Milk Ingredients in Meat Products: Can Autoclaving and In Vitro Gastroduodenal Digestion Mitigate Their IgE-Binding Capacity?
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Production of Milk-Free and Incurred Model Sausages
2.3. Sera of Milk-Allergic Patients
2.4. Simulated In Vitro Gastroduodenal Digestion of Model Sausages
2.4.1. Preparation of Gastroduodenal Fluid Stock Solutions
2.4.2. Assessment of Protein Solubility in Gastroduodenal Fluids before Enzymatic Digestion
2.4.3. In Vitro Gastroduodenal Digestion of Model Sausages
2.5. SDS-PAGE Analysis
2.6. Immunoblot for IgE-Binding Assay
2.7. LC-MS/MS Analysis
2.7.1. Purification of Gastroduodenal Digested Samples
2.7.2. In-Gel Protein Digestion
2.7.3. Protein Identification by Untargeted LC-MS/MS Analysis
2.8. Bioinformatic Analysis of the Residual Immunoreactivity of Milk-Enriched Raw and Processed Sausages after Gastroduodenal Digestion
3. Results and Discussion
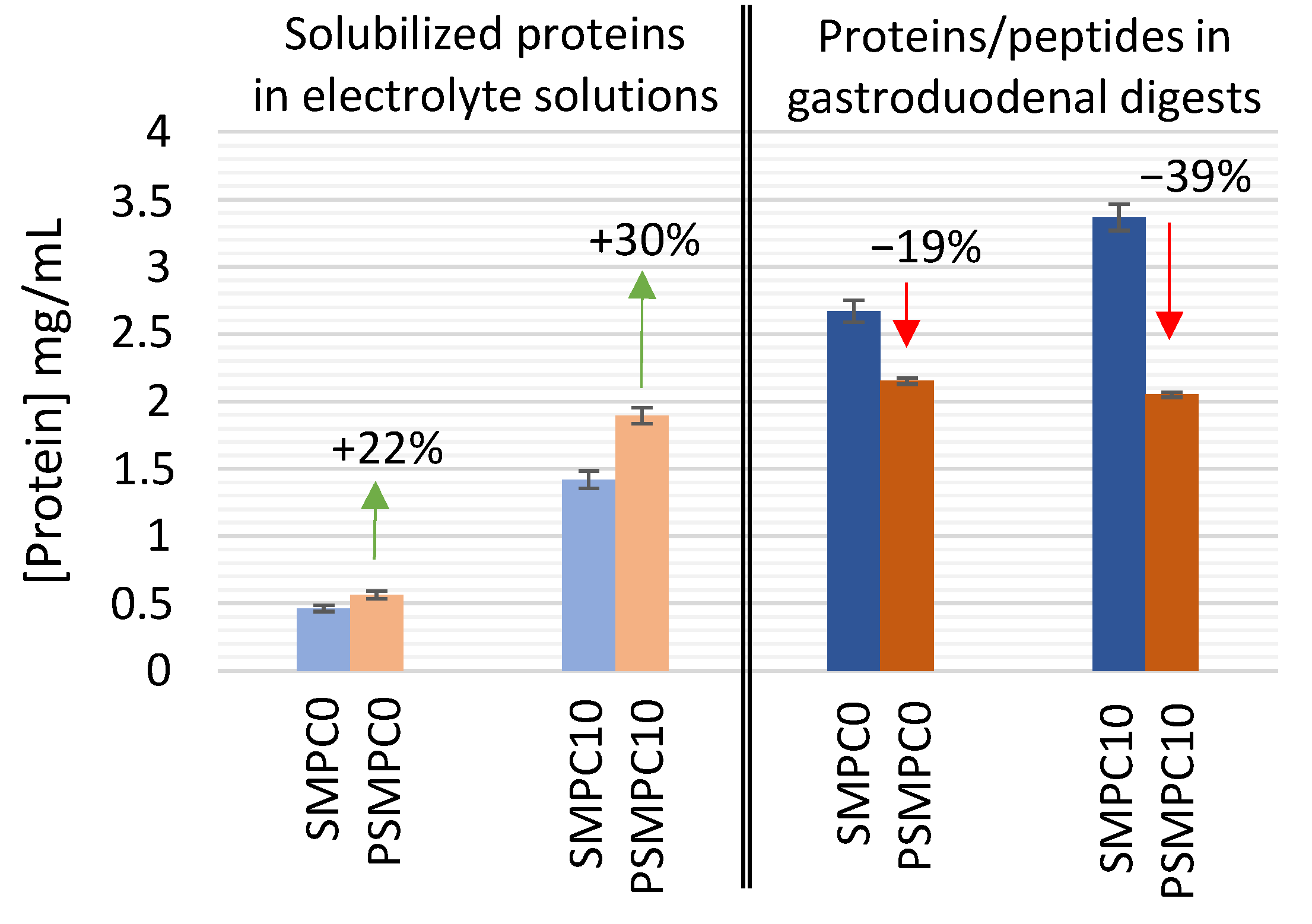
3.1. Effect of Autoclaving on the Solubility and Enzymatic Digestion of Milk Proteins in Model Sausages under Simulated Gastroduodenal Conditions
3.1.1. Assessment of Protein Solubility
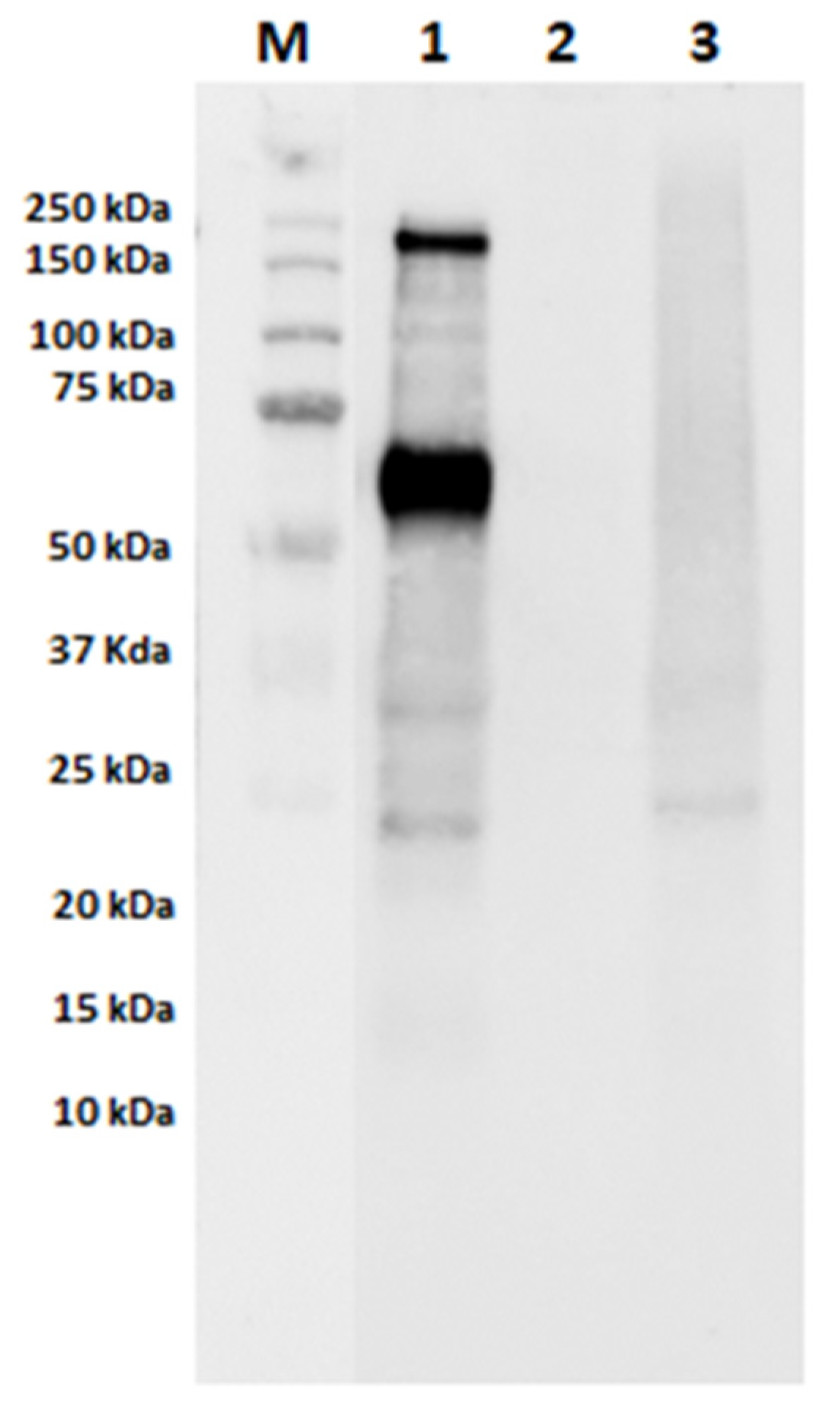
3.1.2. Characterization of the Electrophoretic Profile of Model Sausages Samples upon Technological Treatment
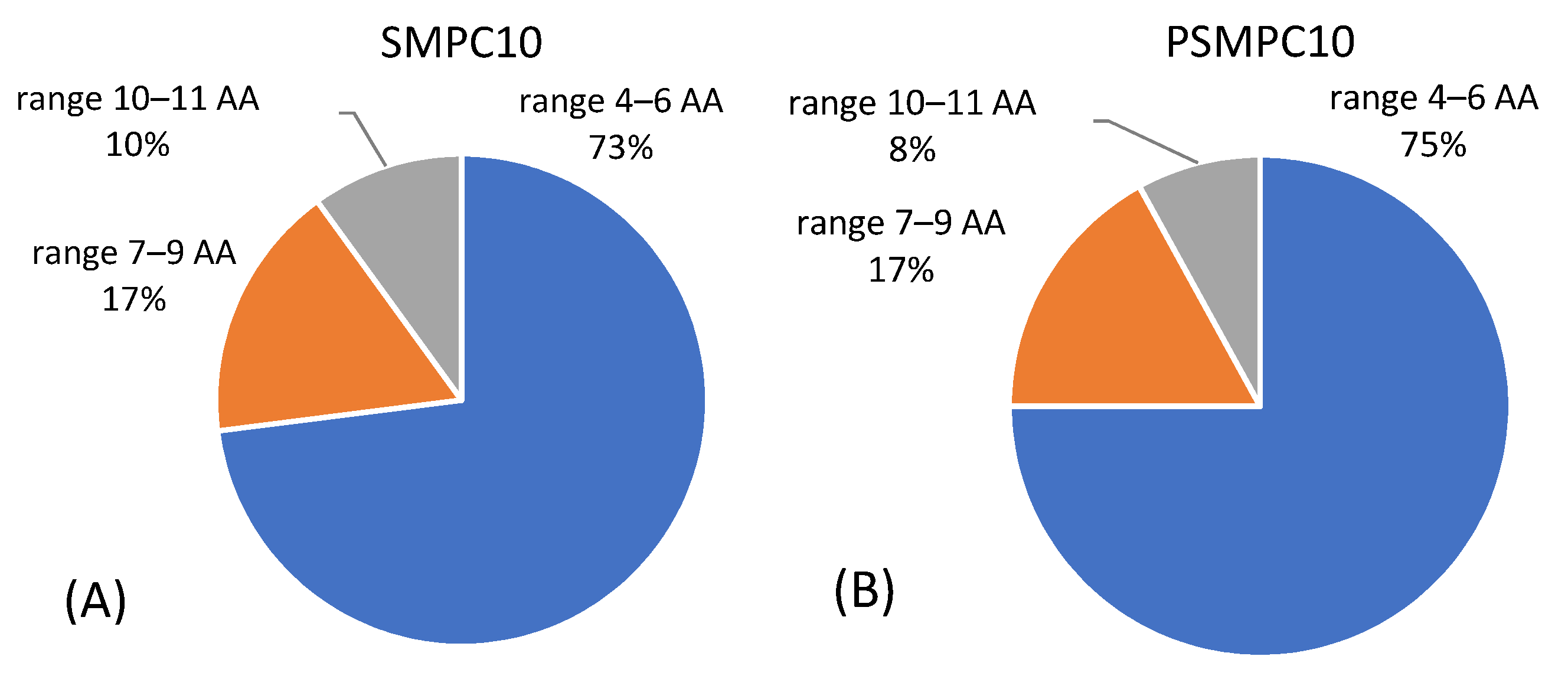
3.1.3. LC-MS/MS Analysis of Sausage Material upon Gastroduodenal Digestion
3.2. Assessment of IgE-Binding Capacity of Autoclaved Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, P. Milk proteins as food ingredients. Int. J. Dairy Technol. 2001, 54, 41–55. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine milk allergens: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef]
- Monaci, L.; Tregoat, V.; van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]
- WHO/IUIS. World Health Organization/International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-Committee. 2020. Available online: http://www.allergen.org/ (accessed on 31 May 2020).
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food processing: The influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Fiocchi, A. Rare, medium, or well done? The effect of heating and food matrix on food protein allergenicity. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Schulten, V.; Lauer, I.; Scheurer, S.; Thalhammer, T.; Bohle, B. A food matrix reduces digestion and absorption of food allergens in vivo. Mol. Nutr. Food Res. 2011, 55, 1484–1491. [Google Scholar] [CrossRef]
- El Mecherfi, K.E.; Curet, S.; Lupi, R.; Larré, C.; Rouaud, O.; Choiset, Y.; Rabesona, H.; Haertlé, T. Combined microwave processing and enzymatic proteolysis of bovine whey proteins: The impact on bovine β-lactoglobulin allergenicity. J. Food Sci. Technol. 2019, 56, 177–186. [Google Scholar] [CrossRef] [PubMed]
- El Mecherfi, K.-E.; Rouaud, O.; Curet, S.; Negaoui, H.; Chobert, J.-M.; Kheroua, O.; Saidi, D.; Haertlé, T. Peptic hydrolysis of bovine beta-lactoglobulin under microwave treatment reduces its allergenicity in an ex vivo murine allergy model. Int. J. Food Sci. Technol. 2015, 50, 356–364. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Markiewicz, L.H.; Szyc, A.; Wasilewska, E. Whey prefermented with beneficial microbes modulates immune response and lowers responsiveness to milk allergens in mouse model. J. Funct. Foods 2019, 54, 41–52. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Pérez-Rodríguez, L.; Pablos-Tanarro, A.; López-Fandiño, R.; Molina, E. Pepsin treatment of whey proteins under high pressure produces hypoallergenic hydrolysates. Innov. Food Sci. Emerg. Technol. 2017, 43, 154–162. [Google Scholar] [CrossRef]
- Orcajo, J.; Lavilla, M.; Martínez-de-Marañón, I. Effect of pulsed light treatment on β-lactoglobulin immunoreactivity. LWT Food Sci. Technol. 2019, 112, 108231. [Google Scholar] [CrossRef]
- Liu, J.; Tu, Z.-C.; Liu, G.-X.; Niu, C.-D.; Yao, H.-L.; Wang, H.; Sha, X.-M.; Shao, Y.-H.; Kaltashov, I.A. Ultrasonic pretreatment combined with dry-state glycation reduced the immunoglobulin E/immunoglobulin G-binding ability of α-lactalbumin revealed by high-resolution mass spectrometry. J. Agric. Food Chem. 2018, 66, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Bloom, K.A.; Sicherer, S.H.; Shreffler, W.G.; Noone, S.; Wanich, N.; Sampson, H.A. Tolerance to extensively heated milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2008, 122, 342–347. [Google Scholar] [CrossRef]
- Bavaro, S.L.; De Angelis, E.; Barni, S.; Pilolli, R.; Mori, F.; Novembre, E.M.; Monaci, L. Modulation of milk allergenicity by baking milk in foods: A proteomic investigation. Nutrients 2019, 11, 1536. [Google Scholar] [CrossRef]
- Bogh, K.L.; Madsen, C.B. Food allergens: Is there a correlation between stability to digestion and allergenicity? Crit. Rev. Food Sci. Nutr. 2016, 56, 1545–1567. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Lu, Y.; Lin, X.; Hu, X.; Liu, L.; He, Z.; Wu, X. Effect of chlorogenic acid covalent conjugation on the allergenicity, digestibility and functional properties of whey protein. Food Chem. 2019, 298, 125024. [Google Scholar] [CrossRef] [PubMed]
- Nitride, C.; Nørgaard, J.; Omar, J.; Emons, H.; Esteso, M.-J.M.; O’Connor, G. An assessment of the impact of extraction and digestion protocols on multiplexed targeted protein quantification by mass spectrometry for egg and milk allergens. Anal. Bioanal. Chem. 2019, 411, 3463–3475. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Liu, T.; Yang, Y.; Zhang, J.; Yin, J.; Ding, X.; Qin, W.; Yang, Y. A rapid immobilized trypsin digestion combined with liquid chromatography—Tandem mass spectrometry for the detection of milk allergens in baked food. Food Control 2019, 102, 179–187. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Digestibility and antigenicity of β-lactoglobulin as affected by heat, pH and applied shear. Food Chem. 2017, 217, 517–523. [Google Scholar] [CrossRef]
- Quintieri, L.; Monaci, L.; Baruzzi, F.; Giuffrida, M.G.; de Candia, S.; Caputo, L. Reduction of whey protein concentrate antigenicity by using a combined enzymatic digestion and ultrafiltration approach. J. Food Sci. Technol. 2017, 54, 1910–1916. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; Giménez, G.; Grishina, G.; Bardina, L.; Sampson, H.A.; Molina, E.; López-Fandiño, R. In vitro digestibility of bovine β-casein with simulated and human oral and gastrointestinal fluids. Identification and IgE-reactivity of the resultant peptides. Food Chem. 2014, 143, 514–521. [Google Scholar] [CrossRef]
- Meena, G.S.; Singh, A.K.; Panjagari, N.R.; Arora, S. Milk protein concentrates: Opportunities and challenges. J. Food Sci. Technol. 2017, 54, 3010–3024. [Google Scholar] [CrossRef] [PubMed]
- Spychaj, A.; Pospiech, E.; Iwańska, E.; Montowska, M. Detection of allergenic additives in processed meat products. J. Sci. Food Agric. 2018, 98, 4807–4815. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- De Angelis, E.; Pilolli, R.; Bavaro, S.L.; Monaci, L. Insight into the gastro-duodenal digestion resistance of soybean proteins and potential implications for residual immunogenicity. Food Funct. 2017, 8, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, S.L.; Di Stasio, L.; Mamone, G.; De Angelis, E.; Nocerino, R.; Canani, R.B.; Logrieco, A.F.; Montemurro, N.; Monaci, L. Effect of thermal/pressure processing and simulated human digestion on the immunoreactivity of extractable peanut allergens. Food Res. Int. 2018, 109, 126–137. [Google Scholar] [CrossRef]
- Dhakal, S.; Liu, C.; Zhang, Y.; Roux, K.H.; Sathe, S.K.; Balasubramaniam, V.M. Effect of high pressure processing on the immunoreactivity of almond milk. Food Res. Int. 2014, 62, 215–222. [Google Scholar] [CrossRef]
- De Angelis, E.; Bavaro, S.L.; Forte, G.; Pilolli, R.; Monaci, L. Heat and pressure treatments on almond protein stability and change in immunoreactivity after simulated human digestion. Nutrients 2018, 10, 1679. [Google Scholar] [CrossRef] [PubMed]
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef]
- Meltretter, J.; Schmidt, A.; Humeny, A.; Becker, C.-M.; Pischetsrieder, M. Analysis of the peptide profile of milk and its changes during thermal treatment and storage. J. Agric. Food Chem. 2008, 56, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; Léonil, J.; Henry, G.; Cauty, C.; Carvalho, A.F.; Bouhallab, S. Heating and glycation of β-lactoglobulin and β-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- Lamberti, C.; Baro, C.; Giribaldi, M.; Napolitano, L.; Cavallarin, L.; Giuffrida, M.G. Effects of two different domestic boiling practices on the allergenicity of cow’s milk proteins. J. Sci. Food Agric. 2018, 98, 2370–2377. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, J.; Yao, M.; Jiang, M.; Luo, Y. Effects of heat treatment on the antigenicity of four milk proteins in milk protein concentrates. Food Agric. Immunol. 2016, 27, 401–413. [Google Scholar] [CrossRef]
- Monaci, L.; Pilolli, R.; De Angelis, E.; Crespo, J.; Novak, N.; Cabanillas, B. Food allergens: Classification, molecular properties, characterization and detection in food sources. In Advances in Food and Nutrition Research; Toldrà, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 93, pp. 113–146. [Google Scholar]
- Bloom, K.A.; Huang, F.R.; Bencharitiwong, R.; Bardina, L.; Ross, A.; Sampson, H.A.; Nowak-Węgrzyn, A. Effect of heat treatment on milk and egg proteins allergenicity. Pediatr. Allergy Immunol. 2014, 25, 740–746. [Google Scholar] [CrossRef] [PubMed]

| Sera | Total IgE (kU/L) | sIgE to Cow’s Milk (kUA/L) | sIgE to Caseins (kUA/L) | sIgE to α-Lactoalbumin (kUA/L) | sIgE to β-Lactoglobulin (kUA/L) | Allergic Reaction Displayed |
|---|---|---|---|---|---|---|
| 1 | 66 | 26.8 | 6.32 | 8.91 | 3.19 | anaphylaxis |
| 2 | 227 | 19 | 17.5 | 3.49 | 0.19 | cough and rhinitis |
| 3 | 4798 | 38.1 | 20.7 | 17.5 | 16.5 | anaphylaxis |
| 4 | 88 | 22.7 | 21.1 | 3.06 | 0.59 | vomit |
| 5 | 1629 | 24.1 | 28.6 | 1.62 | 6.05 | anaphylaxis |
| 6 | 439 | 13.9 | 6.24 | 3.79 | 0.27 | vomit |
| 7 | 115 | >100 | >100 | 20.4 | 5.08 | anaphylaxis |
| Sample | Accession | Description | Coverage | Peptides (Unique) | PSMs a | Score | MW b [kDa] |
|---|---|---|---|---|---|---|---|
| SMC10-Dig | P02666 | β-casein OS c = Bos taurus | 58.04 | 21 (14) | 660 | 106.64 | 25.1 |
| P02668 | k-casein OS = Bos taurus | 32.11 | 5 (1) | 55 | 5.42 | 21.3 | |
| P02662 | αS1-casein OS = Bos taurus | 41.59 | 2 (2) | 103 | 4.23 | 24.5 | |
| PSMC10-Dig | P02666 | β-casein OS = Bos taurus | 62.95 | 18 (11) | 814 | 108.87 | 25.1 |
| P02668 | k-casein OS = Bos taurus | 34.21 | 4 (2) | 59 | 3.84 | 21.3 | |
| P02662 | αS1-casein OS = Bos taurus | 59.81 | 2 (2) | 164 | 2.63 | 24.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, C.; Bavaro, S.L.; De Angelis, E.; Pilolli, R.; Costa, J.; Barni, S.; Novembre, E.; Mafra, I.; Monaci, L. Milk Ingredients in Meat Products: Can Autoclaving and In Vitro Gastroduodenal Digestion Mitigate Their IgE-Binding Capacity? Nutrients 2021, 13, 931. https://doi.org/10.3390/nu13030931
Villa C, Bavaro SL, De Angelis E, Pilolli R, Costa J, Barni S, Novembre E, Mafra I, Monaci L. Milk Ingredients in Meat Products: Can Autoclaving and In Vitro Gastroduodenal Digestion Mitigate Their IgE-Binding Capacity? Nutrients. 2021; 13(3):931. https://doi.org/10.3390/nu13030931
Chicago/Turabian StyleVilla, Caterina, Simona L. Bavaro, Elisabetta De Angelis, Rosa Pilolli, Joana Costa, Simona Barni, Elio Novembre, Isabel Mafra, and Linda Monaci. 2021. "Milk Ingredients in Meat Products: Can Autoclaving and In Vitro Gastroduodenal Digestion Mitigate Their IgE-Binding Capacity?" Nutrients 13, no. 3: 931. https://doi.org/10.3390/nu13030931
APA StyleVilla, C., Bavaro, S. L., De Angelis, E., Pilolli, R., Costa, J., Barni, S., Novembre, E., Mafra, I., & Monaci, L. (2021). Milk Ingredients in Meat Products: Can Autoclaving and In Vitro Gastroduodenal Digestion Mitigate Their IgE-Binding Capacity? Nutrients, 13(3), 931. https://doi.org/10.3390/nu13030931











