Association between Serum Concentration of Carotenoid and Visceral Fat
Abstract
1. Introduction
2. Materials and Methods
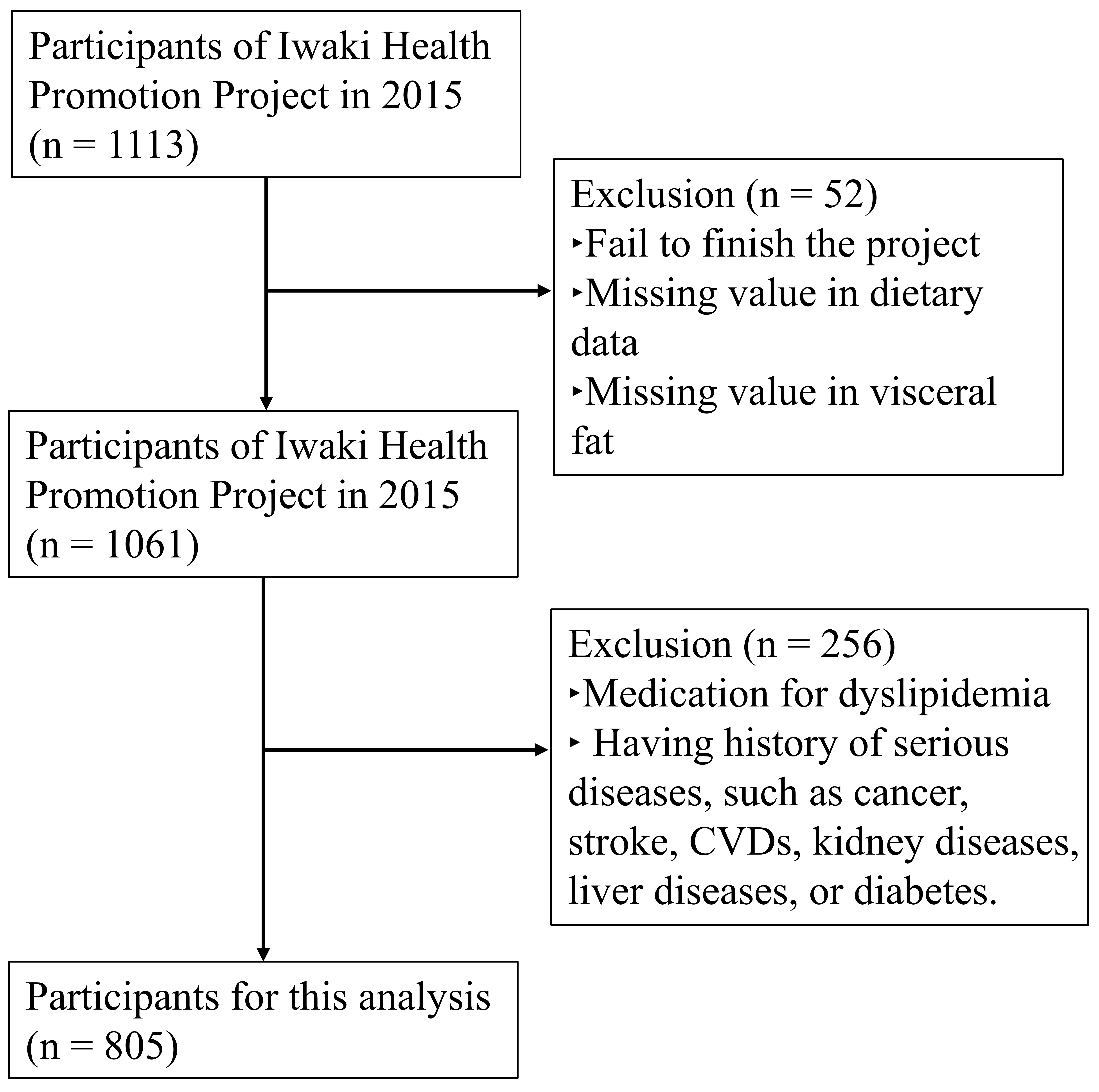
2.1. Study Design and Subjects
2.2. Body Measurements
2.3. Self-Administered Questionnaire
2.4. Blood Sampling and Testing
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Subjects
3.2. Relationship between Serum Carotenoid Concentrations and VFA/BMI
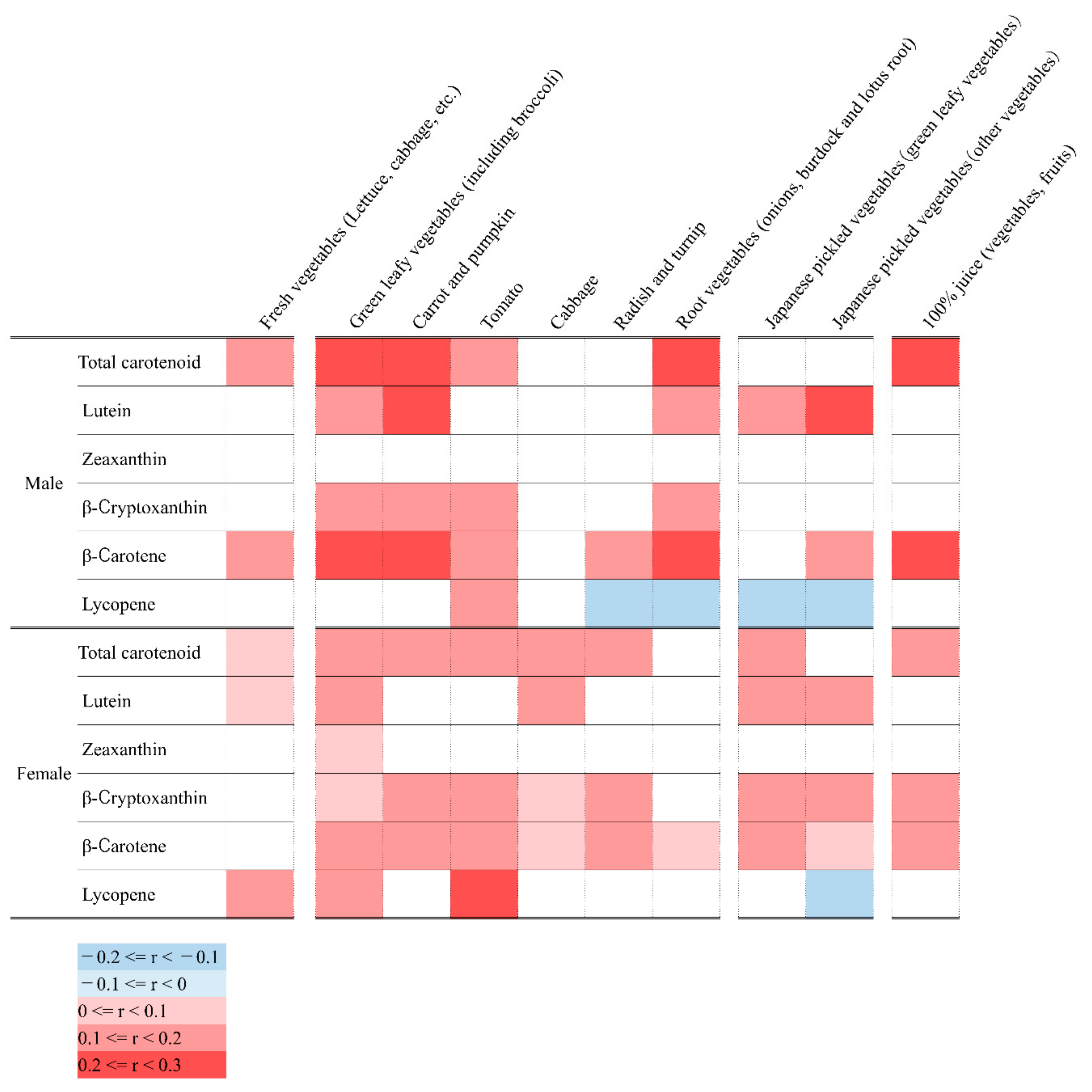
3.3. Relationship between Serum Concentration of Carotenoids and Food Consumed
4. Discussion
4.1. Relationship between Serum Carotenoid Levels and VFA
4.2. Relationship between Serum Concentrations of Lutein, β-Carotene, and β-Cryptoxanthin and VFA
4.3. Relationship between Lycopene Levels and VFA
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Wedick, N.M.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am. J. Clin. Nutr. 2013, 98, 1514–1523. [Google Scholar] [CrossRef]
- Takachi, R.; Inoue, M.; Ishihara, J.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Iso, H.; Tsubono, Y.; Tsugane, S. JPHC Study Group. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Center-Based Prospective Study. Am. J. Epidemiol. 2008, 167, 59–70. [Google Scholar] [CrossRef]
- Oude Griep, L.M.; Verschuren, W.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Colors of fruit and vegetables and 10-year incidence of stroke. Stroke 2011, 42, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Fan, J.H.; Dawsey, S.M.; Sinha, R.; Freedman, N.D.; Taylor, P.R.; Qiao, Y.L.; Abnet, C.C. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci. Rep. 2016, 6, 22619. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Matsumoto, M.; Waki, N.; Suganuma, H.; Takahashi, I.; Kurauchi, S.; Sawada, K.; Tokuda, I.; Misawa, M.; Ando, M.; Itoh, K.; et al. Association between Biomarkers of Cardiovascular Diseases and the Blood Concentration of Carotenoids among the General Population without Apparent Illness. Nutrients 2020, 12, 2310. [Google Scholar] [CrossRef]
- Matsumoto, M.; Suganuma, H.; Shimizu, S.; Hayashi, H.; Sawada, K.; Tokuda, I.; Ihara, K.; Nakaji, S. Skin Carotenoid Level as an Alternative Marker of Serum Total Carotenoid Concentration and Vegetable Intake Correlates with Biomarkers of Circulatory Diseases and Metabolic Syndrome. Nutrients 2020, 12, 1825. [Google Scholar] [CrossRef]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Ryo, M.; Kishida, K.; Nakamura, T.; Yoshizumi, T.; Funahashi, T.; Shimomura, I. Clinical significance of visceral adiposity assessed by computed tomography: A Japanese perspective. World J. Radiol. 2014, 6, 409–416. [Google Scholar] [CrossRef]
- Suzuki, K.; Inoue, T.; Hioki, R.; Ochiai, J.; Kusuhara, Y.; Ichino, N.; Osakabe, K.; Hamajima, N.; Ito, Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin. Nutr. 2006, 25, 780–789. [Google Scholar] [CrossRef]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and Insulin Resistance Are Inversely Associated with Serum and Adipose Tissue Carotenoid Concentrations in Adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Dohke, M.; Mizutome, N.; Kimura, R.; Makuri, A.; Matsui, A.; Hanawa, N.; Oashi, K. Evaluation of metabolic syndrome with respect to the waist circumference and visceral fat area. Ningen Dock 2008, 23, 3. (In Japanese) [Google Scholar]
- Sluijs, I.; Beulens, J.W.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef]
- Ryo, M.; Maeda, K.; Onda, T.; Katashima, M.; Okumiya, A.; Nishida, M.; Yamaguchi, T.; Funahashi, T.; Matsuzawa, Y.; Nakamura, T.; et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care 2005, 28, 451–453. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Imoto, S.; Ihara, K.; Nakaji, S. Association between Nutrients and Visceral Fat in Healthy Japanese Adults: A 2-Year Longitudinal Study Brief Title: Micronutrients Associated with Visceral Fat Accumulation. Nutrients 2019, 11, 2698. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Oshima, S.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Accumulation and clearance of capsanthin in blood plasma after the ingestion of paprika juice in men. J. Nutr. 1997, 127, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Inakuma, T. Quantitation of carotenoids in commonly consumed vegetables in Japan. Food Sci. Technol. Res. 2007, 13, 247–252. [Google Scholar] [CrossRef][Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Aroor, A.R.; DeMarco, V.G. Oxidative stress and obesity: The chicken or the egg? Diabetes 2014, 63, 2216–2218. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: A review. J. Oleo Sci. 2015, 64, 125–132. [Google Scholar] [CrossRef]
- Östh, M.; Öst, A.; Kjolhede, P.; Strålfors, P. The concentration of β-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS ONE 2014, 9, e85610. [Google Scholar] [CrossRef]
- Nakade, M.; Kurotani, M.; Susaki, H. Investigation of supplement usage and awareness in working adults. J. Jpn. Prim. Care Assoc. 2011, 34, 1. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Parikh, S.; Pollock, N.K.; Bhagatwala, J.; Guo, D.H.; Gutin, B.; Zhu, H.; Dong, Y. Adolescent fiber consumption is associated with visceral fat and inflammatory markers. J. Clin. Endocrinol. Metab. 2012, 97, E1451–E1457. [Google Scholar] [CrossRef]
- Geboers, B.; Reijneveld, S.A.; Jansen, C.J.; de Winter, A.F. Health Literacy Is Associated with Health Behaviors and Social Factors Among Older Adults: Results from the LifeLines Cohort Study. J. Health Commun. 2016, 21, 45–53. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Padhi, E.M.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophill (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Marcelino, G.; Machate , D.J.; Freitas, K.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Matsumoto, H.; Kato, M.; Ikoma, Y.; Yano, M.; Nagao, A. Multiple linear regression analysis of the seasonal changes in the serum concentration of beta-cryptoxanthin. J. Nutr. Sci. Vitaminol. 2004, 50, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sugiura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. Serum β-cryptoxanthin and β-carotene derived from Satsuma mandarin and brachial-ankle pulse wave velocity: The Mikkabi cohort study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 808–814. [Google Scholar] [CrossRef]
- Takayanagi, K. Prevention of Adiposity by the Oral Administration of β-Cryptoxanthin. Front. Neurol. 2011, 2. [Google Scholar] [CrossRef]
- Shirakura, Y.; Takayanagi, K.; Mukai, K.; Tanabe, H.; Inoue, M. β-cryptoxanthin suppresses the adipogenesis of 3T3-L1 cells via RAR activation. J. Nutr. Sci. Vitaminol. 2011, 57, 426–431. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yılmaz, I.; Juturu, V. β-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef]
- Takagi, T.; Hayashi, R.; Nakai, Y.; Okada, S.; Miyashita, R.; Yamada, M.; Mihara, Y.; Mizushima, K.; Morita, M.; Uchiyama, K.; et al. Dietary Intake of Carotenoid-Rich Vegetables Reduces Visceral Adiposity in Obese Japanese men-A Randomized, Double-Blind Trial. Nutrients 2020, 12, 2342. [Google Scholar] [CrossRef]
| Measurement Item | All | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Young (20–39 Years) | Middle-Aged (40–59 Years) | Old (≥60 Years) | All | Young (20–39 Years) | Middle-Aged (40–59 Years) | Old (≥60 Years) | ||
| N | 805 | 310 | 103 | 118 | 89 | 495 | 120 | 188 | 187 |
| Age, year | 51.1 ± 14.8 | 49.1 ± 14.8 | 32.5 ± 4.6 | 49.6 ± 5.5 | 67.7 ± 5.8 | 52.3 ± 14.6 ** | 32.5 ± 5.1 | 50 ± 5.8 | 67.3 ± 5.8 |
| Alcohol intake, g/day | 11 ± 19 | 21.8 ± 24.2 | 19.8 ± 25.6 | 25.8 ± 24.8 | 19 ± 20.8 | 4.2 ± 10.1 *** | 4.3 ± 9.9 | 6.2 ± 12.4 | 2.3 ± 6.9 b |
| Smoking, % | 19.5 | 32.6 | 37.9 | 37.3 | 20.2 | 11.3 | 11.7 | 17.6 | 4.8 |
| Habitual exercise, % | 9.2 | 11.3 | 12.6 | 11.9 | 9 | 7.9 | 9.2 | 6.9 | 8 |
| Medication, % | 19.3 | 16.5 | 2.9 | 13.6 | 36 | 21 | 0.8 | 11.2 | 43.9 |
| VFA, cm2 | 79.11 ± 42.28 | 102.81 ± 44.96 | 92.2 ± 47.62 | 108.1 ± 41.09 a | 108.07 ± 45.03 a | 64.27 ± 32.74 *** | 53.13 ± 32.36 | 63.31 ± 31.13 a | 72.4 ± 32.49 ab |
| BMI, kg/m2 | 22.58 ± 3.46 | 23.61 ± 3.23 | 23.14 ± 3.86 | 24.13 ± 2.87 | 23.47 ± 2.8 | 21.94 ± 3.45 *** | 21.22 ± 3.95 | 21.76 ± 3.16 | 22.58 ± 3.28 a |
| Total carotenoid, μg/mL | 1.47 ± 0.72 | 1.15 ± 0.53 | 1.07 ± 0.5 | 1.13 ± 0.53 | 1.28 ± 0.56 a | 1.67 ± 0.75 *** | 1.5 ± 0.68 | 1.61 ± 0.68 | 1.84 ± 0.84 ab |
| Lutein, μg/mL | 0.34 ± 0.14 | 0.3 ± 0.13 | 0.25 ± 0.1 | 0.31 ± 0.12 a | 0.35 ± 0.15 a | 0.36 ± 0.15 *** | 0.3 ± 0.11 | 0.35 ± 0.15 a | 0.41 ± 0.16 ab |
| Zeaxanthin, μg/mL | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 |
| β-Cryptoxanthin, μg/mL | 0.15 ± 0.1 | 0.11 ± 0.06 | 0.1 ± 0.05 | 0.1 ± 0.05 | 0.14 ± 0.08 ab | 0.17 ± 0.11 *** | 0.13 ± 0.06 | 0.17 ± 0.11 a | 0.21 ± 0.12 ab |
| α-Carotene, μg/mL | 0.17 ± 0.16 | 0.13 ± 0.14 | 0.13 ± 0.15 | 0.13 ± 0.13 | 0.14 ± 0.14 | 0.2 ± 0.16 *** | 0.21 ± 0.19 | 0.19 ± 0.12 | 0.21 ± 0.17 |
| β-Carotene, μg/mL | 0.5 ± 0.42 | 0.3 ± 0.28 | 0.25 ± 0.25 | 0.28 ± 0.28 | 0.39 ± 0.28 ab | 0.62 ± 0.44 *** | 0.5 ± 0.36 | 0.57 ± 0.36 a | 0.74 ± 0.52 ab |
| Lycopene, μg/mL | 0.25 ± 0.13 | 0.24 ± 0.13 | 0.28 ± 0.14 | 0.24 ± 0.12 | 0.19 ± 0.13 ab | 0.26 ± 0.14 | 0.3 ± 0.13 | 0.27 ± 0.13 | 0.21 ± 0.12 ab |
| BDHQ | |||||||||
| Total dietary fiber, g/day | 10.74 ± 4.40 | 11.11 ± 4.74 | 9.55 ± 3.52 | 10.58 ± 4.23 | 13.63 ± 5.56 ab | 10.51 ± 4.16 | 8.98 ± 3.75 | 10.36 ± 3.93 a | 11.63 ± 4.33 ab |
| Soluble dietary fiber, g/day | 2.66 ± 1.20 | 2.72 ± 1.32 | 2.33 ± 0.99 | 2.57 ± 1.18 | 3.38 ± 1.56 ab | 2.62 ± 1.13 | 2.26 ± 1.01 | 2.60 ± 1.08 a | 2.86 ± 1.19 a |
| Insoluble dietary fiber, g/day | 7.73 ± 3.05 | 8.04 ± 3.24 | 6.95 ± 2.50 | 7.67 ± 2.90 | 9.79 ± 3.72 ab | 7.54 ± 2.90 * | 6.46 ± 2.61 | 7.42 ± 2.71 a | 8.37 ± 3.04 ab |
| Fresh vegetables, g/day | 25 ± 19.88 | 25.31 ± 20.77 | 23.94 ± 20.38 | 26.56 ± 20.33 | 25.24 ± 21.89 | 24.8 ± 19.32 | 23.26 ± 18.81 | 26.33 ± 19.04 | 24.25 ± 19.91 |
| Green leafy vegetables, g/day | 28.89 ± 28.61 | 26.73 ± 27.81 | 26.09 ± 24.93 | 25.28 ± 29.7 | 29.4 ± 28.5 | 30.25 ± 29.04 | 27.64 ± 25.94 | 31.95 ± 31.01 | 30.21 ± 28.89 |
| Carrot and pumpkin, g/day | 16.77 ± 15.07 | 14.23 ± 13.52 | 12.9 ± 12.13 | 13.84 ± 14.64 | 16.28 ± 13.39 | 18.36 ± 15.78 *** | 17.08 ± 16.07 | 17.33 ± 14.01 | 20.22 ± 17.11 |
| Tomato, g/day | 19.39 ± 21.72 | 18.49 ± 19.96 | 17.35 ± 19.82 | 17.9 ± 19.31 | 0.58 ± 21.01 | 19.95 ± 22.76 | 17.14 ± 16.98 | 20.25 ± 23.81 | 21.45 ± 24.79 |
| Cabbage, g/day | 29.31 ± 26.34 | 30.27 ± 27.24 | 26.29 ± 22.66 | 30.84 ± 25.52 | 34.12 ± 33.34 | 28.71 ± 25.76 | 25.34 ± 23.09 | 27.5 ± 23.32 | 32.1 ± 29.23 |
| Radish and turnip, g/day | 15.31 ± 17.42 | 15.3 ± 18.84 | 12.86 ± 13.64 | 13.57 ± 17.25 | 20.4 ± 24.45 ab | 15.31 ± 16.49 | 13.12 ± 14.23 | 13.47 ± 14.28 | 18.57 ± 19.25 ab |
| Root vegetables, g/day | 29.88 ± 25.06 | 27.52 ± 26.27 | 24.74 ± 20.37 | 25.95 ± 24.16 | 32.83 ± 33.55 | 31.36 ± 24.18 * | 30.29 ± 23.55 | 31.42 ± 24.36 | 31.99 ± 24.49 |
| Pickled green leafy vegetables, g/day | 6.08 ± 8.96 | 6.84 ± 9.05 | 4.59 ± 5.33 | 6.1 ± 7.5 | 10.42 ± 12.67 ab | 5.6 ± 8.88 | 3.36 ± 6.37 | 4.15 ± 6.81 | 8.5 ± 11.08 ab |
| Pickled other vegetables, g/day | 8.17 ± 10.84 | 8.46 ± 11.18 | 3.35 ± 4.66 | 9.39 ± 11.66 a | 13.13 ± 13.39 ab | 7.98 ± 10.62 | 4.27 ± 6.55 | 6.06 ± 8.32 | 12.3 ± 13.09 ab |
| 100% juice (vegetables and fruits), g/day | 42.49 ± 74.38 | 39.83 ± 62.93 | 31.29 ± 50.86 | 40.6 ± 66.35 | 48.68 ± 69.92 | 44.16 ± 80.74 | 39.25 ± 58.06 | 41.69 ± 83.69 | 49.8 ± 89.8 |
| Carotenoids | Male | Female | ||
|---|---|---|---|---|
| VFA | BMI | VFA | BMI | |
| Total carotenoid | 0.003 | 0.050 | −0.151 *** | −0.192 *** |
| Lutein | −0.017 | −0.046 | −0.095 * | −0.179 *** |
| Zeaxanthin | 0.071 | 0.039 | −0.046 | −0.103 * |
| Β-Cryptoxanthin | −0.018 | 0.062 | −0.027 | −0.045 |
| Β-Carotene | 0.014 | 0.086 | −0.138 ** | −0.153 *** |
| Lycopene | 0.070 | 0.062 | −0.122 ** | −0.145 ** |
| Carotenoids | VFA | BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| All | 20–39 | 40–59 | 60– | All | 20–39 | 40–59 | 60– | |
| Total carotenoid | 0.012 | 0.039 | −0.119 | 0.121 | 0.052 | 0.094 | 0.017 | 0.104 |
| Lutein | −0.059 | −0.123 | −0.026 | −0.057 | −0.063 | −0.077 | −0.060 | 0.000 |
| Zeaxanthin | 0.053 | 0.007 | 0.035 | 0.131 | 0.031 | 0.067 | 0.048 | 0.021 |
| β-Cryptoxanthin | −0.012 | −0.001 | −0.036 | 0.058 | 0.073 | 0.114 | 0.093 | 0.101 |
| β-Carotene | 0.041 | 0.050 | −0.068 | 0.155 | 0.117 | 0.147 | 0.059 | 0.198 |
| Lycopene | 0.123 * | 0.189 | −0.058 | 0.212 * | 0.075 | 0.129 | 0.045 | 0.033 |
| Carotenoids | VFA | BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| All | 20–39 | 40–59 | 60– | All | 20–39 | 40–59 | 60– | |
| Total carotenoid | −0.210 *** | −0.256 ** | −0.250 ** | −0.180 * | −0.244 *** | −0.250 *** | −0.273 *** | −0.223 ** |
| Lutein | −0.192 *** | −0.307 ** | −0.168 * | −0.163 * | −0.258 *** | −0.323 *** | −0.234 ** | −0.224 ** |
| Zeaxanthin | −0.039 | −0.147 | −0.043 | 0.017 | −0.010 * | −0.052 | −0.135 | −0.104 |
| β-Cryptoxanthin | −0.110 * | −0.167 | −0.144 | −0.058 | −0.113 * | −0.107 | −0.162 * | −0.065 |
| β-Carotene | −0.239 *** | −0.263 ** | −0.253 ** | −0.226 ** | −0.237 *** | −0.231 * | −0.248 ** | −0.220 ** |
| Lycopene | −0.020 | −0.008 | −0.107 | 0.076 | −0.078 | −0.162 | −0.109 | −0.011 |
| Total Carotenoid | r | Lutein | r | Zeaxanthin | r | β-Cryptoxanthin | r | β-Carotene | r | Lycopene | r | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive Correlation | 1 | Other fruits | 0.27 | Japanese pickled (other vegetables) | 0.27 | Egg | 0.30 | Fruits high in vitamin C | 0.26 | Other fruits | 0.37 | Ham | 0.20 |
| 2 | Carrot and pumpkin | 0.26 | Seaweed | 0.24 | Pork and beef | 0.15 | Other fruits | 0.26 | Carrot and pumpkin | 0.29 | Pasta | 0.19 | |
| 3 | 100% juice | 0.25 | “Natto” | 0.21 | Ham | 0.13 | Citrus | 0.26 | Root vegetables | 0.27 | Hamburger | 0.16 | |
| 4 | Egg | 0.23 | Carrot and pumpkin | 0.20 | Japanese liquor | 0.13 | Japanese sweets | 0.18 | Fruits high in vitamin C | 0.26 | Tomato | 0.15 | |
| 5 | Green leafy vegetables | 0.23 | Root vegetables | 0.19 | - | “Natto” | 0.17 | 100% juice | 0.25 | Stir-fried meat | 0.15 | ||
| Negative Correlation | 1 | Beer | −0.25 | Hamburger | −0.14 | Fatty fish | −0.20 | Beer | −0.35 | Beer | −0.39 | “Natto” | −0.20 |
| 2 | “Ramen” | −0.20 | Coke | −0.14 | Raw fish | −0.13 | “Ramen” | −0.17 | “Ramen” | −0.24 | Japanese sake | −0.20 | |
| 3 | Liver | −0.11 | Ice cream | −0.13 | Fried fish | −0.11 | Japanese liquor | −0.14 | Japanese liquor | −0.15 | Japanese pickled (other vegetables) | −0.17 | |
| 4 | - | - | - | - | Liver | −0.11 | Fatty fish | −0.17 | |||||
| 5 | - | - | - | - | - | Dried fish | −0.16 |
| Total Carotenoid | r | Lutein | r | Zeaxanthin | r | β-Cryptoxanthin | r | β-Carotene | r | Lycopene | r | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive Correlation | 1 | Other fruits | 0.22 | “Natto” | 0.22 | Egg | 0.23 | Citrus | 0.30 | Grilled fish | 0.25 | Tomato | 0.25 |
| 2 | Fruits high in vitamin C | 0.20 | Grilled fish | 0.19 | Coffee | 0.10 | Other fruits | 0.28 | Other fruits | 0.23 | Hamburger | 0.23 | |
| 3 | “Tofu” | 0.20 | “Tofu” | 0.18 | Green leafy vegetables | 0.10 | Fruits high in vitamin C | 0.22 | “Tofu” | 0.21 | Fruits high in vitamin C | 0.15 | |
| 4 | Grilled fish | 0.20 | Green leafy vegetables | 0.17 | - | Grilled fish | 0.21 | Fruits high in vitamin C | 0.21 | Pasta | 0.14 | ||
| 5 | 100% juice | 0.18 | Fatty fish | 0.16 | - | Fatty fish | 0.20 | “Natto” | 0.19 | Fresh vegetables | 0.12 | ||
| Negative Correlation | 1 | Beer | −0.20 | Hamburger | −0.18 | Fried fish | −0.10 | Beer | −0.23 | Beer | −0.30 | Japanese pickled (other vegetables) | −0.18 |
| 2 | “Ramen” | −0.15 | Fried meat | −0.16 | - | Ham | −0.11 | “Ramen” | −0.18 | Boiled fish | −0.15 | ||
| 3 | Fried meat | −0.12 | Coke | −0.13 | - | “Ramen” | −0.10 | Japanese liquor | −0.14 | Grilled fish | −0.15 | ||
| 4 | Japanese liquor | −0.10 | Ham | −0.13 | - | Fried food | −0.09 | Fried meat | −0.13 | Rice | −0.15 | ||
| 5 | Coke | −0.10 | Fried meat | −0.12 | - | - | Hamburger | −0.12 | Dried fish | −0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, M.; Suganuma, H.; Ozato, N.; Shimizu, S.; Katashima, M.; Katsuragi, Y.; Mikami, T.; Itoh, K.; Nakaji, S. Association between Serum Concentration of Carotenoid and Visceral Fat. Nutrients 2021, 13, 912. https://doi.org/10.3390/nu13030912
Matsumoto M, Suganuma H, Ozato N, Shimizu S, Katashima M, Katsuragi Y, Mikami T, Itoh K, Nakaji S. Association between Serum Concentration of Carotenoid and Visceral Fat. Nutrients. 2021; 13(3):912. https://doi.org/10.3390/nu13030912
Chicago/Turabian StyleMatsumoto, Mai, Hiroyuki Suganuma, Naoki Ozato, Sunao Shimizu, Mitsuhiro Katashima, Yoshihisa Katsuragi, Tatsuya Mikami, Ken Itoh, and Shigeyuki Nakaji. 2021. "Association between Serum Concentration of Carotenoid and Visceral Fat" Nutrients 13, no. 3: 912. https://doi.org/10.3390/nu13030912
APA StyleMatsumoto, M., Suganuma, H., Ozato, N., Shimizu, S., Katashima, M., Katsuragi, Y., Mikami, T., Itoh, K., & Nakaji, S. (2021). Association between Serum Concentration of Carotenoid and Visceral Fat. Nutrients, 13(3), 912. https://doi.org/10.3390/nu13030912






