Ghrelin Receptors Enhance Fat Taste Responsiveness in Female Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and High-Fat-Diet Feeding
2.2. Immunohistochemistry
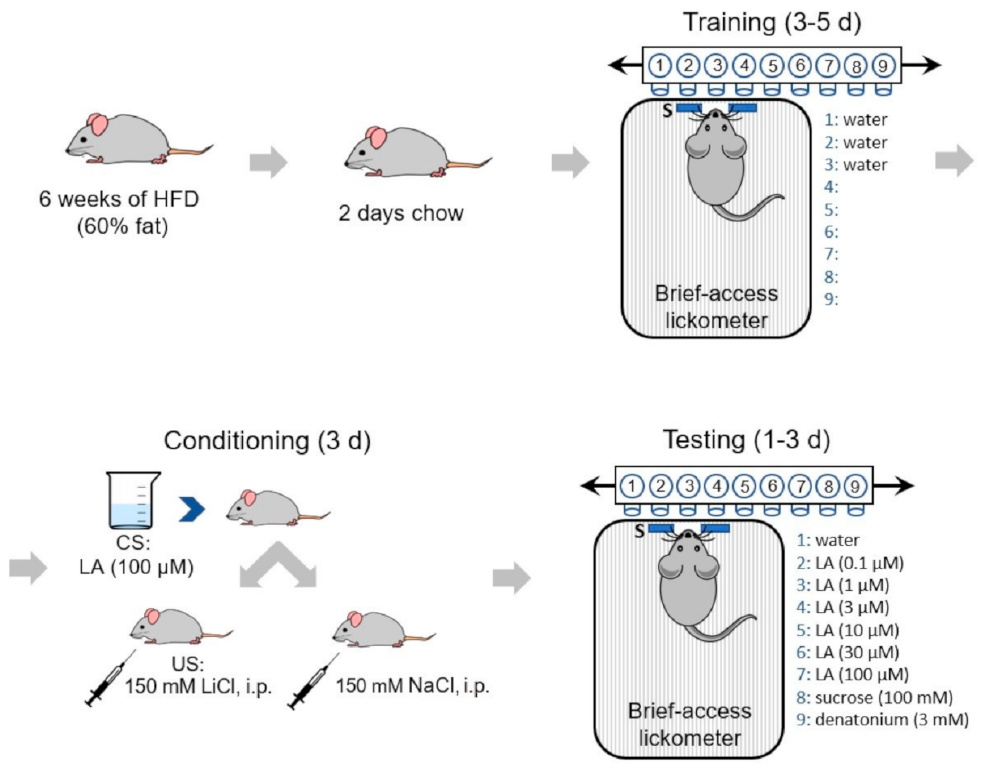
2.3. Conditioned Taste Aversion (CTA) Assay
2.4. Stimuli
2.5. Statistics
3. Results
3.1. GHS-R Is Expressed Predominantly in Type II Taste Cells
3.2. Ghsr−/− Males and Females Express Divergent Metabolic Phenotypes
3.3. Female Ghsr−/− Mice Show Reduced Avoidance to Linoleic Acid in CTA Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albarran-Zeckler, R.G.; Sun, Y.; Smith, R.G. Physiological roles revealed by ghrelin and ghrelin receptor deficient mice. Peptides 2011, 32, 2229–2235. [Google Scholar] [CrossRef] [Green Version]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Drazen, D.L.; Vahl, T.P.; D’Alessio, D.A.; Seeley, R.J.; Woods, S.C. Effects of a fixed meal pattern on ghrelin secretion: Evidence for a learned response independent of nutrient status. Endocrinology 2006, 147, 23–30. [Google Scholar] [CrossRef]
- LeSauter, J.; Hoque, N.; Weintraub, M.; Pfaff, D.W.; Silver, R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc. Natl. Acad. Sci. USA 2009, 106, 13582–13587. [Google Scholar] [CrossRef] [Green Version]
- Fu, O.; Iwai, Y.; Narukawa, M.; Ishikawa, A.W.; Ishii, K.K.; Murata, K.; Yoshimura, Y.; Touhara, K.; Misaka, T.; Minokoshi, Y.; et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun. 2019, 10, 4560. [Google Scholar] [CrossRef] [PubMed]
- Zverev, Y.P. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 2004, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Al Massadi, O.; Lopez, M.; Tschop, M.; Dieguez, C.; Nogueiras, R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017, 40, 167–180. [Google Scholar] [CrossRef]
- Zigman, J.M.; Nakano, Y.; Coppari, R.; Balthasar, N.; Marcus, J.N.; Lee, C.E.; Jones, J.E.; Deysher, A.E.; Waxman, A.R.; White, R.D.; et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Investig. 2005, 115, 3564–3572. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Butte, N.F.; Garcia, J.M.; Smith, R.G. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 2008, 149, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef] [Green Version]
- Groschl, M.; Topf, H.G.; Bohlender, J.; Zenk, J.; Klussmann, S.; Dotsch, J.; Rascher, W.; Rauh, M. Identification of ghrelin in human saliva: Production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 2005, 51, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Cong, W.N.; Daimon, C.M.; Wang, R.; Tschop, M.H.; Sevigny, J.; Martin, B.; Maudsley, S. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS ONE 2013, 8, e76553. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sevigny, J.; Tschop, M.H.; Maudsley, S.; et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE 2010, 5, e12729. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M.; Ganot, D.; Hamosh, P. Rat lingual lipase. Characteristics of enzyme activity. J Biol Chem 1979, 254, 12121–12125. [Google Scholar] [CrossRef]
- Kawai, T.; Fushiki, T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R447–R454. [Google Scholar] [CrossRef] [Green Version]
- Chow, C.K. Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mattes, R.D. Oral exposure to butter, but not fat replacers elevates postprandial triacylglycerol concentration in humans. J. Nutr. 2001, 131, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Is there a fatty acid taste? Annu. Rev. Nutr. 2009, 29, 305–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Cartoni, C.; Yasumatsu, K.; Ohkuri, T.; Shigemura, N.; Yoshida, R.; Godinot, N.; le Coutre, J.; Ninomiya, Y.; Damak, S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 2010, 30, 8376–8382. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, T.A.; Fontenot, D.T.; Liu, L.; Zhang, H.; Monroe, W.T. Fatty acid modulation of K+ channels in taste receptor cells: Gustatory cues for dietary fat. Am. J. Physiol. 1997, 272, C1203–C1210. [Google Scholar] [CrossRef]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Shah, B.P.; Hansen, D.R.; Park-York, M.; Gilbertson, T.A. Activation of oral trigeminal neurons by fatty acids is dependent upon intracellular calcium. Pflugers Arch. 2012, 464, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Shah, B.P.; Croasdell, S.; Gilbertson, T.A. Transient receptor potential channel type M5 is essential for fat taste. J. Neurosci. 2011, 31, 8634–8642. [Google Scholar] [CrossRef] [Green Version]
- Stratford, J.M.; Curtis, K.S.; Contreras, R.J. Chorda tympani nerve transection alters linoleic acid taste discrimination by male and female rats. Physiol. Behav. 2006, 89, 311–319. [Google Scholar] [CrossRef]
- Lee, J.H.; Lin, L.; Xu, P.; Saito, K.; Wei, Q.; Meadows, A.G.; Bongmba, O.Y.; Pradhan, G.; Zheng, H.; Xu, Y.; et al. Neuronal Deletion of Ghrelin Receptor Almost Completely Prevents Diet-Induced Obesity. Diabetes 2016, 65, 2169–2178. [Google Scholar] [CrossRef] [Green Version]
- Janssen, S.; Laermans, J.; Verhulst, P.J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Schele, E.; Bake, T.; Rabasa, C.; Dickson, S.L. Centrally Administered Ghrelin Acutely Influences Food Choice in Rodents. PLoS ONE 2016, 11, e0149456. [Google Scholar] [CrossRef] [Green Version]
- Disse, E.; Bussier, A.L.; Veyrat-Durebex, C.; Deblon, N.; Pfluger, P.T.; Tschop, M.H.; Laville, M.; Rohner-Jeanrenaud, F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol. Behav. 2010, 101, 277–281. [Google Scholar] [CrossRef]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36- and GPR120-mediated Ca(2)(+) signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014, 146, 995–1005. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhao, X.; Feng, J.; Liou, A.P.; Anthony, S.; Pechhold, S.; Sun, Y.; Lu, H.; Wank, S. Postprandial inhibition of gastric ghrelin secretion by long-chain fatty acid through GPR120 in isolated gastric ghrelin cells and mice. Am. J. Physiol. Gastrointest Liver Physiol. 2012, 303, G367–G376. [Google Scholar] [CrossRef] [Green Version]
- Engelstoft, M.S.; Park, W.M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nohr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Dahir, N.S.; Calder, A.N.; McKinley, B.J.; Liu, Y.; Gilbertson, T.A. Sex Differences in Fat Taste Responsiveness are Modulated by Estradiol. Am. J. Physiol. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Brown, L.M.; Zigman, J.M.; Kemp, C.J.; Strader, A.D.; Benoit, S.C.; Woods, S.C.; Mangiaracina, M.; Geary, N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 2007, 56, 1051–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratford, J.M.; Curtis, K.S.; Contreras, R.J. Linoleic acid increases chorda tympani nerve responses to and behavioral preferences for monosodium glutamate by male and female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R764–R772. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.M.; Nuessle, T.M.; Garneau, N.L.; Smutzer, G.; Mattes, R.D. No Difference in Perceived Intensity of Linoleic Acid in the Oral Cavity between Obese and Nonobese Individuals. Chem. Senses 2015, 40, 557–563. [Google Scholar] [CrossRef]
- Pittman, D.W.; Smith, K.R.; Crawley, M.E.; Corbin, C.H.; Hansen, D.R.; Watson, K.J.; Gilbertson, T.A. Orosensory detection of fatty acids by obesity-prone and obesity-resistant rats: Strain and sex differences. Chem. Senses 2008, 33, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Ruiz, N.R.; Lopez-Diaz, J.A.; Wall-Medrano, A.; Jimenez-Castro, J.A.; Angulo, O. Oral fat perception is related with body mass index, preference and consumption of high-fat foods. Physiol. Behav. 2014, 129, 36–42. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Frazao, R.; Dungan Lemko, H.M.; da Silva, R.P.; Ratra, D.V.; Lee, C.E.; Williams, K.W.; Zigman, J.M.; Elias, C.F. Estradiol modulates Kiss1 neuronal response to ghrelin. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E606–E614. [Google Scholar] [CrossRef] [Green Version]





| PLCß2-GFP, n | GHS-R(+), n | Co-Expressing, n (%) | |
| Circumvallate | 101 | 97 | 69 (71.1) |
| Fungiform | 12 | 8 | 8 (100) |
| GAD67-GFP, n | GHS-R(+), n | Co-Expressing, n (%) | |
| Circumvallate | 114 | 103 | 3 (2.9) |
| Fungiform | 9 | 24 | 1 (4.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calder, A.N.; Yu, T.; Dahir, N.S.; Sun, Y.; Gilbertson, T.A. Ghrelin Receptors Enhance Fat Taste Responsiveness in Female Mice. Nutrients 2021, 13, 1045. https://doi.org/10.3390/nu13041045
Calder AN, Yu T, Dahir NS, Sun Y, Gilbertson TA. Ghrelin Receptors Enhance Fat Taste Responsiveness in Female Mice. Nutrients. 2021; 13(4):1045. https://doi.org/10.3390/nu13041045
Chicago/Turabian StyleCalder, Ashley N., Tian Yu, Naima S. Dahir, Yuxiang Sun, and Timothy A. Gilbertson. 2021. "Ghrelin Receptors Enhance Fat Taste Responsiveness in Female Mice" Nutrients 13, no. 4: 1045. https://doi.org/10.3390/nu13041045
APA StyleCalder, A. N., Yu, T., Dahir, N. S., Sun, Y., & Gilbertson, T. A. (2021). Ghrelin Receptors Enhance Fat Taste Responsiveness in Female Mice. Nutrients, 13(4), 1045. https://doi.org/10.3390/nu13041045







