The Effects of a Low Sodium Meal Plan on Blood Pressure in Older Adults: The SOTRUE Randomized Feasibility Trial
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Intervention
2.3. Primary Outcome: Seated Systolic Blood Pressure
2.4. Secondary Outcomes
2.5. Safety, Symptoms, Compliance, and Palatability
2.6. Other Covariates
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
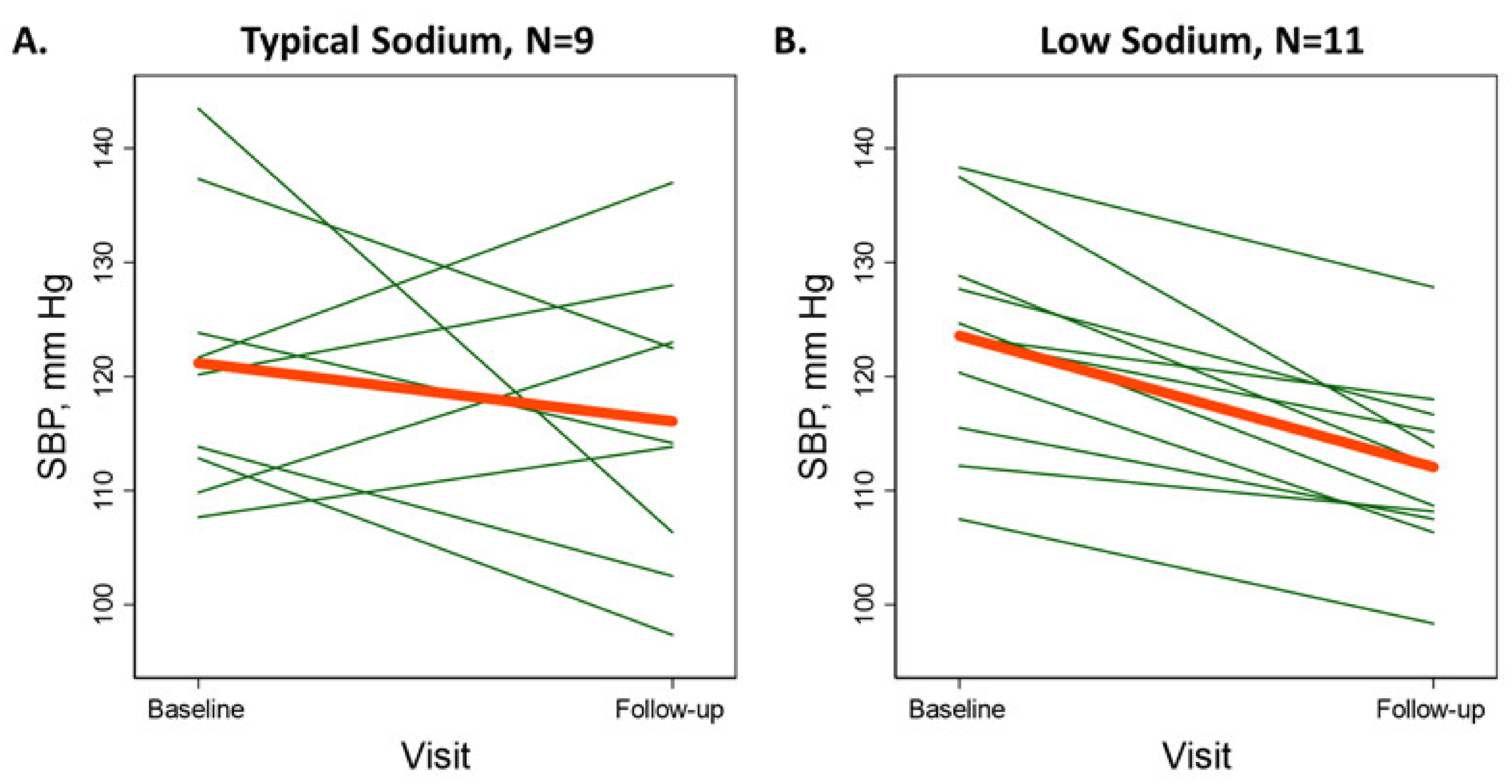
3.2. Primary Outcome: Seated Systolic Blood Pressure
3.3. Secondary Outcomes
3.4. Adverse Events and Symptoms
3.5. Correlation between BP Changes and Urinary Sodium and Potassium Excretion
3.6. Pre-stated Subgroups and Sensitivity Analyses
3.7. Adherence, Palatability, and Blinding
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CI | confidence interval |
| DBP | diastolic blood pressure |
| SBP | systolic blood pressure |
| OH | orthostatic hypotension |
| TUG | timed-up-and-go test |
References
- Muntner, P.; Carey, R.M.; Gidding, S.; Jones, D.W.; Taler, S.J.; Wright, J.T.; Whelton, P.K. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J. Am. Coll. Cardiol. 2018, 71, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B. Coronary heart disease risk factors in the elderly. Am. J. Geriatr. Cardiol. 2002, 11, 101–107. [Google Scholar] [CrossRef]
- Gangavati, A.; Hajjar, I.; Quach, L.; Jones, R.N.; Kiely, D.K.; Gagnon, P.; Lipsitz, L.A. Hypertension, Orthostatic Hypotension, and the Risk of Falls in a Community-Dwelling Elderly Population: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J. Am. Geriatr. Soc. 2011, 59, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Appel, L.J.; Sacco, R.L.; Anderson, C.A.; Antman, E.M.; Campbell, N.; Dunbar, S.B.; Frohlich, E.D.; Hall, J.E.; Jessup, M.; et al. Sodium, Blood Pressure, and Cardiovascular Disease. Circulation 2012, 126, 2880–2889. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Mugavero, K.; Bowman, B.A.; Frieden, T.R. Dietary Sodium and Cardiovascular Disease Risk—Measurement Matters. N. Engl. J. Med. 2016, 375, 580–586. [Google Scholar] [CrossRef]
- Johns, D.M.; Bayer, R.; Galea, S.; Juraschek, S.P.; Mukamal, K.J.; Sahni, S. Sodium-Intake Reduction and the Food Industry. N. Engl. J. Med. 2019, 381, 1788. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Rosenberg, R.E.; Simonovich, S.D.; Belza, B. Food Access Patterns and Barriers among Midlife and Older Adults with Mobility Disabilities. J. Aging Res. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Center on Budget and Policy Priorities. United States Federal Rental Assistance Fact Sheet. Published online December 2019. Available online: https://www.cbpp.org/research/housing/federal-rental-assistance-fact-sheets#US (accessed on 14 March 2021).
- HUD Handbook 4350.3: Occupancy Requirements of Subsidized Multifamily Housing Programs. Published Online November 2013. Available online: https://www.hud.gov/program_offices/administration/hudclips/handbooks/hsgh/4350.3 (accessed on 2 January 2020).
- Losby, J.L.; Patel, D.; Schuldt, J.; Hunt, G.S.; Stracuzzi, J.C.; Johnston, Y. Sodium-Reduction Strategies for Meals Prepared for Older Adults. J. Public Health Manag. Pr. 2014, 20, S23–S30. [Google Scholar] [CrossRef]
- Kwok, T.; Liddle, J.; Hastie, I.R. Postural hypotension and falls. Postgrad Med. J. 1995, 71, 278–280. [Google Scholar] [CrossRef][Green Version]
- NHANES Questionnaires, Datasets, and Related Documentation 1999–2004. Published 2004; 1999. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2003 (accessed on 8 March 2020).
- Hu, J.-R.; Sahni, S.; Mukamal, K.J.; Millar, C.L.; Wu, Y.; Appel, L.J.; Juraschek, S.P. Dietary Sodium Intake and Sodium Density in the United States: Estimates From NHANES 2005–2006 and 2015–2016. Am. J. Hypertens. 2020, 33, 825–830. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of Predictive Equations for Resting Metabolic Rate in Healthy Nonobese and Obese Adults: A Systematic Review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire: Validity Evidence Supporting its Use for Classifying Healthy Adults into Active and Insufficiently Active Categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Altman, D.G. Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- White, H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica 1980, 48, 817. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Ishak, A.; Mukamal, K.J.; Cohen, M.L.; Beach, J.L. Impact of Clinic-Based Blood Pressure Approaches on Blood Pressure Measurement. Am. J. Hypertens. 2020, 33, 26–30. [Google Scholar] [CrossRef]
- Moore, C.G.; Carter, R.E.; Nietert, P.J.; Stewart, P.W. Recommendations for Planning Pilot Studies in Clinical and Translational Research. Clin. Transl. Sci. 2011, 4, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Appel, L.J.; Espeland, M.A.; Applegate, W.B.; Ettinger, W.H.; Kostis, J.B.; Kumanyika, S.; Lacy, C.R.; Johnson, K.C.; Folmar, S.; et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: A randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998, 279, 839–846. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Sodium intake among adults—United States, 2005–2006. MMWR Morb. Mortal. Wkly Rep. 2010, 59, 746–749. [Google Scholar]
- Juraschek, S.P.; Miller, E.R.; Weaver, C.M.; Appel, L.J. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J. Am. Coll. Cardiol. 2017, 70, 2841–2848. [Google Scholar] [CrossRef]
- Babcock, M.C.; Robinson, A.T.; Migdal, K.U.; Watso, J.C.; Wenner, M.M.; Stocker, S.D.; Farquhar, W.B. Reducing Dietary Sodium to 1000 mg per Day Reduces Neurovascular Transduction Without Stimulating Sympathetic Outflow. Hypertension 2019, 73, 587–593. [Google Scholar] [CrossRef]
- Matthews, E.L.; Brian, M.S.; Edwards, D.G.; Stocker, S.D.; Wenner, M.M.; Farquhar, W.B. Blood pressure responses to dietary sodium: Association with autonomic cardiovascular function in normotensive adults. Auton. Neurosci. 2017, 208, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.W.; Appel, L.J.; Mueller, N.T.; Tang, O.; Miller, E.R.; Juraschek, S.P. Effects of sodium intake on postural lightheadedness: Results from the DASH-sodium trial. J. Clin. Hypertens. 2019, 21, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Illigens, B.M.; Lapusca, R.; Campagnolo, M.; Abuzinadah, A.R.; Bonyhay, I.; Sinn, D.-I.; Miglis, M.; White, J.; Gibbons, C.H. Symptom Recognition Is Impaired in Patients With Orthostatic Hypotension. Hypertension 2020, 75, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Woodward, M.; Sacks, F.M.; Carey, V.J.; Miller, E.R.; Appel, L.J. Time Course of Change in Blood Pressure From Sodium Reduction and the DASH Diet. Hypertension 2017, 70, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.A.; Beasley, J.M.; Appel, L.J.; Guenther, P.M.; McFadden, M.; Greene, T.; Tooze, J.A. Relationship of Sodium Intake and Blood Pressure Varies With Energy Intake: Secondary Analysis of the DASH (Dietary Approaches to Stop Hypertension)-Sodium Trial. Hypertension 2018, 71, 858–865. [Google Scholar] [CrossRef]
- Jones, D.W.; Luft, F.C.; Whelton, P.K.; Alderman, M.H.; Hall, J.E.; Peterson, E.D.; Califf, R.M.; McCarron, D.A. Can We End the Salt Wars With a Randomized Clinical Trial in a Controlled Environment? Hypertension 2018, 72, 10–11. [Google Scholar] [CrossRef]
| Typical Sodium | Low Sodium | |||
|---|---|---|---|---|
| Targeted | Prepared | Targeted | Prepared | |
| Density target, mg/kcal | >2.0 | * | <0.95 | * |
| High Calorie | ||||
| Energy, kcal/d | 2250 | 2262 | 2250 | 2167 |
| Carbohydrates, % | 45–55 | 52 | 45–55 | 50 |
| Sodium, mg/d | >4500 | 4395 | <2100 | 2096 |
| Potassium, mg/d | ~4500 | 3319 | ~4500 | 3233 |
| Medium Calorie | ||||
| Energy, kcal/d | 2000 | 2091 | 2000 | 1977 |
| Carbohydrates, % | 45–55 | 52 | 45–55 | 49 |
| Sodium, mg/d | >4000 | 4071 | <1900 | 1920 |
| Potassium, mg/d | ~4000 | 3223 | ~4000 | 3047 |
| Low Calorie | ||||
| Energy, kcal/d | 1750 | 1861 | 1750 | 1842 |
| Carbohydrates, % | 45–55 | 52 | 45–55 | 49 |
| Sodium, mg/d | >3500 | 3589 | <1650 | 1643 |
| Potassium, mg/d | ~3500 | 2953 | ~3500 | 2966 |
| Typical Sodium, N = 9 | Low Sodium, N = 11 | |
|---|---|---|
| Mean (SD) or % | Mean (SD) or % | |
| Age, yr | 79.6 (9.4) | 76.9 (6.1) |
| Female, % | 100 | 91 |
| White, % | 100 | 91 |
| Systolic blood pressure, mmHg | 121.1 (12.2) | 123.5 (9.7) |
| Diastolic blood pressure, mmHg | 62.2 (7.5) | 65.3 (11.1) |
| Hypertension, % | 56 | 55 |
| Hypertension medication use, % | 56 | 64 |
| Self-reported orthostatic hypotension, % | 33 | 9 |
| Measured orthostatic hypotension, % | 22 | 0 |
| Falls fracture/emergency room, % | 11 | 46 |
| Falls & hospitalization | 11 | 9 |
| Months since last fall (among fallers) | 28.0 (23.0) | 28.7 (34.2) |
| How often (1 never, 5 every time) | ||
| Concerned about falling | 1.7 (0.9) | 1.5 (1.2) |
| Brace yourself after standing to avoid falling | 2.7 (1.8) | 1.4 (1.2) |
| Pause before taking a step forward after standing | 2.4 (1.9) | 1.5 (1.2) |
| Take time in process of standing to avoid falling | 2.1 (1.5) | 1.5 (1.2) |
| Body mass index, kg/m2 | 31.9 (7.9) | 32.3 (7.2) |
| Obesity, % | 67 | 64 |
| Diabetes-related condition *, % | 33 | 27 |
| Medications for pre/diabetes, % | 33 | 18 |
| Cardiovascular disease, % | 44 | 9 |
| Hyperlipidemia, % | 56 | 64 |
| Gastrointestinal conditions (GERD, h. pylori, diverticulitis, colitis, hiatal hernia), % | 11 | 36 |
| History of cancer, % | 22 | 9 |
| Montreal Cognitive Assessment (MOCA) score | 23.6 (1.9) | 24.3 (3.3) |
| Physical activity score (scale 0–42) ** | 22.8 (13.7) | 15.9 (15.1) |
| Timed-up-and-go, sec | 13.4 (3.9) | 14.0 (6.2) |
| Current alcohol use, % | 44 | 46 |
| Estimated calorie intake, kcal/d | 1785.1 (311.4) | 1849.0 (243.6) |
| Typical Sodium, N = 9 | Low Sodium, N = 11 | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Difference | Baseline | Follow-up | Difference | |||
| Mean (SD) | Mean (SD) | Mean (95% CI) | p | Mean (SD) | Mean (SD) | Mean (95% CI) | p | |
| Seated SBP, mmHg | 121.1 (12.2) | 116.1 (13.0) | −5.0 (−18.1, 8.1) | 0.41 | 123.5 (9.7) | 112.1 (7.6) | −11.5 (−15.2, −7.7) | <0.001 ** |
| Seated DBP, mmHg | 62.2 (7.5) | 60.4 (8.0) | −1.8 (−7.4, 3.9) | 0.50 | 65.3 (11.1) | 60.3 (9.6) | −5.0 (−8.4, −1.6) | 0.009 ** |
| Supine SBP, mmHg | 130.7 (13.6) | 127.8 (20.2) | −2.9 (−19.4, 13.6) | 0.70 | 127.0 (10.1) | 120.2 (9.2) | −6.8 (−15.5, 1.9) | 0.12 ** |
| Supine DBP, mmHg | 69.4 (7.3) | 67.3 (12.2) | −2.1 (−10.6, 6.4) | 0.59 | 70.3 (8.6) | 67.8 (9.8) | −2.5 (−7.8, 2.9) | 0.34 |
| Standing SBP, mmHg | 122.9 (14.3) | 110.2 (23.5) | −12.7 (−26.7, 1.3) | 0.08** | 125.2 (14.0) | 110.3 (12.8) | −14.9 (−23.0, −6.8) | 0.002 ** |
| Standing DBP, mmHg | 63.8 (10.3) | 61.6 (16.0) | −2.2 (−11.0, 6.5) | 0.58 | 69.6 (11.1) | 62.8 (9.0) | −6.8 (−12.0, −1.7) | 0.016 ** |
| Timed-Up-and-Go, sec | 13.4 (3.9) | 13.8 (4.9) | 0.3 (−1.3, 1.9) | 0.67 | 14.0 (6.2) | 13.8 (5.6) | −0.2 (−1.1, 0.8) | 0.70 |
| Orthostatic hypotension, % | 22.2 | 55.6 | 33.3 (−21.3, 88.0) | 0.38 | 0.0 | 27.3 | 27.3 (−8.1, 62.7) | 0.25 ** |
| Orthostatic symptoms, % | 33.3 | 11.1 | −22.2 (−60.5, 16.1) | 0.50 | 27.3 | 27.3 | 0.0 (−34.3, 34.3) | 1.00 |
| Body mass index, kg/m2 | 31.9 (7.9) | 32.1 (7.9) | 0.2 (−0.2, 0.5) | 0.29 | 32.3 (7.2) | 32.3 (7.2) | 0.0 (−0.2, 0.3) | 0.79 |
| Baseline | Follow-Up | %-Difference | Baseline | Follow-Up | %-Difference | |||
| Mean (SE) * | Mean (SE) * | Mean (95% CI) | p | Mean (SE) * | Mean (SE) * | Mean (95% CI) | p | |
| Urinary sodium | 53.0 (0.7) | 54.3 (0.5) | 2.4 (−21.3, 33.3) | 0.84 | 66.9 (0.5) | 46.4 (0.6) | −30.6 (−43.7, −14.4) | 0.003 ** |
| Urinary potassium | 66.2 (0.3) | 64.7 (0.4) | −2.3 (−25.8, 28.5) | 0.85 | 56.8 (0.4) | 62.1 (0.4) | 9.4 (−17.7, 45.5) | 0.50 |
| Urinary creatinine | 107.8 (0.5) | 96.2 (0.5) | −10.8 (−40.9, 34.8) | 0.55 | 101.4 (0.6) | 101.6 (0.6) | 0.1 (−14.4, 17.1) | 0.99 |
| Urinary sodium-creatinine | 0.5 (0.8) | 0.6 (0.8) | 14.8 (−29.2, 86.1) | 0.54 | 0.7 (0.6) | 0.5 (0.7) | −30.7 (−47.9, −7.8) | 0.018 ** |
| Urinary potassium-creatinine | 0.6 (0.4) | 0.7 (0.4) | 9.5 (−27.3, 64.9) | 0.63 | 0.6 (0.6) | 0.6 (0.5) | 9.3 (−10.4, 33.2) | 0.35 |
| Mean (95% CI) | p | |
|---|---|---|
| Seated systolic blood pressure, mmHg | −4.78 (−14.41, 4.85) | 0.31 |
| Seated diastolic blood pressure, mmHg | −2.35 (−7.97, 3.28) | 0.39 |
| Supine systolic blood pressure, mmHg | −6.82 (−21.54, 7.91) | 0.34 |
| Supine diastolic blood pressure, mmHg | −0.10 (−9.24, 9.04) | 0.98 |
| Standing systolic blood pressure, mmHg | −1.68 (−16.33,12.96) | 0.81 |
| Standing diastolic blood pressure, mmHg | −3.33 (−12.87, 6.20) | 0.47 |
| Timed-Up-and-Go, sec | −0.47 (−2.20, 1.26) | 0.58 |
| Orthostatic hypotension, OR | 0.28 (0.04, 2.08) | 0.21 * |
| Orthostatic symptoms, OR | 5.87 (0.28,123.01) | 0.25 * |
| Body mass index, kg/m2 | −0.13 (−0.51, 0.25) | 0.48 |
| %-Difference (95% CI) | p | |
| Urinary sodium | −29.7 (−47.6, −5.6) | 0.02 * |
| Urinary potassium | 2.3 (−26.3, 41.9) | 0.89 |
| Urinary creatinine | 10.8 (−22.2, 58.0) | 0.57 |
| Urinary sodium-creatinine | −36.0 (−60.3, 3.4) | 0.07 * |
| Urinary potassium-creatinine | −4.4 (−31.2, 32.9) | 0.79 |
| All primary and secondary analyses were adjusted for baseline values | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juraschek, S.P.; Millar, C.L.; Foley, A.; Shtivelman, M.; Cohen, A.; McNally, V.; Crevatis, R.; Post, S.M.; Mukamal, K.J.; Lipsitz, L.A.; et al. The Effects of a Low Sodium Meal Plan on Blood Pressure in Older Adults: The SOTRUE Randomized Feasibility Trial. Nutrients 2021, 13, 964. https://doi.org/10.3390/nu13030964
Juraschek SP, Millar CL, Foley A, Shtivelman M, Cohen A, McNally V, Crevatis R, Post SM, Mukamal KJ, Lipsitz LA, et al. The Effects of a Low Sodium Meal Plan on Blood Pressure in Older Adults: The SOTRUE Randomized Feasibility Trial. Nutrients. 2021; 13(3):964. https://doi.org/10.3390/nu13030964
Chicago/Turabian StyleJuraschek, Stephen P., Courtney L. Millar, Abby Foley, Misha Shtivelman, Alegria Cohen, Virginia McNally, Robert Crevatis, Stephen M. Post, Kenneth J. Mukamal, Lewis A. Lipsitz, and et al. 2021. "The Effects of a Low Sodium Meal Plan on Blood Pressure in Older Adults: The SOTRUE Randomized Feasibility Trial" Nutrients 13, no. 3: 964. https://doi.org/10.3390/nu13030964
APA StyleJuraschek, S. P., Millar, C. L., Foley, A., Shtivelman, M., Cohen, A., McNally, V., Crevatis, R., Post, S. M., Mukamal, K. J., Lipsitz, L. A., Cluett, J. L., Davis, R. B., & Sahni, S. (2021). The Effects of a Low Sodium Meal Plan on Blood Pressure in Older Adults: The SOTRUE Randomized Feasibility Trial. Nutrients, 13(3), 964. https://doi.org/10.3390/nu13030964






