Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. CPP Crystal-Induced Arthritis Development
2.3. Drugs
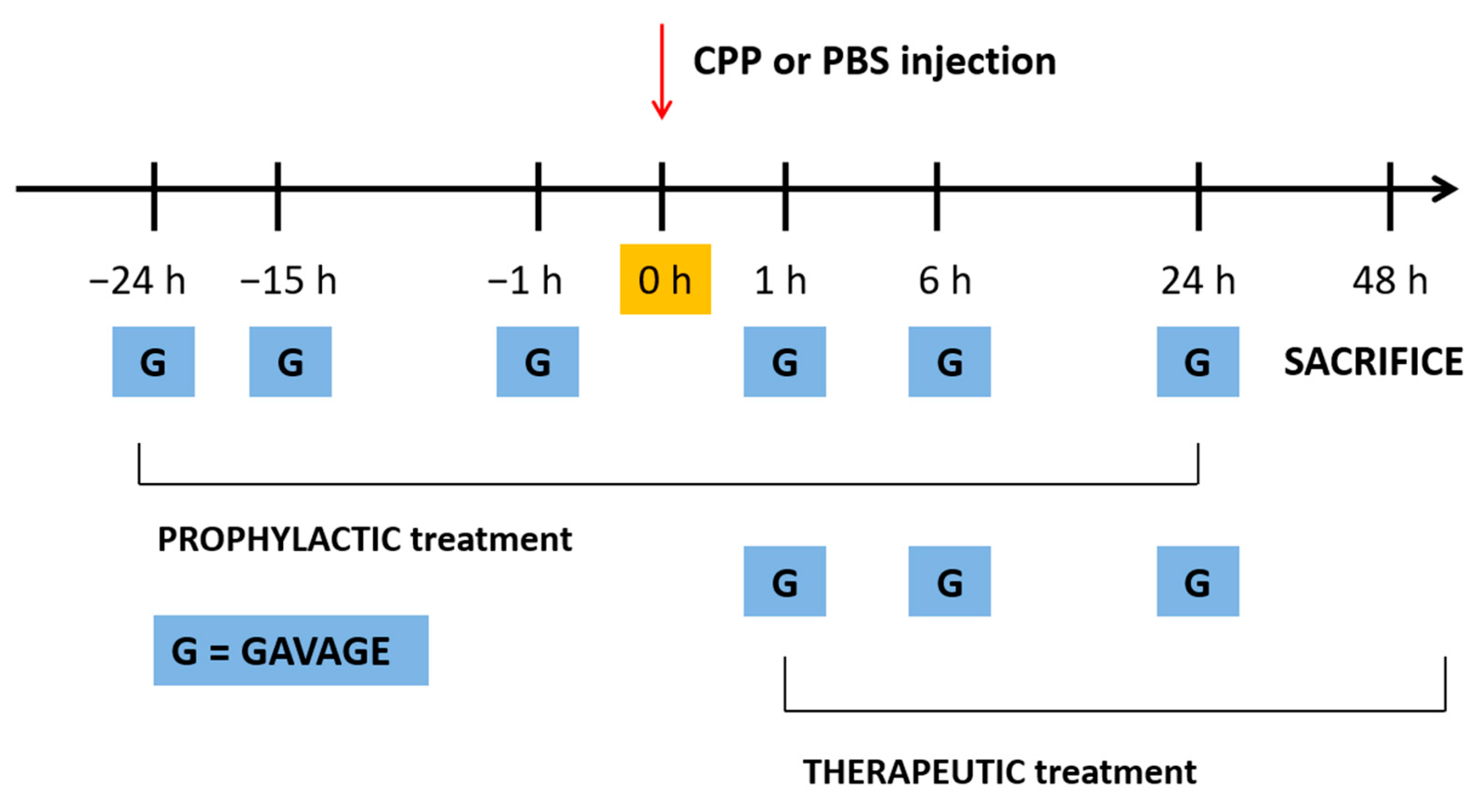
2.4. Treatment with PD and Colchicine
2.5. A Priori Sample Size Calculation, Primary and Secondary Outcomes
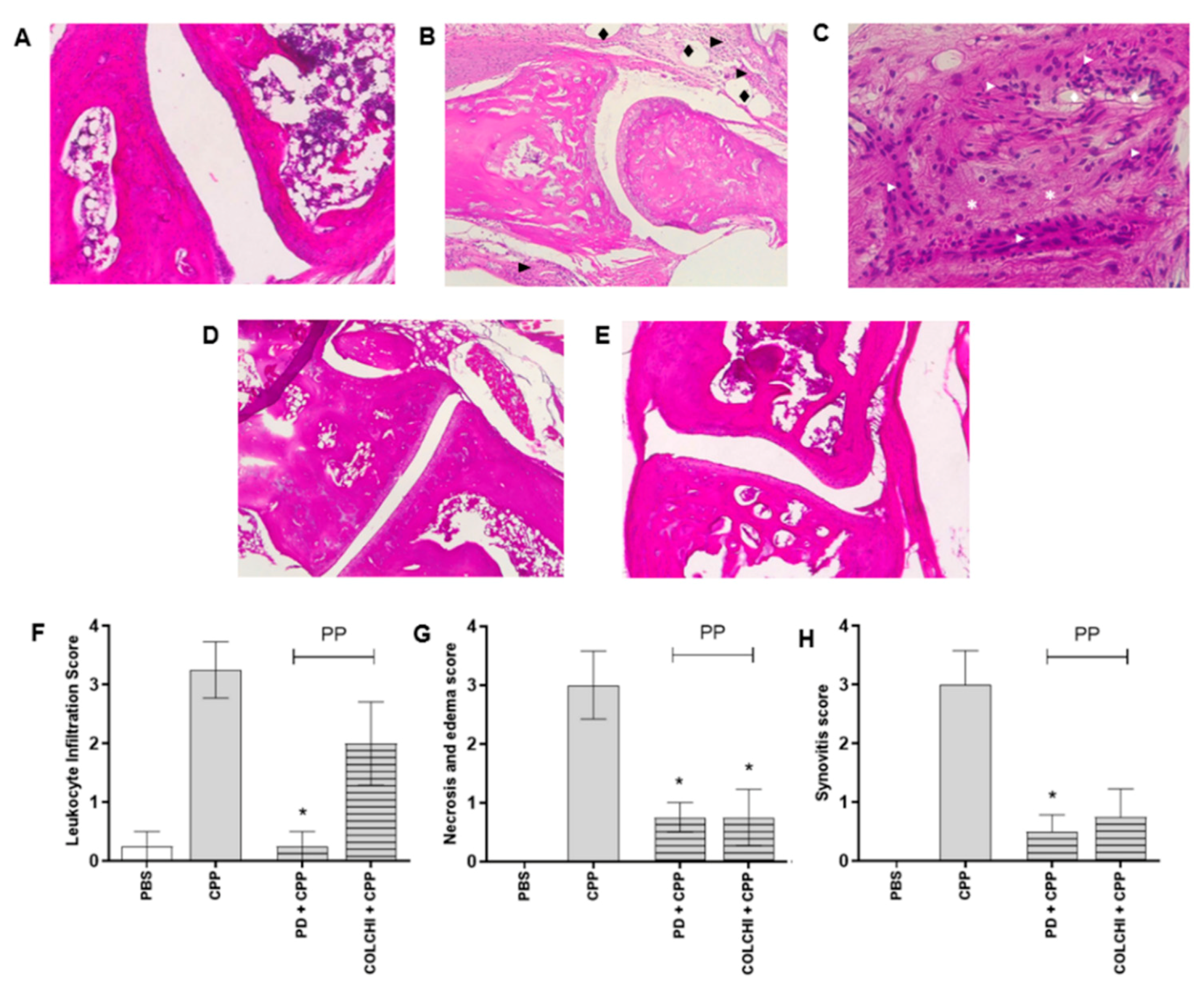
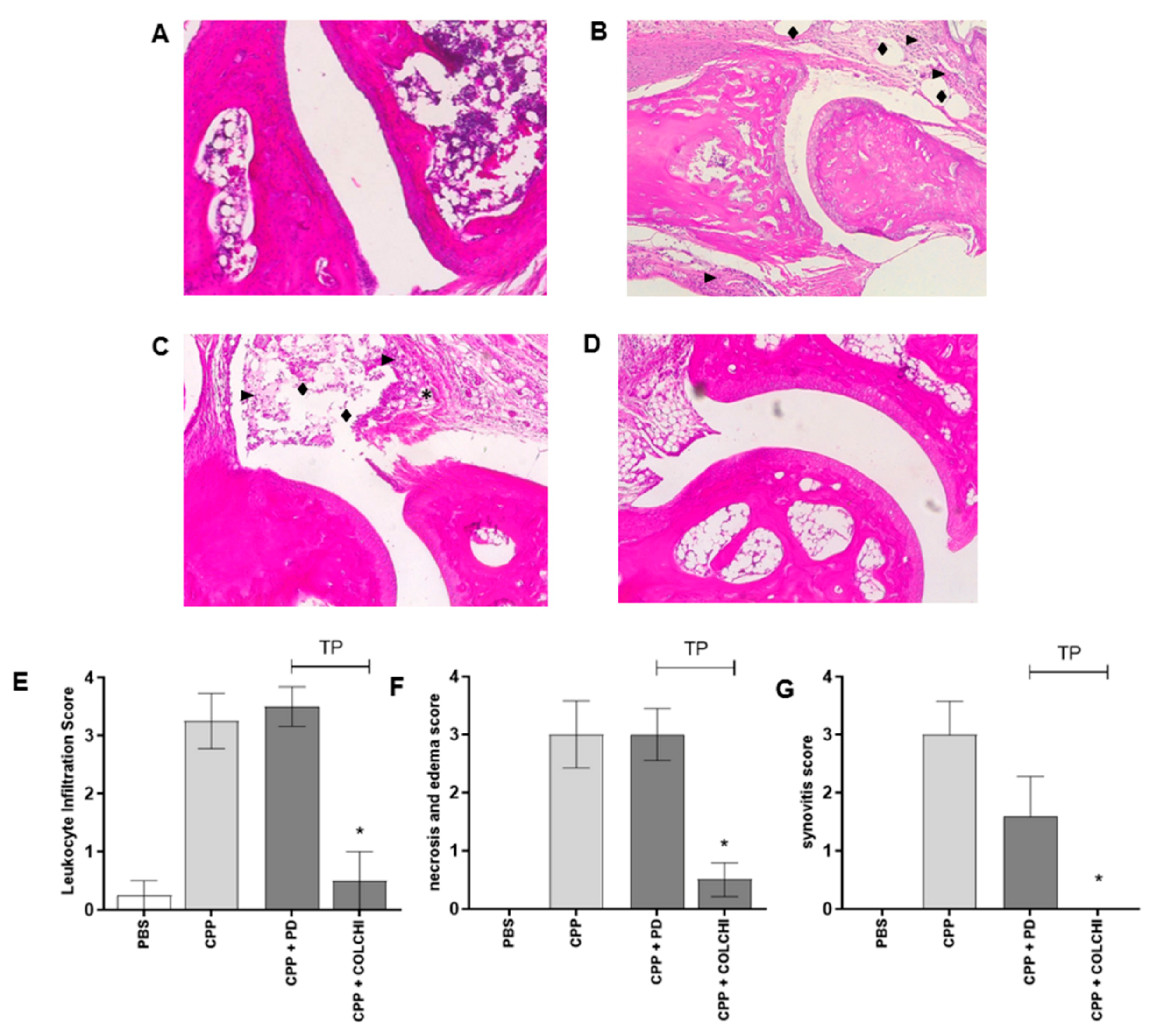
2.6. Histological Assessment
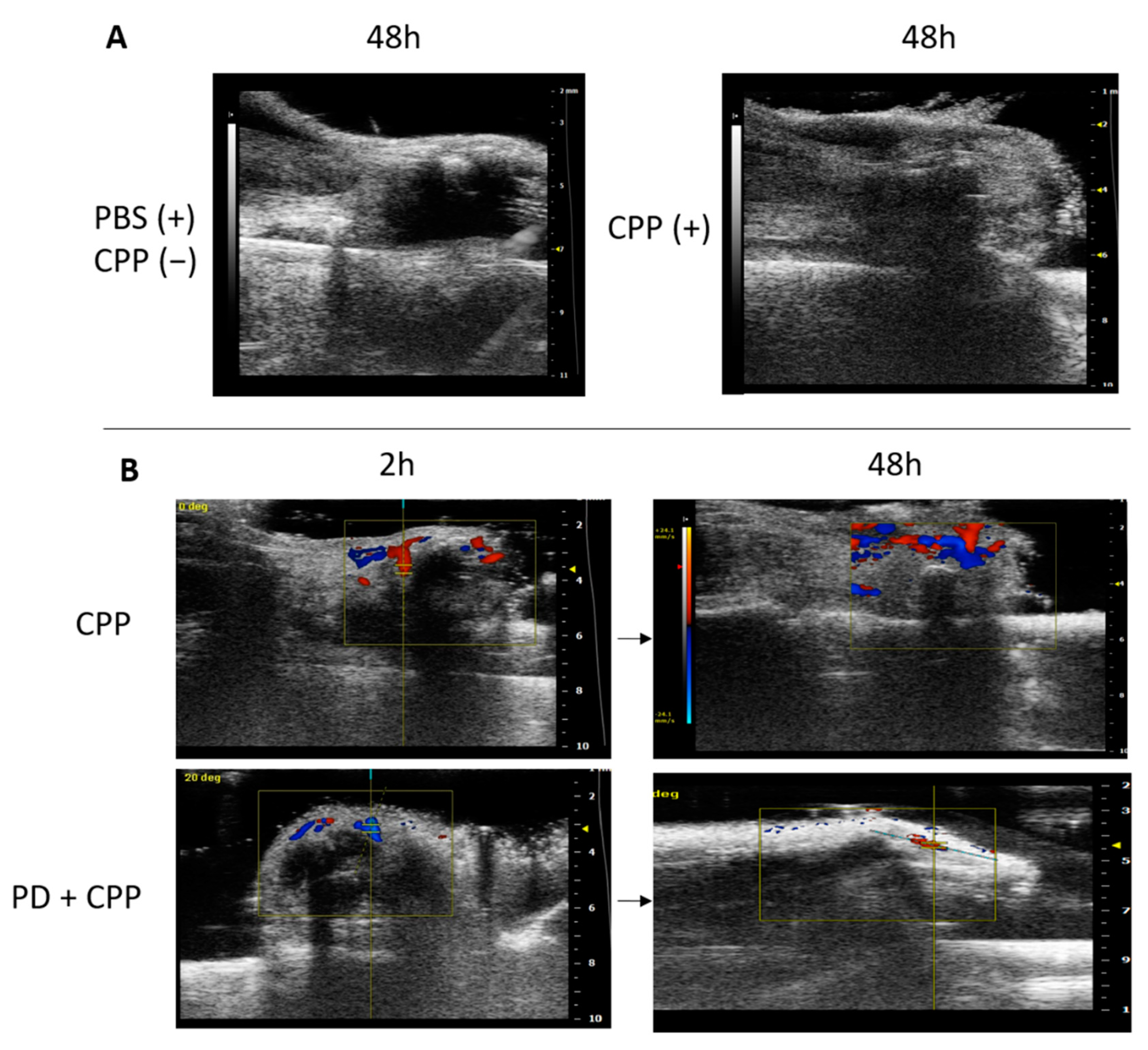
2.7. Ultrasound Assessment
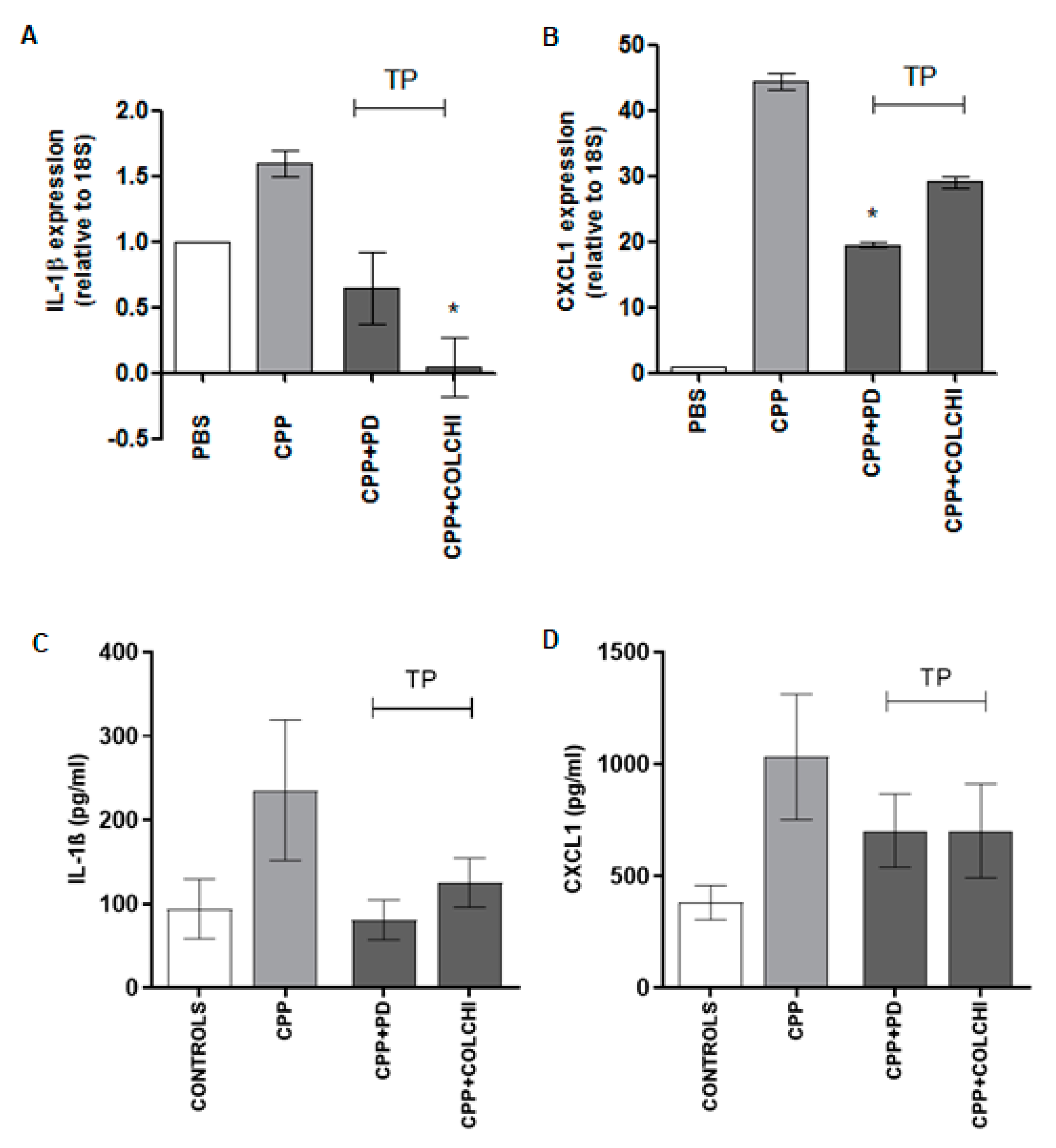
2.8. RNA Extraction from Ankle Joint and RT-PCR
- IL-1ß Forward: 5′-CGCAGCAGCACATCAACAAG-3′
- Reverse: 5′-GTGCTCATGTCCTCATCCTG-3′
- CXCL1 Forward: 5′-ATCCAGAGCTTGAAGGTGTTG-3′
- Reverse: 5′-GTCTGTCTTCTTTCTCCGTTACTT-3′
- 18. S Forward: 5′ GGGAGCCTGAGAAACGGC 3′
- Reverse: 5′ GGGTCGGGAGTGGGTAATTT 3′
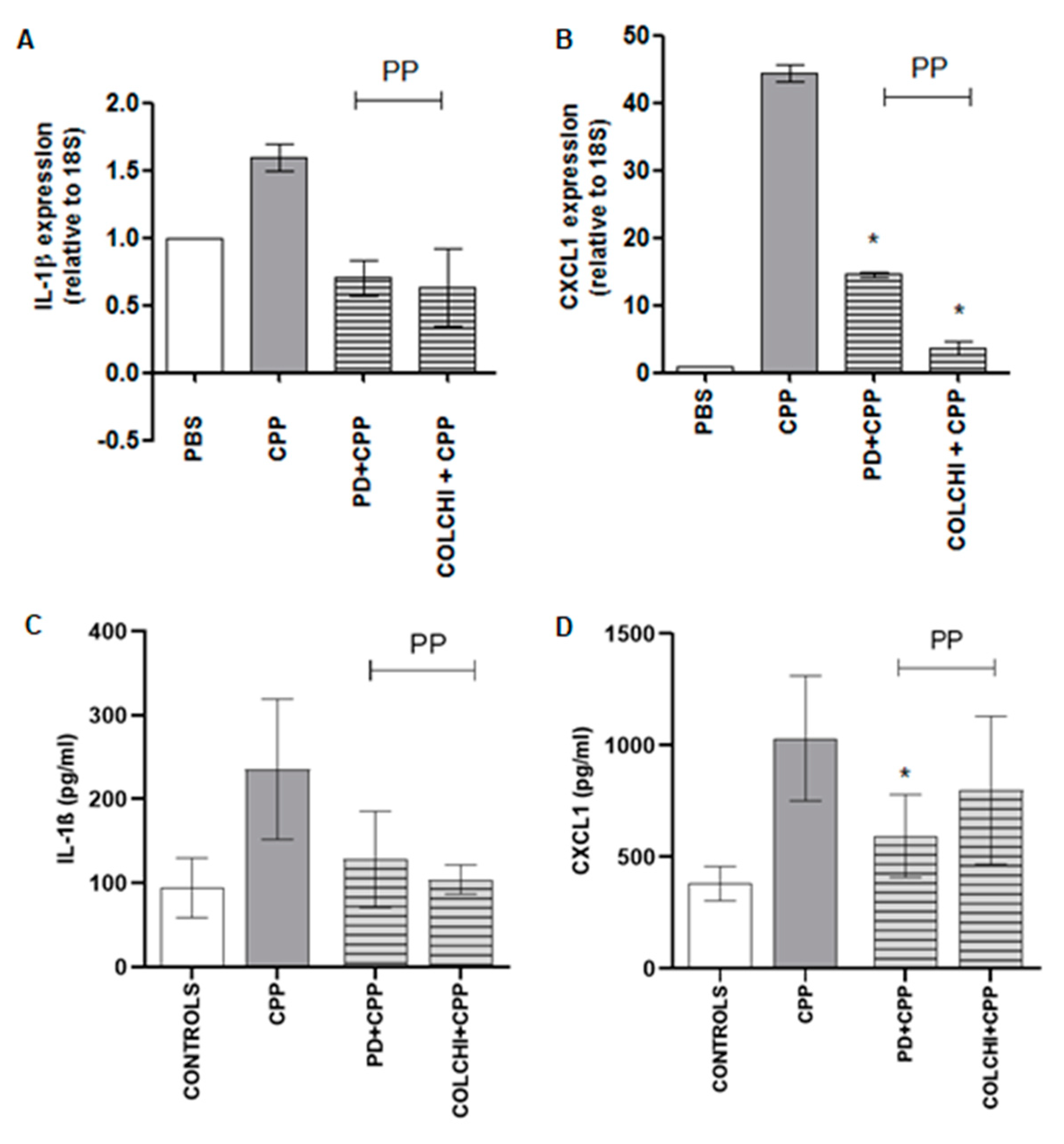
2.9. Serum Cytokine Determination
2.10. Statistical Analyses
3. Results
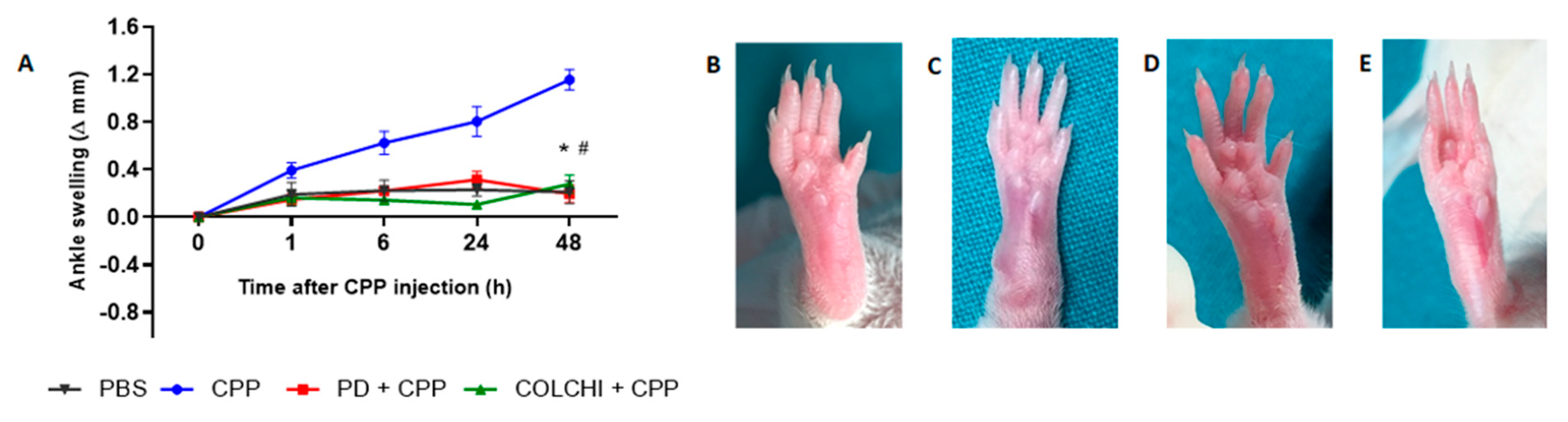
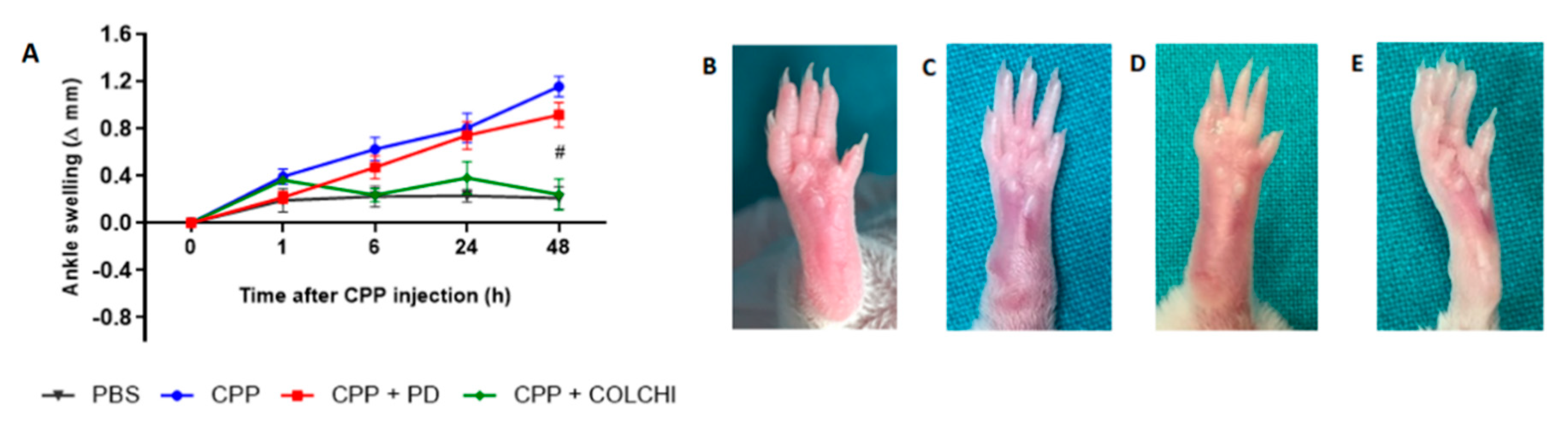
3.1. Prophylactic Oral Treatment with PD Prevents CPP Crystal-Induced Arthritis in Mice
3.2. Therapeutic Oral Treatment with PD Does Not Affect CPP Crystal-Induced Arthritis in Mice
3.3. Ultrasound Evaluation of CPP Crystal-Induced Arthritis in Mice Treated Prophylactically with Polydatin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Oliviero, F.; Bindoli, S.; Scanu, A.; Feist, E.; Doria, A.; Galozzi, P.; Sfriso, P. Autoinflammatory Mechanisms in Crystal-Induced Arthritis. Front. Med. 2020, 7, 166. [Google Scholar] [CrossRef]
- Abhishek, A.; Neogi, T.; Choi, H.; Doherty, M.; Rosenthal, A.K.; Terkeltaub, R. Unmet needs and the path forward in joint disease associated with calcium pyrophosphate crystal deposition. Arthritis Rheumatol. 2018, 70, 1182–1191. [Google Scholar] [CrossRef]
- Andrés, M.; Sivera, F.; Pascual, E. Therapy for CPPD: Options and evidence. Curr. Rheumatol. Rep. 2018, 20, 31. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, M. Update on calcium pyrophosphate deposition. Clin. Exp. Rheumatol. 2016, 34, 32–38. [Google Scholar]
- Zhang, W.; Doherty, M.; Pascual, E.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: Management. Ann. Rheum. Dis. 2011, 70, 571–575. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Wang, J.; Wu, Q.; Yan, H. Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-κB pathway after Mycoplasma pneumoniae infection. J. Cell Biochem. 2019, 120, 10137–10144. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Shen, L.; Yang, L.; Diao, W.; Yang, Z.; Zhang, Y.; Yu, S.; Li, Y. Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via Sonic hedgehog signaling pathway. Int. Immunopharmacol. 2018, 64, 256–263. [Google Scholar] [CrossRef]
- Martano, M.; Stiuso, P.; Facchiano, A.; De Maria, S.; Vanacore, D.; Restucci, B.; Rubini, C.; Caraglia, M.; Ravagnan, G.; Lo Muzio, L. Aryl hydrocarbon receptor, a tumor grade-associated marker of oral cancer, is directly downregulated by polydatin: A pilot study. Oncol. Rep. 2018, 40, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, G.; De Filippis, A.; Cartenì, M.; De Maria, S.; Cozza, V.; Petrazzuolo, M.; Tufano, M.A.; Donnarumma, G. Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation 2013, 36, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Zamudio-Cuevas, Y.; Belluzzi, E.; Andretto, L.; Scanu, A.; Favero, M.; Ramonda, R.; Ravagnan, G.; López-Reyes, A.; Spinella, P.; et al. Polydatin and resveratrol inhibit the inflammatory process induced by urate and pyrophosphate crystals in thp-1 cells. Foods 2019, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Starkl, P.; Balbino, B.; Sibilano, R.; Gaudenzio, N.; Rogalla, S.; Sensarn, S.; Kang, D.; Raghu, H.; Sokolove, J.; et al. The tyrosine kinase inhibitor imatinib mesylate suppresses uric acid crystal-induced acute gouty arthritis in mice. PLoS ONE 2017, 12, e0185704. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)). Method Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Galozzi, P.; Maschio, L.; Carraro, S.; Scanu, A.; Facco, M.; Oliviero, F. M2 macrophages as resolvers of crystal-induced inflammation. Rheumatology 2021. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, B.Y.; Nie, J.; Zhao, G.H.; Zhuo, J.Y.; Yuan, J.; Li, Y.C.; Wang, L.L.; Chen, Z.W. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp. Med. 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, J.; Xu, D.; Lu, Y. Polydatin prevents LPS-induced acute kidney injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 2019, 137, 103688. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Jo, K.; Lee, T.G.; Hyun, S.W.; Kim, J.S.; Kim, C.S. Polydatin Inhibits NLRP3 Inflammasome in Dry Eye Disease by Attenuating Oxidative Stress and Inhibiting the NF-κB Pathway. Nutrients 2019, 11, 2792. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tang, Q.; Jin, J.; Zheng, G.; Xu, J.; Huang, W.; Li, X.; Shang, P.; Liu, H. Polydatin inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018, 9, 1701–1712. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Chen, L.X.; Fang, L.; Zhang, Q. Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of NADPH oxidase and NF-κB activities. BMC Complement Med. 2020, 20, 378. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Huang, Y.; Li, H.; Yan, S.; Lin, J.; Chen, Y.; Wu, L.; Liu, B.; Wang, G.; et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int. J. Biol. Sci. 2018, 14, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, J.; Mo, J.; Guo, L.; Wu, X.; Bao, Y. Polydatin Inhibits adipose tissue inflammation and ameliorates lipid metabolism in high-fat-fed mice. BioMed Res. Int. 2019, 2019, 7196535. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, H.P.; Liu, C.Y.; Yu, L.T.; Wei, D.N.; Zhang, Z.C.; Lu, K.; Zhao, K.S.; Maegele, M.; Cai, D.Z.; et al. Polydatin prevents the induction of secondary brain injury after traumatic brain injury by protecting neuronal mitochondria. Neural. Regen. Res. 2019, 14, 1573–1582. [Google Scholar] [CrossRef]
- Yang, Q.B.; He, Y.L.; Zhong, X.W.; Xie, W.G.; Zhou, J.G. Resveratrol ameliorates gouty inflammation via upregulation of sirtuin 1 to promote autophagy in gout patients. Inflammopharmacology 2019, 27, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Lu, L.; Zou, H. Sirt1 inhibits gouty arthritis via activating PPARγ. Clin. Rheumatol. 2019, 38, 3235–3242. [Google Scholar] [CrossRef]
- Reber, L.L.; Marichal, T.; Sokolove, J.; Starkl, P.; Gaudenzio, N.; Iwakura, Y.; Karasuyama, H.; Schwartz, L.B.; Robinson, W.H.; Tsai, M.; et al. Contribution of mast cell-derived interleukin-1β to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014, 66, 2881–2891. [Google Scholar] [CrossRef]
- McCarthy, G.M.; Dunne, A. Calcium crystal deposition diseases—Beyond gout. Nat. Rev. Rheumatol. 2018, 14, 592–602. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliviero, F.; Galozzi, P.; Scanu, A.; Galuppini, F.; Lazzarin, V.; Brocco, S.; Ravagnan, G.; Sfriso, P.; Ramonda, R.; Spinella, P.; et al. Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice. Nutrients 2021, 13, 929. https://doi.org/10.3390/nu13030929
Oliviero F, Galozzi P, Scanu A, Galuppini F, Lazzarin V, Brocco S, Ravagnan G, Sfriso P, Ramonda R, Spinella P, et al. Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice. Nutrients. 2021; 13(3):929. https://doi.org/10.3390/nu13030929
Chicago/Turabian StyleOliviero, Francesca, Paola Galozzi, Anna Scanu, Francesca Galuppini, Vanni Lazzarin, Silvia Brocco, Giampietro Ravagnan, Paolo Sfriso, Roberta Ramonda, Paolo Spinella, and et al. 2021. "Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice" Nutrients 13, no. 3: 929. https://doi.org/10.3390/nu13030929
APA StyleOliviero, F., Galozzi, P., Scanu, A., Galuppini, F., Lazzarin, V., Brocco, S., Ravagnan, G., Sfriso, P., Ramonda, R., Spinella, P., Punzi, L., Pennelli, G., & Luisetto, R. (2021). Polydatin Prevents Calcium Pyrophosphate Crystal-Induced Arthritis in Mice. Nutrients, 13(3), 929. https://doi.org/10.3390/nu13030929









