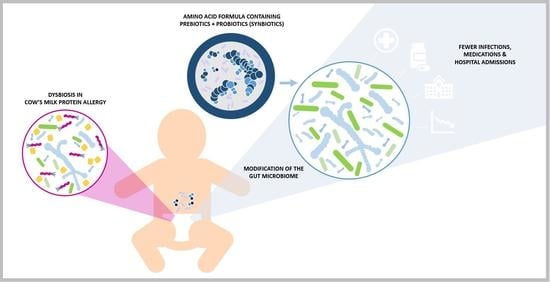
Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Selection of Studies for the Systematic Literature Review
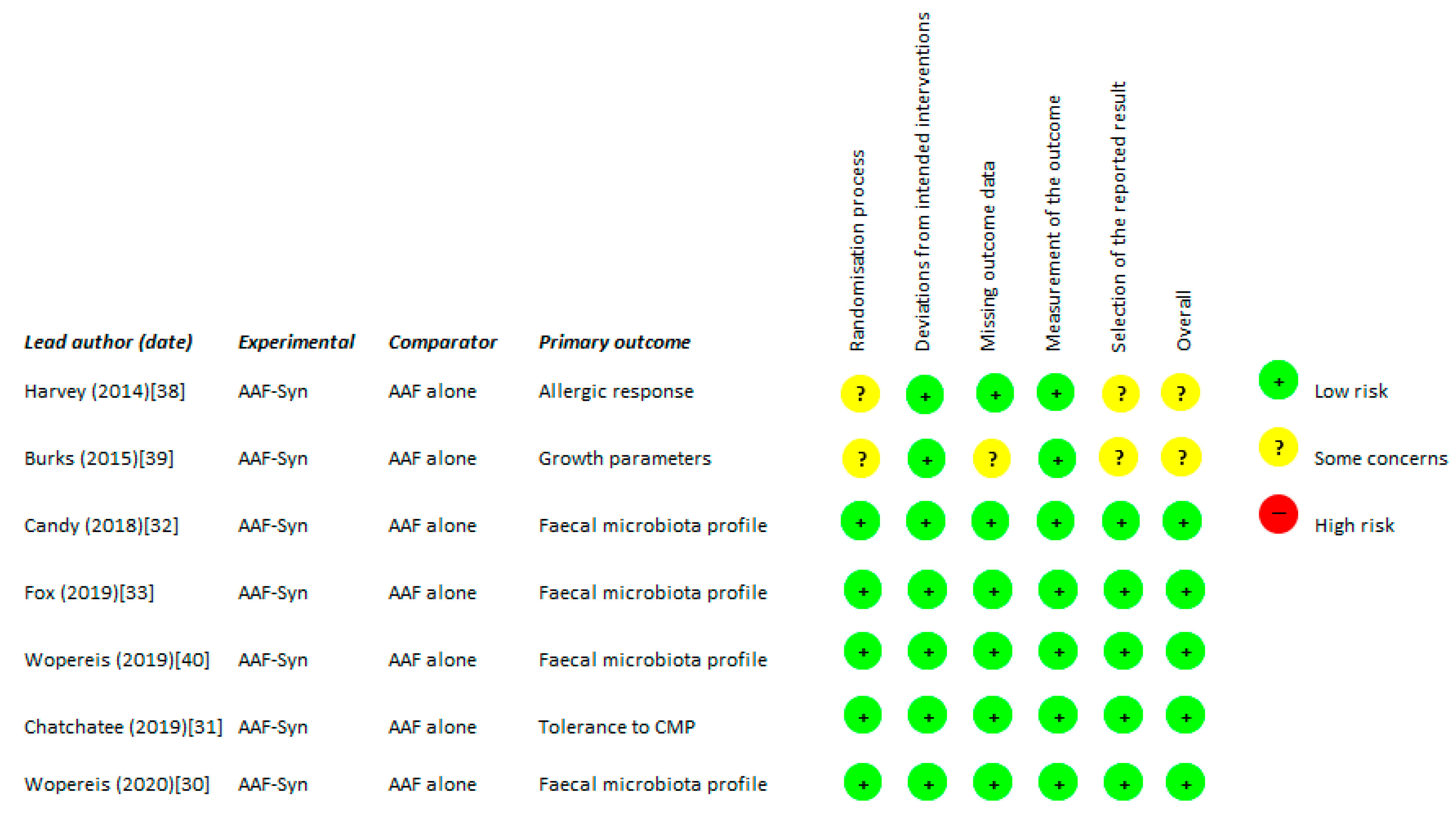
2.2. Quality Assessment
2.3. Data Extraction and Outcome Measures
2.4. Statistical Methods
2.5. Simple Cost Analysis
3. Results
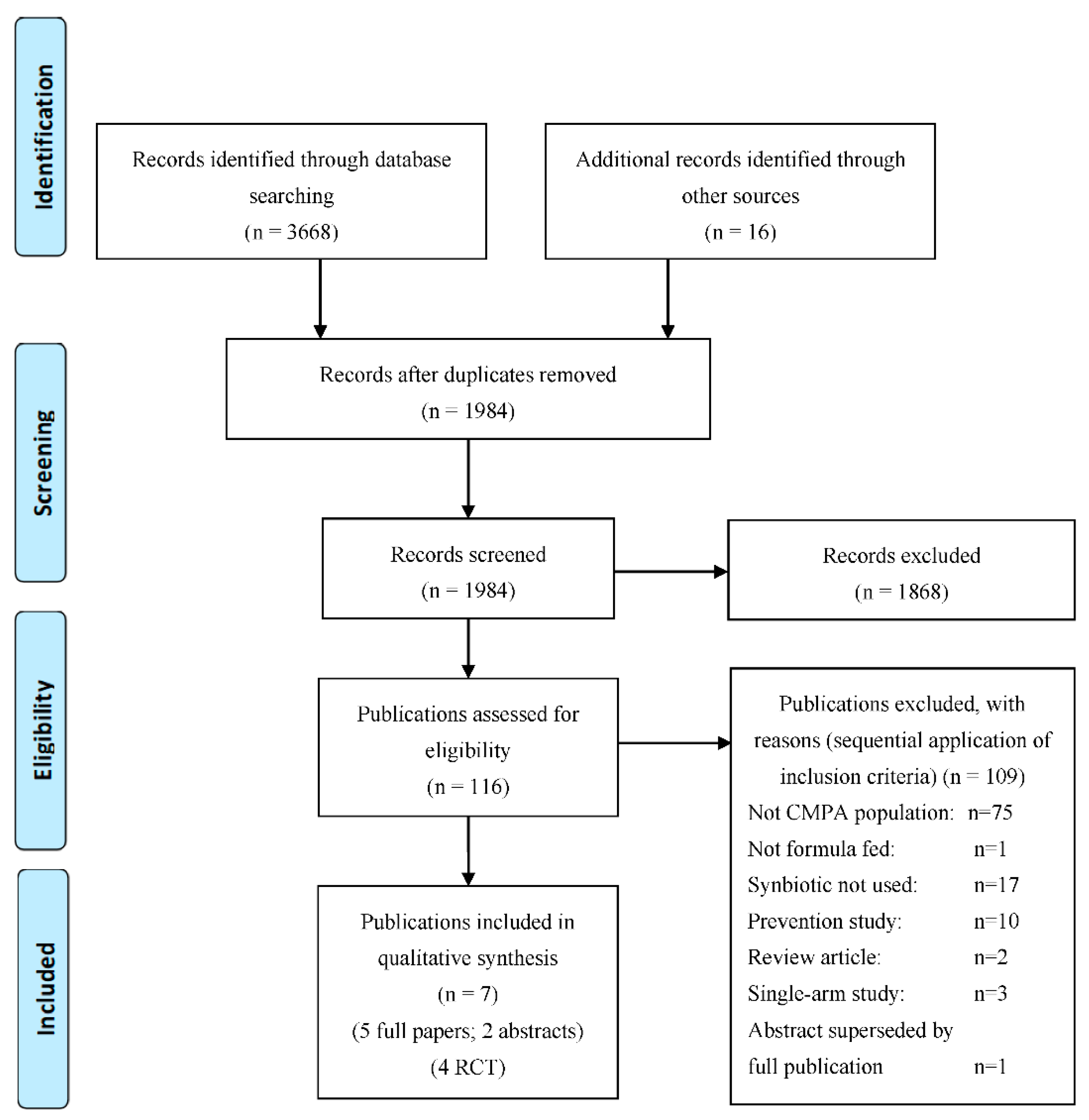
3.1. Overall Search Findings (n = 7)
3.2. Description of Publications Included in the Systematic Review (n = 7)
3.3. Outcomes from Studies Comparing AAF with Synbiotics with AAF Alone
3.3.1. Clinical Symptoms & Allergenicity
3.3.2. Infections and Hospital Admissions
Simple Cost Analysis Based on Hospital Admission Data
3.3.3. Medication Use
3.3.4. Change in Gut Microbiota Profile
3.3.5. Other Outcomes
Stool Characteristics
Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A.; The EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy 2014, 69, 992–1007. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; Von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. World Allergy Organ. J. 2010, 3, 57–161. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Gupta, R.; Sheikh, A.; Strachan, D.P.; Anderson, H.R. Burden of allergic disease in the UK: Secondary analyses of national databases. Clin. Exp. Allergy 2004, 34, 520–526. [Google Scholar] [CrossRef]
- Fox, M.; Voordouw, J.; Mugford, M.; Cornelisse, J.; Antonides, G.; Frewer, L. Social and Economic Costs of Food Allergies in Europe: Development of a Questionnaire to Measure Costs and Health Utility. Health Serv. Res. 2009, 44, 1662–1678. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Kim, H.; Gerdts, J.; Protudjer, J.L.P. Milk allergy most burdensome in multi-food allergic children. Pediatr. Allergy Immunol. 2020, 31, 827–834. [Google Scholar] [CrossRef]
- Meyer, R.; Godwin, H.; Dziubak, R.; Panepinto, J.A.; Foong, R.-X.M.; Bryon, M.; Lozinsky, A.C.; Reeve, K.; Shah, N. The impact on quality of life on families of children on an elimination diet for Non-immunoglobulin E mediated gastrointestinal food allergies. World Allergy Organ. J. 2017, 10, 8. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: Espghan gi committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Prac. 2018, 6, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 1–4. [Google Scholar] [CrossRef]
- Shreiner, A.; Huffnagle, G.B.; Noverr, M.C. The “Microflora Hypothesis” of Allergic Disease. Adv. Exp. Med. Biol. 2009, 635, 113–134. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef]
- Thompson-Chagoyan, O.C.; Vieites, J.M.; Maldonado, J.; Edwards, C.; Gil, A. Changes in faecal microbiota of infants with cow’s milk protein allergy—A Spanish prospective case-control 6-month follow-up study. Pediatr. Allergy Immunol. 2010, 21, e394–e400. [Google Scholar] [CrossRef] [PubMed]
- Balmer, S.E.; Wharton, B.A. Diet and faecal flora in the newborn: Breast milk and infant formula. Arch. Dis. Child. 1989, 64, 1672–1677. [Google Scholar] [CrossRef]
- Harmsen, H.J.M.; Wildeboer–Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of Intestinal Flora Development in Breast-Fed and Formula-Fed Infants by Using Molecular Identification and Detection Methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. [Google Scholar] [CrossRef]
- Stark, P.L.; Lee, A. The Microbial Ecology of the Large Bowel of Breastfed and Formula-fed Infants During the First Year of Life. J. Med. Microbiol. 1982, 15, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.A.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Gandhi, S.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Prebiotics. World Allergy Organ. J. 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Qamer, S.; Deshmukh, M.; Patole, S. Probiotics for cow’s milk protein allergy: A systematic review of randomized controlled trials. Eur. J. Nucl. Med. Mol. Imaging 2019, 178, 1139–1149. [Google Scholar] [CrossRef]
- Moro, G.; Mosca, F.; Miniello, V.; Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G. Effects of a new mixture of prebiotics on faecal flora and stools in term infants. Acta Paediatr. 2007, 92, 77–79. [Google Scholar] [CrossRef]
- Sierra, C.; Bernal, M.-J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuño, I.; Espín, B.; Vasallo, M.-I.; Gil, D.; Vidal, M.-L.; et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: A multicentre, randomised, double-blind and placebo-controlled trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Van de Broek, H.; Plaskie, K.; Wesling, F.; Roger, L.; McCartney, A.; Assam, P. Physiological and Bifidogenic Effects of Prebiotic Supplements in Infant Formulae. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Ahanchian, H.; Nouri, Z.; Jafari, S.A.; Moghiman, T.; Amirian, M.H.; Ezzati, A.; Kianifar, H.R. Synbiotics in Children with Cow’s Milk Allergy: A Randomized Controlled Trial. Iran J. Pediatr. 2014, 24, 29–34. [Google Scholar]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 192–198. [Google Scholar] [CrossRef]
- Peldan, P.; Kukkonen, A.K.; Savilahti, E.; Kuitunen, M. Perinatal probiotics decreased eczema up to 10 years of age, but at 5-10 years, allergic rhino-conjunctivitis was increased. Clin. Exp. Allergy 2017, 47, 975–979. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, H.; Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.; Sangsupawanich, P.; de Weerd, H.; Kakourou, A.; Roeselers, G.; et al. Increased and enriched Bifidobacterium community in gut microbiota of infants with IgE-mediated CMA receiving a specific synbiotic-containing amino acid-based formula. In Proceedings of the FAAM-EUROBAT, Digital Event, 16–17 October 2020. [Google Scholar]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.; Sangsupawanich, P.; Van Ampting, M.; Nijhuis, M.; Harthoorn, L.; Landgford, J.; et al. Tolerance development in infants with IgE mediated cow’s milk allergy receiving amino acid-based formula including specific synbiotics: A multi-center randomized controlled clinical trial (PRESTO). In Proceedings of the PAAM, Florence, Italy, 17–19 October 2019. [Google Scholar]
- Candy, D.C.A.; Van Ampting, M.T.J.; Nijhuis, M.M.O.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E.; et al. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr. Res. 2018, 83, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.T.; ASSIGN Study Group; Wopereis, H.; Van Ampting, M.T.J.; Nijhuis, M.M.O.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Candy, D.C.A.; Shah, N.; et al. A specific synbiotic-containing amino acid-based formula in dietary management of cow’s milk allergy: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Introduction to Meta-analysis (Statistics in Practice); John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- NHS Improvement. 2020/21 National Tariff Payment System: National Prices and Prices for Blended Payments. Available online: https://improvement.nhs.uk/resources/national-tariff/#h2-supporting-documents (accessed on 11 January 2021).
- Monthly Index of Medical Specialties (MIMS) Online. Available online: https://www.mims.co.uk (accessed on 11 January 2021).
- Harvey, B.M.; Langford, J.E.; Harthoorn, L.F.; Gillman, S.A.; Green, T.D.; Schwartz, R.H.; Burks, A.W. Effects on growth and tolerance and hypoallergenicity of an amino acid–based formula with synbiotics. Pediatr. Res. 2013, 75, 343–351. [Google Scholar] [CrossRef]
- Burks, A.W.; Harthoorn, L.F.; Van Ampting, M.T.J.; Nijhuis, M.M.O.; Langford, J.E.; Wopereis, H.; Goldberg, S.B.; Ong, P.Y.; Essink, B.J.; Scott, R.B.; et al. Synbiotics-supplemented amino acid-based formula supports adequate growth in cow’s milk allergic infants. Pediatr. Allergy Immunol. 2015, 26, 316–322. [Google Scholar] [CrossRef]
- Wopereis, H.; Van Ampting, M.T.J.; Cetinyurek-Yavuz, A.; Slump, R.; Candy, D.C.A.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; et al. A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow’s milk allergic infants: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 27. [Google Scholar] [CrossRef]
- Van Der Aa, L.B.; Van Aalderen, W.M.C.; Heymans, H.S.A.; Smitt, J.H.S.; Nauta, A.J.; Knippels, L.M.J.; Ben Amor, K.; Sprikkelman, A.B. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef]
- Van Der Aa, L.B.; Heymans, H.S.; Van Aalderen, W.M.; Smitt, J.H.S.; Knol, J.; Ben Amor, K.; Goossens, D.A.; Sprikkelman, A.B. Group Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Van Der Aa, L.B.; Lutter, R.; Heymans, H.S.A.; Smids, B.S.; Dekker, T.; Van Aalderen, W.M.C.; Smitt, J.H.S.; Knippels, L.M.J.; Garssen, J.; Nauta, A.J.; et al. No detectable beneficial systemic immunomodulatory effects of a specific synbiotic mixture in infants with atopic dermatitis. Clin. Exp. Allergy 2011, 42, 531–539. [Google Scholar] [CrossRef]
- Abrahamse-Berkeveld, M.; Alles, M.; Franke-Beckmann, E.; Helm, K.; Knecht, R.; Köllges, R.; Sandner, B.; Knol, J.; Ben Amor, K.; Bufe, A. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J. Nutr. Sci. 2016, 5, e42. [Google Scholar] [CrossRef]
- Schallreuter, K.; Levenig, C.; Berger, J.; Umbert, J.; Winkelmann, R.; Wegener, L.; Correia, O.; Chosidow, O.; Saiag, P.; Bastuji-Garin, S.; et al. Severity Scoring of Atopic Dermatitis: The SCORAD Index. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Agostoni, C.; Axelsson, I.; Braegger, C.; Goulet, O.; Koletzko, B.; Michaelsen, K.F.; Rigo, J.; Shamir, R.; Szajewska, H.; Turck, D.; et al. Probiotic Bacteria in Dietetic Products for Infants: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 365–374. [Google Scholar] [CrossRef]
- Juntti, S.T.H. Cow’s Milk Allergy is Associated with Recurrent Otitis Media During Childhood. Acta Oto-Laryngologica 1999, 119, 867–873. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Zhang, J.; Zeng, L.; Wang, Y.; Zheng, Q.Y. Risk Factors for Chronic and Recurrent Otitis Media–A Meta-Analysis. PLoS ONE 2014, 9, e86397. [Google Scholar] [CrossRef]
- Luong, A.; Roland, P.S. The Link Between Allergic Rhinitis and Chronic Otitis Media with Effusion in Atopic Patients. Otolaryngol. Clin. N. Am. 2008, 41, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hurst, D.S. The Role of Allergy in Otitis Media with Effusion. Otolaryngol. Clin. N. Am. 2011, 44, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.M.; Lee, J.; Conboy, K.; Ellis, E.; Li, P. Further Observations on the Role of IgE-Mediated Hypersensitivity in Recurrent Otitis Media with Effusion. Otolaryngol. Neck Surg. 1985, 93, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Koch, T.; Philipp, A. Allergic origin of recurrent middle ear effusion and adenoids in young children. HNO 1991, 39, 182–184. [Google Scholar] [PubMed]
- Zernotti, M.E.; Pawankar, R.; Ansotegui, I.; Badellino, H.; Croce, J.S.; Hossny, E.; Ebisawa, M.; Rosario, N.; Borges, M.S.; Zhang, Y.; et al. Otitis media with effusion and atopy: Is there a causal relationship? World Allergy Organ. J. 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- NHS Digital Prescribing and Medicines Team. Prescription Cost Analysis: England —2018 Data Tables. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018 (accessed on 11 January 2021).
- NHS Scotland Information Services Division. Prescribing & Medicines: Prescription Cost Analysis—Financial Year 2015/16. Available online: https://www.isdscotland.org/Health-Topics/Prescribing-and-medicines/Community-Dispensing/Prescription-Cost-Analysis/ (accessed on 11 January 2021).
- NHS Wales Shared Services Partnership. Prescription Cost Analysis Yearly Data 2019. Available online: https://nwssp.nhs.wales/ourservices/primary-care-services/general-information/data-and-publications/prescription-cost-analysis/ (accessed on 11 January 2021).
- HSC Business Services Organisation. Prescription Cost Analysis Northern Ireland 2019. Available online: http://www.hscbusiness.hscni.net/services/1806.htm (accessed on 11 January 2021).
- Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR): Report 2019 to 2020; Public Health England: London, UK, 2020. [Google Scholar]
- Phavichitr, N.; COLOR Study Group; Wang, S.; Chomto, S.; Tantibhaedhyangkul, R.; Kakourou, A.; Intarakhao, S.; Jongpiputvanich, S.; Roeselers, G.; Knol, J. Impact of synbiotics on gut microbiota during early life: A randomized, double-blind study. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
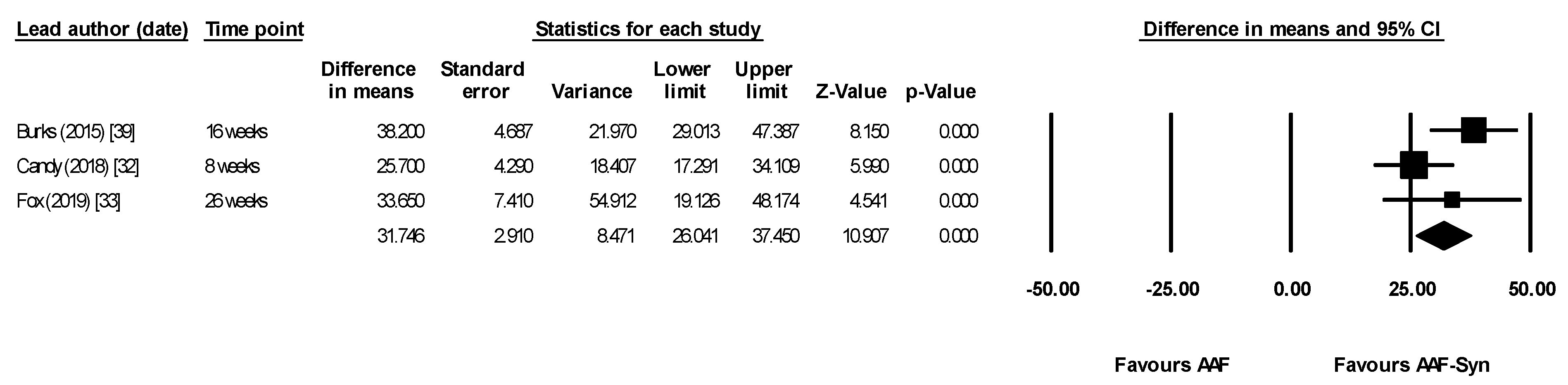
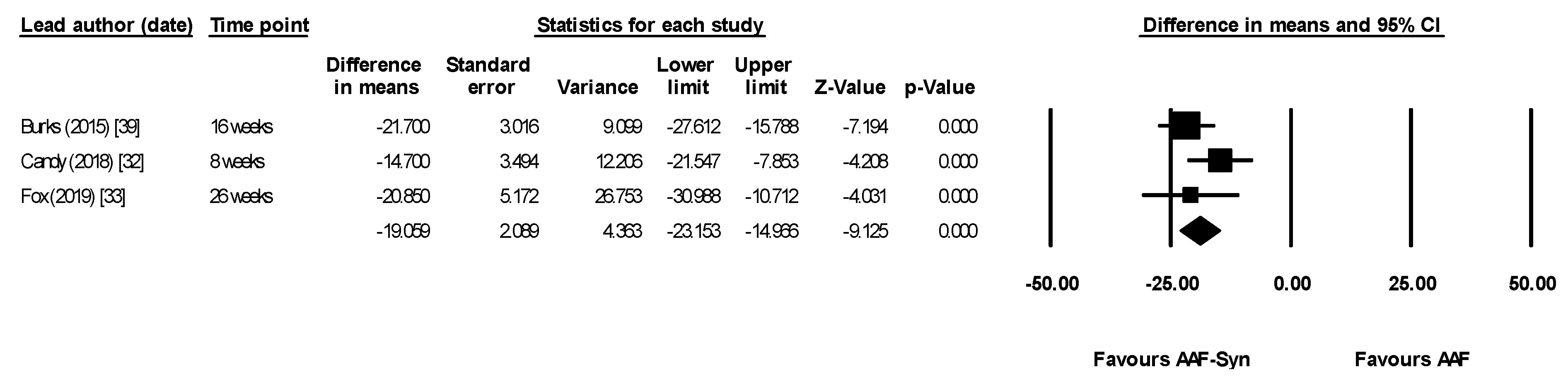
| Lead Author (Date) | Population and Type of Study | Male | Mean Age (Months) | Amount of Formula Consumed/Day (mL) Mean ± SD | n AAF-Syn | n AAF | Intervention Duration | Timepoint Outcomes Measured |
|---|---|---|---|---|---|---|---|---|
| Harvey (2014) [38] Full paper | Infants with IgE mediated CMPA aged 0–36 months One arm DBPCCFC and 7 day feeding period | 61% | 17.3, range 3.3–46.9 | Not reported | 30 | 30 | 7 days | 7 days |
| Full-term healthy infants aged 3–16 months, RCT¶ | 67% | 10.6, range 3–16 | AAF-Syn: 349 ± 127§; AAF: 331 ± 124§ | 59 | 56 | 16 weeks | 2, 4, 8, 12 & 16 weeks | |
| Burks (2015) [39] Full paper | Infants with IgE or non-IgE mediated CMPA aged 0–8 months, RCT | 62% | 4.5, range 0.6–8.9 | Not reported. Intake was reported as comparable in both groups | 54 | 56 | 16 weeks | 4 & 16 weeks |
| Candy (2018) [32] ASSIGN study, full paper | Infants with non-IgE mediated CMPA aged 0–13 months, RCT Included breast-fed healthy reference group (not randomised) | 73% | 6, range 1.2–12.8 | Week 8 AAF-Syn 652 ± 176; AAF 639 ± 212 | 35 | 36 | 8 weeks | 4 & 8 weeks |
| Fox (2019) [33] † ASSIGN study, full paper | Infants with non-IgE mediated CMPA aged 0–13 months 26-week follow-up of Candy (2018) | 73% | 6, range 1.2–12.8 | Week 8 AAF-Syn 652 ± 176; AAF 639 ± 212 | 35 | 36 | 8 weeks | 8, 12 & 26 weeks |
| Wopereis (2019) [40] † ASSIGN study, full paper | Infants with non-IgE mediated CMPA aged 0–13 months Gene-sequencing analysis from Candy (2018) and Fox (2019) | 73% | 6, range 1.2–12.8 | Week 8 AAF-Syn 652 ± 176; AAF 639 ± 212 | 35 | 36 | 8 weeks | 8, 12 & 26 weeks |
| Chatchatee (2019) [31] PRESTO study ‡, conference abstract | Infants with confirmed IgE mediated CMPA aged 0–13 months, RCT | 72% | 9.36, SD 2.53 | At 12 months: AAF-Syn: 547 ± 302; AAF: 530 ± 308 | 80 | 89 | 12 months | 12 months |
| Wopereis (2020) [30] PRESTO study ‡, conference abstract | Infants with confirmed IgE mediated CMPA aged 0–13 months, RCT |
| Lead Author (Date) | Population | Clinical Symptoms | Infections & Hospital Admissions | Medication Usage | Gut Microbiota | Stool Characteristics | Growth | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Allergy † | GI | Resp ‡ | Antibiotics | Other Medication § | ||||||
| Harvey (2014) [38] | Infants with IgE mediated CMPA aged 0–36 months | PO = | ||||||||
| Healthy infants aged 3–16 months# | ✓✓ | ✓ | ✓✓ | PO = | ||||||
| Burks (2015) [39] | Infants with IgE or non IgE mediated CMPA aged 0–8 months | = | = | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | PO = | |
| Candy (2018) [32] ASSIGN study | Infants with non-IgE mediated CMPA aged 0–13 months | = | = | = | ✓ | ✓✓ | ✓ | PO ✓✓ | ✓✓ | = |
| Fox (2019) [33] ASSIGN study | 26-week follow-up of Candy (2018) | = | = | = | ✓✓ | ✓✓ | PO ✓✓ ¶ | = | = | |
| Wopereis (2019) [40] ASSIGN study | Gene-sequencing analysis from Candy (2018) and Fox (2019) | PO ✓✓ | ||||||||
| Chatchatee (2019) [31] PRESTO study | Infants with confirmed IgE mediated CMPA aged 0–13 months | ✓✓ | ||||||||
| Wopereis (2020) [30] PRESTO study | Infants with confirmed IgE mediated CMPA aged 0–13 months | PO ✓ | ||||||||
| Lead Author (Date) | AAF-Syn | AAF Alone | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
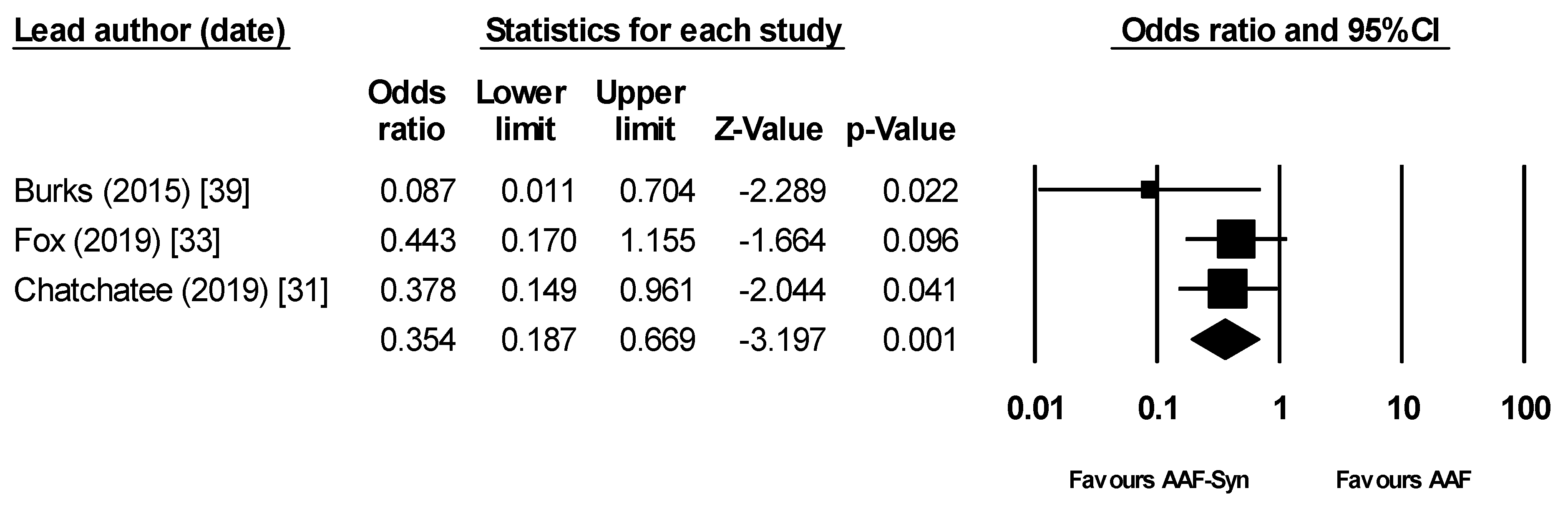
| Burks (2015) [39] | 1/54 | 1.9% | 10/56 | 17.9% |
| Candy (2018) [32] † (ASSIGN study) | 10/35 | 28.6% | 12/36 | 33.3% |
| Fox (2019) [33] (ASSIGN study) | 15/35 | 42.9% | 22/35 | 62.9% |
| Chatchatee (2019) [31] (PRESTO study) | 7/80 ‡ | 8.8% | 18/89 ‡ | 20.2% |
| Pooled Result † | 23/169 | 13.6% (6.7% §) | 50/180 | 27.8% (16.3% §) |
| Percentage reduction | 51.0% (58.6% §) | |||
| Lead Author (Date) | Outcome Measures | Comparison of Findings in AAF-Syn vs. AAF Groups |
|---|---|---|
| Burks (2015) [39] | Systemic antibacterial and functional GI medication use included as exploratory outcome | Results AAF-Syn vs. AAF, 16-week event rates: Systemic antibacterial use: 17% vs. 34%, p = 0.049
|
| Candy (2018) [32] ASSIGN study | Systemic anti-infective & concomitant medication use included as exploratory outcome | Results AAF-Syn vs. AAF, 8-week event rates: Overall concomitant medication use: 60% vs. 78% †, p = 0.117
|
| Fox (2019) [33] ‡ ASSIGN study | Concomitant medication use included as exploratory outcome | Results AAF-Syn vs. AAF, 26-week event rates: Overall concomitant medication use: 71% vs. 83%, p = 0.39
|
| Lead Author (Date) | Outcome Measures | Comparison of Findings in AAF-Syn vs. AAF Groups | Statistical Comparison | Conclusions |
|---|---|---|---|---|
| Burks (2015) [39] | Secondary outcome was change in proportion of faecal BSp, CH & ER/CC | Mean AAF-Syn vs. AAF. Baseline proportions similar in both groups
| All differences between groups at 16 weeks statistically significant | “The indigenous gut microbiota of [CMPA] infants receiving an AAF can be influenced by synbiotics. As expected, synbiotics in the test formula increased Bifidobacterium, a genus typically predominant in the GI tract of breastfed infants” “…It can therefore be hypothesized that abolishing this gut microbiota dysbiosis may decrease [CMPA] risk or [CMPA] persistence…” |
| Candy (2018) [32] ASSIGN study | Primary outcome was change in proportion of faecal BSp & ER/CC Baseline measures were used as covariates for ANCOVA | Median, AAF-Syn vs. AAF. Baseline proportions not given At 8 weeks
| Between groups comparison for both BSp and ER/CC were statistically significant at 8 weeks | “The primary objective of modifying gut microbiota using an AAF including [synbiotics] for 8 weeks in subjects with suspected non-IgE [CMPA] was achieved.” “…The current study showed that microbial composition of infants with suspected non-IgE [CMPA] who received the test formula was closer to the profile of the HBR group than those infants receiving control formula.” |
| Fox (2019) [33] ASSIGN study Subset of infants who continued intervention for 26 weeks | 26-week extension study of Candy (2018) [32] | The between-group differences in microbiota composition seen at week 8 (primary trial endpoint) were maintained with longer study follow-up. At weeks 12 and 26, the AAF-Syn group had a higher percentage of BSp and a lower percentage of ER/CC compared with the AAF group. Mean AAF-Syn vs. AAF: At baseline (0 weeks)
| Between groups comparison for both BSp and ER/CC were statistically significant at 26 weeks | “…In conclusion, use of the AAF including specific synbiotics investigated in this study resulted in a sustained improvement in gut microbiota composition over 26 weeks…” “…it may suggest that the effects on gut microbiota by AAF including synbiotics can even be maintained in a [CMPA] population receiving systemic antibiotics.” |
| Wopereis (2019) [40] ASSIGN study | Detailed genomic characterisation of faecal microbiota, population from Candy (2018) [32] and Fox (2019) [33]. Primary outcome was the assessment of bacterial species diversity over time. | Diversity in faecal microbiota increased over time in both groups. The effect was less pronounced in the AAF-Syn group. Mean difference per week from week 0 to 26
At 12 weeks:
| Significant improvement in faecal microbial diversity | “…AAF including the specific synbiotics offers an effective nutritional strategy to modulate the gut microbiota of infants with suspected non-IgE mediated [CMPA] closer to a healthy breastfed profile…” “The AAF including synbiotics compared to the AAF without synbiotics showed a more gradual increment over time of bacterial diversity, which is also typically observed in longitudinal studies investigating early life gut microbiota development of breastfed infants as compared to formula-fed infants.” |
| Wopereis (2020) [30] PRESTO study | Detailed genomic characterisation of faecal microbiota; abundances of BSp, LSp and adult-type genera; faecal bacterial species diversity | At 6 and 12 months, compared to AAF, AAF-Syn was associated with:
| p-values not reported | “The predominant abundance of Bifidobacterium in subjects receiving [AAF-Syn] was reflected in lower overall diversity at 6 and 12 months.” “…Subjects receiving [AAF-Syn] showed increased diversity of species within the genus Bifidobacterium compared to AAF at 6 and 12 months.” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorensen, K.; Cawood, A.L.; Gibson, G.R.; Cooke, L.H.; Stratton, R.J. Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 935. https://doi.org/10.3390/nu13030935
Sorensen K, Cawood AL, Gibson GR, Cooke LH, Stratton RJ. Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(3):935. https://doi.org/10.3390/nu13030935
Chicago/Turabian StyleSorensen, Katy, Abbie L. Cawood, Glenn R. Gibson, Lisa H. Cooke, and Rebecca J. Stratton. 2021. "Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis" Nutrients 13, no. 3: 935. https://doi.org/10.3390/nu13030935
APA StyleSorensen, K., Cawood, A. L., Gibson, G. R., Cooke, L. H., & Stratton, R. J. (2021). Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy: A Systematic Review and Meta-Analysis. Nutrients, 13(3), 935. https://doi.org/10.3390/nu13030935