, B.; Maher, A.R.; O’Hanlon, C.E.; Sloan, J.; Vaughan, C.A.; Dudley, W.; Shanman, R.M.; et al. Omega-3 Fatty Acids for Major Depressive Disorder: A Systematic Review; RAND Corporation: Santa Monica, CA, USA, 2015. [Google Scholar]
- Bloch, M.H.; Hannestad, J. Omega-3 fatty acids for the treatment of depression: Systematic review and meta-analysis. Mol. Psychiatry 2011, 17, 1272–1282. [Google Scholar] [CrossRef]
- Luo, X.-D.; Feng, J.-S.; Yang, Z.; Huang, Q.-T.; Lin, J.-D.; Yang, B.; Su, K.-P.; Pan, J.-Y. High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: A network meta-analysis. BMC Psychiatry 2020, 20, 24. [Google Scholar] [CrossRef]
- Appleton, K.M.; Sallis, H.M.; Perry, R.; Ness, A.R.; Churchill, R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev. 2015, Cd004692. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Harris, W.S. A Randomized Placebo-Controlled Trial of Omega-3 and Sertraline in Depressed Patients With or at Risk for Coronary Heart Disease. J. Clin. Psychiatry 2019, 80, 80. [Google Scholar] [CrossRef] [PubMed]
- Trebatická, J.; Hradečná, Z.; Surovcová, A.; Katrenčíková, B.; Gushina, I.; Waczulíková, I.; Sušienková, K.; Garaiova, I.; Šuba, J.; Ďuračková, Z. Omega-3 fatty-acids modulate symptoms of depressive disorder, serum levels of omega-3 fatty acids and omega-6/omega-3 ratio in children. A randomized, double-blind and controlled trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef] [PubMed]
- Jahangard, L.; Sadeghi, A.; Ahmadpanah, M.; Holsboer-Trachsler, E.; Bahmani, D.S.; Haghighi, M.; Brand, S. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders—Results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatr. Res. 2018, 107, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Khalili, H.; Abbasian, L.; Arbabi, M.; Ghaeli, P. Effect of Omega-3 Fatty Acids on Depressive Symptoms in HIV-Positive Individuals. Ann. Pharmacother. 2016, 50, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Kuang, L.; Meng, H.; Zhou, X. Omega-3 fatty acids for the treatment of depressive disorders in children and adolescents: A meta-analysis of randomized placebo-controlled trials. Child. Adolesc. Psychiatry Ment. Health 2019, 13, 36. [Google Scholar] [CrossRef]
- Bae, J.-H.; Kim, G. Systematic review and meta-analysis of omega-3-fatty acids in elderly patients with depression. Nutr. Res. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. Available online: https://handbook-5-1.cochrane.org/ (accessed on 9 February 2021).
- Viswanathan, M.; Patnode, C.; Berkman, N.; Bass, E.; Chang, S.; Hartling, L.; Murad, H.; Treadwell, J.; Kane, R. Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions. Methods Guide for Comparative Effectiveness Reviews; AHRQ: Rockville, MD, USA, 2017. [Google Scholar]
- Beck, A.T.; Steer, R.A. Beck Depression Inventory Manual; The Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Hamilton, M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Davey Smith, G. Investigating and dealing with publication and other biases. In Systematic Reviews in Health Care: Meta-Analysis in Context; Egger, M., Smith, G.D., Altman, D.G., Eds.; BMJ Publishing Group: London, UK, 2001; pp. 189–208. [Google Scholar]
- Watanabe, N.; Matsuoka, Y.; Kumachi, M.; Hamazaki, K.; Horikoshi, M.; Furukawa, T.A. Omega-3 fatty acids for a better mental state in working populations—Happy Nurse Project: A 52-week randomized controlled trial. J. Psychiatr. Res. 2018, 102, 72–80. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Haberka, M.; Mizia-Stec, K.; Mizia, M.; Gieszczyk, K.; Chmiel, A.; Sitnik-Warchulska, K.; Gąsior, Z. Effects of n-3 polyunsaturated fatty acids on depressive symptoms, anxiety and emotional state in patients with acute myocardial infarction. Pharmacol. Rep. 2013, 65, 59–68. [Google Scholar] [CrossRef]
- Mazereeuw, G.; Herrmann, N.; Oh, P.I.; Ma, D.W.; Wang, C.T.; Kiss, A.; Lanctôt, K.L. Omega-3 Fatty Acids, Depressive Symptoms, and Cognitive Performance in Patients with Coronary Artery Disease. J. Clin. Psychopharmacol. 2016, 36, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R.C. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Khajehnasiri, F.; Akhondzadeh, S.; Mortazavi, S.B.; Allameh, A.; Sotoudeh, G.; Khavanin, A.; Zamanian, Z. Are Supplementation of Omega-3 and Ascorbic Acid Effective in Reducing Oxidative Stress and Depression among Depressed Shift Workers? Int. J. Vitam. Nutr. Res. 2015, 85, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Pelucchi, C.; La Vecchia, C.; Montorfano, G.; Negroni, M.; Berra, B.; Politi, P.; Rizzo, A.M. Long chain omega 3 polyunsaturated fatty acids supplementation in the treatment of elderly depression: Effects on depressive symptoms, on phospholipids fatty acids profile and on health-related quality of life. J. Nutr. Heal. Aging 2011, 15, 37–44. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Corsetto, P.A.; Montorfano, G.; Opizzi, A.; Faliva, M.; Giacosa, A.; Ricevuti, G.; Pelucchi, C.; Berra, B.; Rondanelli, M. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: Omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr. J. 2012, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Antypa, N.; Smelt, A.H.M.; Strengholt, A.; Van Der Does, A.J.W. Effects of omega-3 fatty acid supplementation on mood and emotional information processing in recovered depressed individuals. J. Psychopharmacol. 2012, 26, 738–743. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Pelucchi, C.; La Vecchia, C.; Montorfano, G.; Negroni, M.; Berra, B.; Politi, P.; Rizzo, A.M. Effect of Omega-3 Fatty Acids Supplementation on Depressive Symptoms and on Health-Related Quality of Life in the Treatment of Elderly Women with Depression: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2010, 29, 55–64. [Google Scholar] [CrossRef]
- Shinto, L.; Marracci, G.; Mohr, D.C.; Bumgarner, L.; Murchison, C.; Senders, A.; Bourdette, D. Omega-3 Fatty Acids for Depression in Multiple Sclerosis: A Randomized Pilot Study. PLoS ONE 2016, 11, e0147195. [Google Scholar] [CrossRef] [PubMed]
- Tajalizadekhoob, Y.; Sharifi, F.; Fakhrzadeh, H.; Mirarefin, M.; Ghaderpanahi, M.; Badamchizade, Z.; Azimipour, S. The effect of low-dose omega 3 fatty acids on the treatment of mild to moderate depression in the elderly: A double-blind, randomized, placebo-controlled study. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Geleijnse, J.M.; Kromhout, D. Effects of n−3 fatty acids on depressive symptoms and dispositional optimism after myocardial infarction. Am. J. Clin. Nutr. 2011, 94, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Tayama, J.; Ogawa, S.; Nakaya, N.; Sone, T.; Hamaguchi, T.; Takeoka, A.; Hamazaki, K.; Okamura, H.; Yajima, J.; Kobayashi, M.; et al. Omega-3 polyunsaturated fatty acids and psychological intervention for workers with mild to moderate depression: A double-blind randomized controlled trial. J. Affect. Disord. 2019, 245, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Kovacs, M. The children’s depression inventory (CDI). Psychopharmacolol. Bull. 1985, 21, 995–998. [Google Scholar]
- Khajehnasiri, F.; Mortazavi, S.B.; Allameh, A.; Akhondzadeh, S. Effect of omega-3 and ascorbic acid on inflammation markers in depressed shift workers in Shahid Tondgoyan Oil Refinery, Iran: A randomized double-blind placebo-controlled study. J. Clin. Biochem. Nutr. 2013, 53, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Freed, R.D.; Alonso, C.M.; Senger, S.; Stadterman, J.; Davison, B.A.; Klein, R.G. A Double-Blind Placebo-Controlled Trial of Omega-3 Fatty Acids as a Monotherapy for Adolescent Depression. J. Clin. Psychiatry 2018, 79, 26. [Google Scholar] [CrossRef]
- Ginty, A.T.; Conklin, S.M. Short-term supplementation of acute long-chain omega-3 polyunsaturated fatty acids may alter depression status and decrease symptomology among young adults with depression: A preliminary randomized and placebo controlled trial. Psychiatry Res. 2015, 229, 485–489. [Google Scholar] [CrossRef]
- Appleton, K.M.; Rogers, P.J.; Ness, A.R. Updated systematic review and meta-analysis of the effects of n−3 long-chain polyunsaturated fatty acids on depressed mood. Am. J. Clin. Nutr. 2010, 91, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Whellan, D.J.; Adams, K.F.; Babyak, M.A.; Boyle, S.H.; Wilson, J.L.; Patel, C.B.; Rogers, J.G.; Harris, W.S.; O’Connor, C.M. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients. JACC Heart Fail. 2018, 6, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Lespérance, F.; Frasure-Smith, N.; St-André, E.; Turecki, G.; Lespérance, P.; Wisniewski, S.R. The Efficacy of Omega-3 Supplementation for Major Depression: A Randomized Controlled Trial. J. Clin. Psychiatry 2010, 72, 1054–1062. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Chang, S.-S.; Yang, H.-T.; Chen, H.-T.; Chien, Y.-C.; Yang, B.; Su, H.; Su, K.-P. Omega-3 polyunsaturated fatty acids in cardiovascular diseases comorbid major depressive disorder—Results from a randomized controlled trial. Brain Behav. Immun. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, A.; Khatami, M.-R.; Dashti-Khavidaki, S.; Razeghi, E.; Noorbala, A.-A.; Hashemi-Nazari, S.-S.; Mansournia, M.-A. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: A randomized, placebo-controlled clinical trial. Eur. J. Clin. Pharmacol. 2014, 70, 655–665. [Google Scholar] [CrossRef]
- Appleton, K.M.; Sallis, H.M.; Perry, R.E.; Ness, A.R.; Churchill, R.C. ω-3 Fatty acids for major depressive disorder in adults: An abridged Cochrane review. BMJ Open 2016, 6, e010172. [Google Scholar] [CrossRef]
- Schefft, C.; Kilarski, L.L.; Bschor, T.; Köhler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef]
- Martins, J.G.; Bentsen, H.; Puri, B.K. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: A critique of Bloch and Hannestad and updated meta-analysis. Mol. Psychiatry 2012, 17, 1144–1149. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Bai, Z.-G.; Bo, A.; Wu, S.-J.; Gai, Q.-Y.; Chi, I. Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: A systematic review and meta-analysis. J. Affect. Disord. 2018, 241, 241–248. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Mischoulon, D.; Freeman, M.P.; Matsuoka, Y.; Hibbeln, J.R.; Belmaker, R.H.; Su, K.-P. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Mol. Psychiatry 2012, 17, 1161–1163. [Google Scholar] [CrossRef]
- Umhau, J.C.; Zhou, W.; Carson, R.E.; Rapoport, S.I.; Polozova, A.; Demar, J.; Hussein, N.; Bhattacharjee, A.K.; Ma, K.; Esposito, G.; et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res. 2009, 50, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.L.; Fehling, K.; Martinson, M.A.; Rapaport, M.H. A Double-Blind, Randomized Controlled Clinical Trial Comparing Eicosapentaenoic Acid Versus Docosahexaenoic Acid for Depression. J. Clin. Psychiatry 2014, 76, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.; Kettle, C.; Hayes, D.; Dennis, C.; Tucci, J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit. Rev. Food Sci. Nutr. 2017, 57, 212–223. [Google Scholar] [CrossRef] [PubMed]
| Lead Author, Publication Date | Country | Sample Size | Duration (Weeks) | Diet/Supplement (per Day) | Mean Age ± SD | Sex (%) | Health Status | Severity of Depression | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | f | m | |||||||
| Andrieu et al., 2017 | France | 1525 | 144 (36 months) | Group 1: capsule: 800 mg DHA 225 mg EPA + multidomain intervention | Group 3: placebo capsule: paraffin oil + multidomain intervention | 75.3 ±4.4 | 64 | 36 | Spontaneous memory complaints or limits in one instrumental activity of daily life or slow gait speed | Nondepressed and mild depression |
| Group 2: capsule: 800 mg DHA 225 mg EPA | Group 4: placebo capsule: paraffin oil | |||||||||
| Antypa et al., 2011 | Netherlands | 71 | 4 | Fish oil capsule: 1740 mg EPA 250 mg DHA | Placebo capsule: olive oil | 24.65 | 81.65 | 18.35 | Mild to moderate | |
| Bot et al., 2010 | Netherlands | 25 | 12 | Capsule: 1000 mg EPA | Placebo capsule: rapeseed oil + medium chain triglycerides | 54.05 | 52 | 48 | Diabetes mellitus 1 or 2 | MDD |
| Carney et al., 2019 | USA | 144 | 10 | Capsule: 2000 mg EPA sertraline (50 mg/day) | Placebo capsule sertraline (50 mg/day) | 59.5 | 38.89 | 61.11 | With or at risk of coronary heart disease | MDD |
| Chang et al., 2019 | China | 59 | 12 | Capsule: 2000 mg EPA 1000 mg DHA | Placebo capsule: 300 mg soybean oil | 61.5 ± 9 | 36 | 64 | CVD | MDD |
| Gabbay et al., 2019 | USA | 48 | 10 | Capsule: starting with 1200 mg, which was increased 600 mg every 2 weeks, up to a maximum of 3600 mg (2400 mg EPA, 1200 mg DHA) | Placebo capsule: 1:1 ratio of corn and soybean oils, consisting mainly n-6-PUFA (50%) and MUFA (25%) | 16.05 ± 2.079 | 58.3 | 41.7 | MDD | |
| Gharekhani et al., 2014 | Iran | 54 | 16 | Capsule: 1080 mg EPA 720 mg DHA | Placebo capsule: paraffin oil | 57 | 44.44 | 55.56 | Hemodialysis patients | Mild, moderate, severe |
| Giltay et al., 2011 | Netherlands | 4116 | 160 | Group 1: margarine spread: 400 mg EPA + DHA 2 mg ALA | Group 4: placebo margarine: oleic acid | 68.725 | 20.8 | 79.2 | Myocardial infarct survivors | Mild, moderate, severe |
| Group 2: margarine spread: 400 mg EPA + DHA | ||||||||||
| Group 3: margarine spread: 2 mg ALA | ||||||||||
| Ginty et al., 2015 | USA | 21 | 3 | Capsule: 1000 mg EPA 400 g DHA | Placebo capsule: corn oil | 20.2 | 78 | 22 | Mild to moderate | |
| Haberka et al., 2013 | Poland | 52 | 4 | Capsule: 46 5 mg EPA 375 mg DHA | Standard therapy, no placebo | 58 | 13.46 | 86.54 | Acute myocardial infarction | Minimal to moderate |
| Jahangard et al., 2018 | Iran | 50 | 12 | Capsule: 1000 mg n3-PUFA sertraline (50–200 mg/day | Placebo capsule sertraline (50–200 mg/day) | 42.46 | 68 | 32 | MDD | |
| Jiang et al., 2018 | USA | 108 | 12 | Group 1: capsule: 2000 mg 2:1 EPA:DHA | Group 3: placebo capsule: corn oil | 57.91 | 53.7 | 46.3 | Chronic heart failure | MDD |
| Group 2: capsule: 2000 mg EPA | ||||||||||
| Khajehnasiri et al., 2012 * | Iran | 136 | 15 | Group 1: softgel: 360 mg EPA 240 mg DHA + capsule: 500 mg vit.C | Group 3: softgel placebo: paraffin oil + capsule: 500 mg vit.C | 30.75 | 0 | 100 | Mild to moderate | |
| Group 2: softgel: 360 mg EPA 240 mg DHA | Group 4: placebo softgel + placebo capsule | |||||||||
| Lespérance et al., 2011 | Canada | 432 | 8 | Capsule: 1050 mg EPA 150 mg DHA | Placebo capsule: sunflower oil 2% fish oil | 46 | 68.5 | 31.50 | Only participants with specific comorbidities excluded | Major depressive episode |
| Mazereeuw et al., 2016 | Canada | 92 | 12 | Capsule: 1200 mg EPA 600 mg DHA 100 mg other n-3 PUFA | Placebo capsule: 1:1 soybean/corn oil blend | 61.7 ± 8.7 | 24 | 76 | Coronary heart disease (in cardiac rehabilitation) | Nondepressed and minor to major depression |
| Mischoulon et al., 2015 | USA | 177 | 8 | Group 1: capsule: 1060 mg EPA 274 mg DHA | Group 3: placebo capsule: 1000 mg soybean oil 50% LA (n-6-PUFA) 8% LA (n-3-PUFA) | 45.8 ± 12.5 | 59.3 | 40.7 | MDD | |
| Group 2: capsule: 450 mg DHA 90 mg EPA | ||||||||||
| Mozaffari-Khosravi et al., 2013 | Iran | 62 | 12 | Group 1: capsule: 1000 mg EPA | Placebo capsule: coconut oil | 35.1 ± 1.2 | 61.3 | 38.7 | Mild to moderate | |
| Group 2: capsule: 1000 mg DHA | ||||||||||
| Ravi et al., 2016 | Iran | 100 | 8 | Capsule: 720 mg EPA 480 mg DHA | Placebo capsule: olive oil | 39.67 | 35 | 65 | HIV positive | Moderate to severe depression |
| Rondanelli et al., 2010 † | Italy | 46 | 8 | Fish oil capsule: 1670 mg EPA 830 g DHA/day | Placebo capsule: paraffin oil | 83.95 | 100 | 0 | Only participants with specific comorbidities excluded | MDD |
| Shinto et al., 2016 | USA | 31 | 12 | Fish oil capsule: 1950 mg EPA 1350 mg DHA | Placebo capsule: soybean oil, 1% fish oil | 51.3 | 18 | 82 | MDD | |
| Sinn et al., 2012 | Australia | 50 | 24 | Group 1: fish oil capsule: 1670 mg EPA 160 mg DHA | Group 3: safflower oil placebo capsule:2200 mg LA (n-6 PUFA) | 74.03 | 32 | 68 | Self-reported memory loss, comorbidities, e.g., diabetes mellitus | Nondepressed and mild depression |
| Group 2: fish oil capsule: 1550 mg DHA 400 mg EPA | ||||||||||
| Tajalizadekhoob et al., 2011 | Iran | 66 | 24 | Fish oil capsule: 180 mg EPA 120 mg DHA | Placebo capsule: coconut oil | 69.685 | 69.70 | 30.30 | Comorbidities, e.g., diabetes mellitus, hypertension, CVD, thyroid dysfunctions | Mild to moderate |
| Tayama et al., 2019 | Japan | 79 | 12 | Capsule: 1064 mg EPA 558 mg DHA pysychoeducation | Placebo capsule: 705 mg rapseed oil 375 mg soybean oil 375 mg olive oil 45 mg fish oil psychoeducation | 40.4 | 47.78 | 52.22 | Only participants with specific comorbidities excluded | Mild to moderate |
| Trebaticka et al., 2020 | Slovakia | 58 | 12 | Fish oil emulsion: 1000 mg EPA 750 mg DHA | Placebo emulsion: sunflower oil with 2467 mg n-6 LA | 15.6 ±1.6 | 79.31 | 20.69 | Only participants with specific comorbidities excluded | Depressive disorder with/without anxiety disorder |
| Watanabe et al., 2018 | Japan | 80 | 13 | Group 1: capsule: 1200 mg EPA 600 mg DHA + stress management program | Group 3: placebo capsule: 47% rapeseed oil 25% soybean oil 25% olive oil 3% fish oil + stressmanagement program | 30.1 ±8.4 | 100 | 0 | Nondepressed or mild depression | |
| Group 2: capsule: 1200 mg EPA 600 mg DHA + psychoeducation | Group 4: placebo capsule: 47% rapeseed oil 25% soybean oil 25% olive oil 3% fish oil + psychoeducation | |||||||||
| Lead Author, Year of Publication | Random Sequence Generation | Allocation Concealment | Selective Reporting | Blinding of Participants/Personnel | Blinding of Outcome Assessment | Incomplete Outcome | Other Bias | Study † Quality |
|---|---|---|---|---|---|---|---|---|
| Andrieu et al., 2017 | + | + | + | + | + | + | + | Good |
| Antypa et al., 2011 | + | + | + | + | + | + | + | Good |
| Bot et al., 2010 | ? | + | + | + | + | ? | + | Fair |
| Carney et al., 2019 | + | + | + | + | + | + | + | Good |
| Chang et al., 2019 | ? | ? | + | ? | ? | + | + | Poor |
| Gabbay et al., 2019 | ? | + | + | + | + | + | + | Good |
| Gharekhani et al., 2014 | + | ? | + | − | ? | + | + | Poor |
| Giltay et al., 2011 | + | + | + | + | + | − | + | Fair |
| Ginty et al., 2015 | + | + | − | + | + | − | + | Poor |
| Haberka et al., 2013 | + | ? | ? | − | + | + | + | Poor |
| Jahangard et al., 2018 | + | + | + | + | ? | + | + | Good |
| Jiang et al., 2018 | + | + | + | + | + | ? | + | Good |
| Khajehnasiri et al., 2013 | + | + | + | + | + | − | + | Fair |
| Lesperance et al., 2010 | + | + | + | + | + | ? | + | Good |
| Mazereeuw et al., 2016 | + | + | + | + | + | + | + | Good |
| Mischoulon et al., 2015 | + | + | + | + | ? | ? | + | Fair |
| Mozaffari-Khosravi et al., 2012 | + | + | + | + | + | − | + | Fair |
| Ravi et al., 2016 | + | + | + | + | + | − | + | Fair |
| Rondanelli et al., 2010 | + | + | + | + | + | + | + | Good |
| Shinto et al., 2016 | + | + | + | + | + | − | + | Fair |
| Sinn et al., 2012 | + | ? | + | + | + | − | + | Poor |
| Tajalizadekhoob et al., 2011 | + | + | + | + | + | − | + | Fair |
| Tayama et al., 2018 | + | + | + | + | + | − | + | Fair |
| Trebatická et al., 2020 | + | + | + | + | + | + | + | Good |
| Watanabe et al., 2018 | + | ? | + | + | + | + | + | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolters, M.; von der Haar, A.; Baalmann, A.-K.; Wellbrock, M.; Heise, T.L.; Rach, S. Effects of n-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1070. https://doi.org/10.3390/nu13041070
Wolters M, von der Haar A, Baalmann A-K, Wellbrock M, Heise TL, Rach S. Effects of n-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(4):1070. https://doi.org/10.3390/nu13041070
Chicago/Turabian StyleWolters, Maike, Annkathrin von der Haar, Ann-Kristin Baalmann, Maike Wellbrock, Thomas L. Heise, and Stefan Rach. 2021. "Effects of n-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-Analysis" Nutrients 13, no. 4: 1070. https://doi.org/10.3390/nu13041070
APA StyleWolters, M., von der Haar, A., Baalmann, A.-K., Wellbrock, M., Heise, T. L., & Rach, S. (2021). Effects of n-3 Polyunsaturated Fatty Acid Supplementation in the Prevention and Treatment of Depressive Disorders—A Systematic Review and Meta-Analysis. Nutrients, 13(4), 1070. https://doi.org/10.3390/nu13041070






