Probiotic Escherichia coli Ameliorates Antibiotic-Associated Anxiety Responses in Mice
Abstract
1. Introduction
2. Materials and Methods
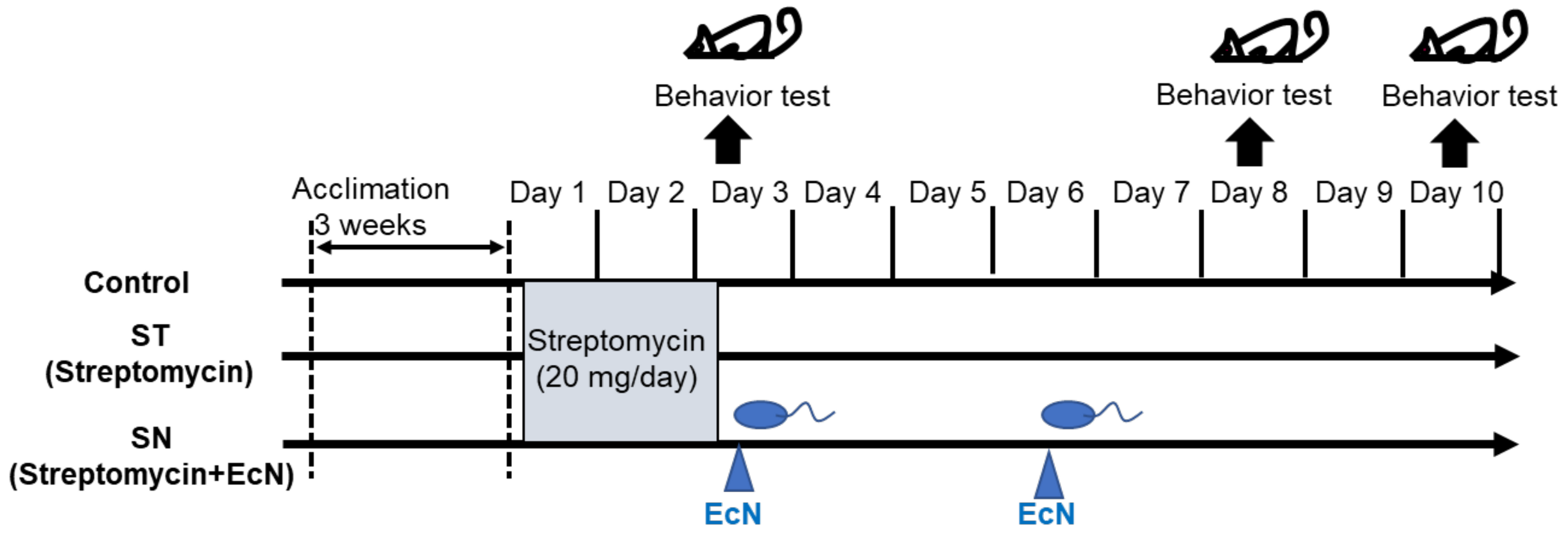
2.1. Animal Experiments
2.2. Behavior Tests for Anxiety
2.3. Statistical Analysis
3. Results
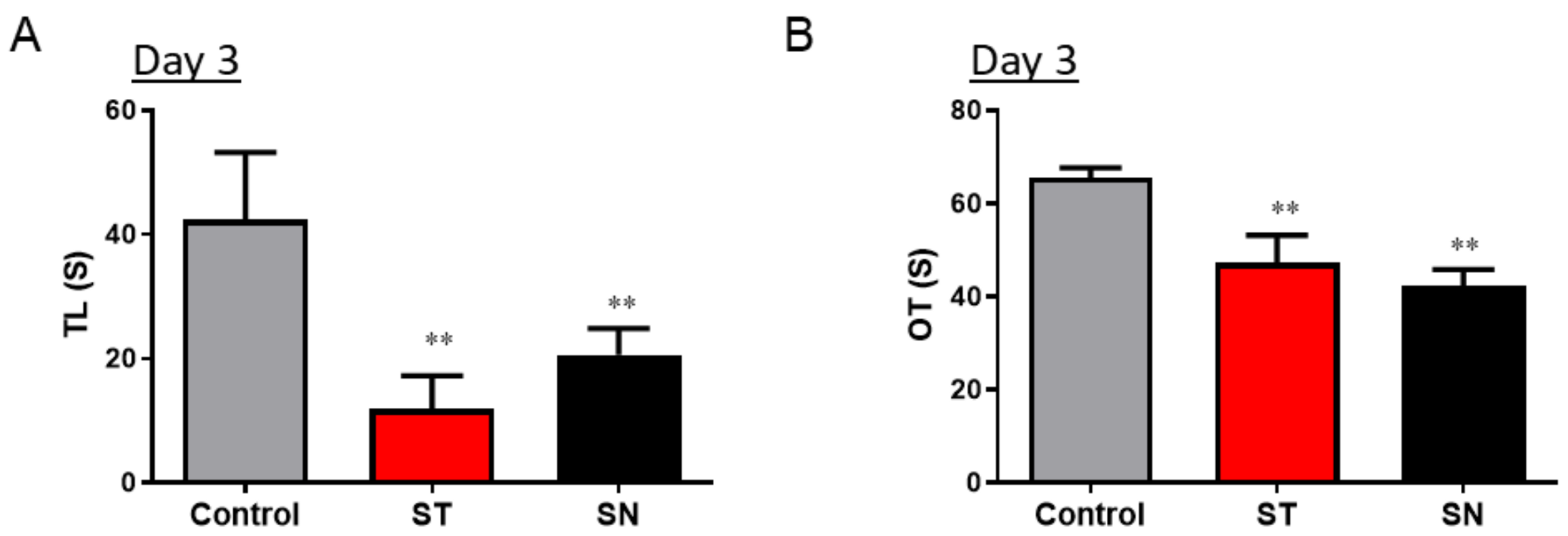
3.1. Streptomycin Treatment Causes Acute Anxiety in Mice
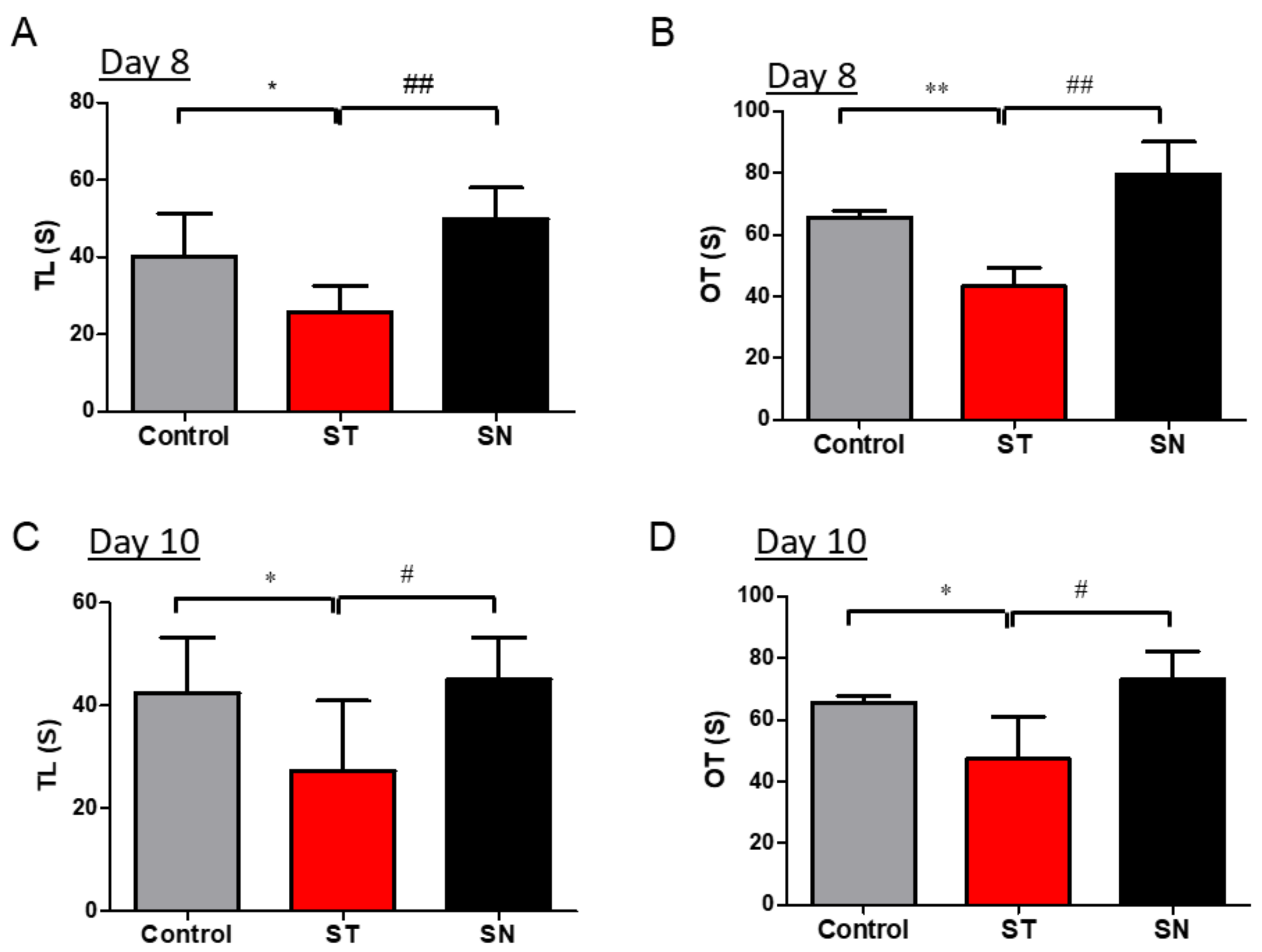
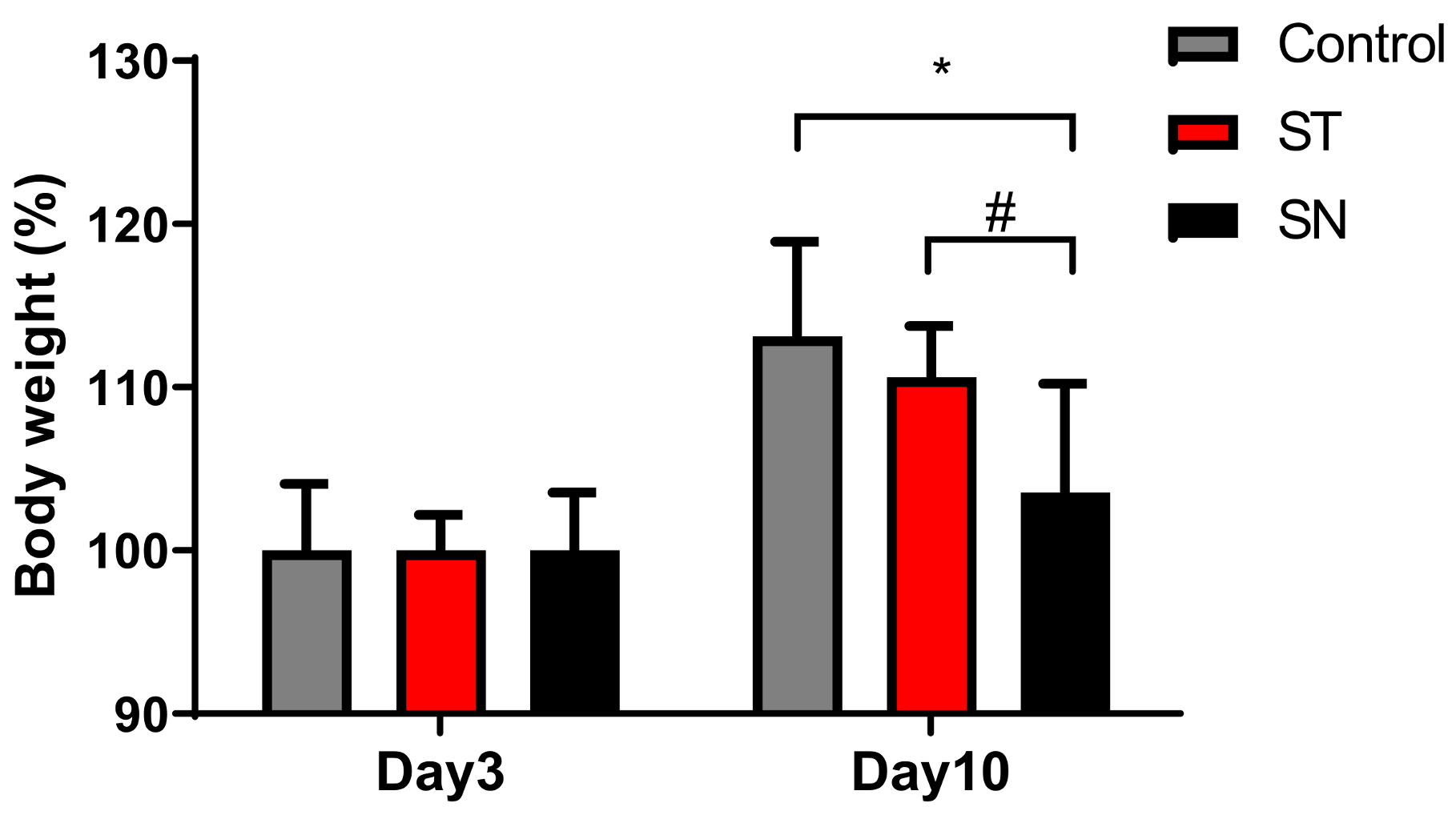
3.2. EcN Supplementation Alleviates Streptomycin-Induced Anxiety in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Prayle, A.; Watson, A.; Fortnum, H.; Smyth, A. Side effects of aminoglycosides on the kidney, ear and balance in cystic fibrosis. Thorax 2010, 65, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Bazett, M.; Bergeron, M.E.; Haston, C.K. Streptomycin treatment alters the intestinal microbiome, pulmonary T cell profile and airway hyperresponsiveness in a cystic fibrosis mouse model. Sci. Rep. 2016, 6, 19189. [Google Scholar] [CrossRef]
- Pindling, S.; Azulai, D.; Zheng, B.; Dahan, D.; Perron, G.G. Dysbiosis and early mortality in zebrafish larvae exposed to subclinical concentrations of streptomycin. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, H.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018, 11, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. BMJ 2002, 324, 1361. [Google Scholar]
- Duncker, S.C.; Lorentz, A.; Schroeder, B.; Breves, G.; Bischoff, S.C. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet. Immunol. Immunopathol. 2006, 111, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schutz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef]
- Hancock, V.; Dahl, M.; Klemm, P. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J. Med. Microbiol. 2010, 59, 392–399. [Google Scholar] [CrossRef]
- Blum, G.; Marre, R.; Hacker, J. Properties of Escherichia coli strains of serotype O6. Infection 1995, 23, 234–236. [Google Scholar] [CrossRef]
- Schulze, J.; Sonnenborn, U. Re.: Oral administration of a certain strain of live Escherichia coli for intestinal disorders? Infection 1995, 23, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Schulze, U.S.J. The non-pathogenic Escherichia coli strain Nissle 1917—Features of a versatile probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar]
- Kim, J.; Moon, Y. Worm-Based Alternate Assessment of Probiotic Intervention against Gut Barrier Infection. Nutrients 2019, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kim, J.; Ahn, J.H.; Moon, Y. Nononcogenic restoration of the intestinal barrier by E. coli-delivered human EGF. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Adediran, J.; Leatham-Jensen, M.P.; Mokszycki, M.E.; Frimodt-Moller, J.; Krogfelt, K.A.; Kazmierczak, K.; Kenney, L.J.; Conway, T.; Cohen, P.S. An Escherichia coli Nissle 1917 missense mutant colonizes the streptomycin-treated mouse intestine better than the wild type but is not a better probiotic. Infect. Immun. 2014, 82, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Maltby, R.; Leatham-Jensen, M.P.; Gibson, T.; Cohen, P.S.; Conway, T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS ONE 2013, 8, e53957. [Google Scholar] [CrossRef]
- Schinner, S.A.; Mokszycki, M.E.; Adediran, J.; Leatham-Jensen, M.; Conway, T.; Cohen, P.S. Escherichia coli EDL933 requires gluconeogenic nutrients to successfully colonize the intestines of streptomycin-treated mice precolonized with E. coli Nissle 1917. Infect. Immun. 2015, 83, 1983–1991. [Google Scholar] [CrossRef]
- Abdulkadir, S.A.; Qu, Z.; Garabedian, E.; Song, S.K.; Peters, T.J.; Svaren, J.; Carbone, J.M.; Naughton, C.K.; Catalona, W.J.; Ackerman, J.J.; et al. Impaired prostate tumorigenesis in Egr1-deficient mice. Nat. Med. 2001, 7, 101–107. [Google Scholar] [CrossRef]
- Henker, J.; Muller, S.; Laass, M.W.; Schreiner, A.; Schulze, J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: An open-label pilot study. Z. Gastroenterol. 2008, 46, 874–875. [Google Scholar] [CrossRef]
- Matthes, H.; Krummenerl, T.; Giensch, M.; Wolff, C.; Schulze, J. Clinical trial: Probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement. Altern. Med. 2010, 10, 1–8. [Google Scholar] [CrossRef]
- Plassmann, D.; Schulte-Witte, H. Treatment of irritable bowel syndrome with Escherichia coli strain Nissle 1917 (EcN): A retrospective survey. Med. Klin. 2007, 102, 888–892. [Google Scholar]
- Gronbach, K.; Eberle, U.; Muller, M.; Olschlager, T.A.; Dobrindt, U.; Leithauser, F.; Niess, J.H.; Doring, G.; Reimann, J.; Autenrieth, I.B.; et al. Safety of probiotic Escherichia coli strain Nissle 1917 depends on intestinal microbiota and adaptive immunity of the host. Infect. Immun. 2010, 78, 3036. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Mackos, A.R.; Varaljay, V.A.; Maltz, R.; Gur, T.L.; Bailey, M.T. Role of the Intestinal Microbiota in Host Responses to Stressor Exposure. Int. Rev. Neurobiol. 2016, 131, 1–19. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; Macqueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Lurie, I.; Yang, Y.X.; Haynes, K.; Mamtani, R.; Boursi, B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: A nested case-control study. J. Clin. Psychiatry 2015, 76, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Lavebratt, C.; Yang, L.L.; Giacobini, M.; Forsell, Y.; Schalling, M.; Partonen, T.; Gissler, M. Early exposure to antibiotic drugs and risk for psychiatric disorders: A population-based study. Transl. Psychiatry 2019, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ye, J.; Wen, Y.; Li, P.; Cheng, B.; Cheng, S.; Liu, L.; Zhang, L.; Ma, M.; Qi, X.; et al. Long-term antibiotic use during early life and risks to mental traits: An observational study and gene-environment-wide interaction study in UK Biobank cohort. Neuropsychopharmacology 2020. [Google Scholar] [CrossRef] [PubMed]
- Ceylani, T.; Jakubowska-Dogru, E.; Gurbanov, R.; Teker, H.T.; Gozen, A.G. The effects of repeated antibiotic administration to juvenile BALB/c mice on the microbiota status and animal behavior at the adult age. Heliyon 2018, 4, e00644. [Google Scholar] [CrossRef]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Dauge, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, B.P.; Krych, L.; Pedersen, T.B.; Plath, N.; Redrobe, J.P.; Hansen, A.K.; Nielsen, D.S.; Pedersen, C.S.; Larsen, C.; Sorensen, D.B. Investigating the long-term effect of subchronic phencyclidine-treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiol. Behav. 2015, 141, 32–39. [Google Scholar] [CrossRef]
- Chao, L.; Liu, C.; Sutthawongwadee, S.; Li, Y.; Lv, W.; Chen, W.; Yu, L.; Zhou, J.; Guo, A.; Li, Z.; et al. Effects of Probiotics on Depressive or Anxiety Variables in Healthy Participants under Stress Conditions or With a Depressive or Anxiety Diagnosis: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2020, 11, 421. [Google Scholar] [CrossRef]
- Partrick, K.A.; Rosenhauer, A.M.; Auger, J.; Arnold, A.R.; Ronczkowski, N.M.; Jackson, L.M.; Lord, M.N.; Abdulla, S.M.; Chassaing, B.; Huhman, K.L. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 2021, 11, 3763. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.F.; Ma, C.L.; Wei, H.; Li, B.M.; Luo, J. Alleviation of Anxiety/Depressive-Like Behaviors and Improvement of Cognitive Functions by Lactobacillus plantarum WLPL04 in Chronically Stressed Mice. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6613903. [Google Scholar] [CrossRef]
- Kruis, W.; Chrubasik, S.; Boehm, S.; Stange, C.; Schulze, J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int. J. Colorectal. Dis. 2012, 27, 467–474. [Google Scholar] [CrossRef][Green Version]
- Tannock, G.W.; Tiong, I.S.; Priest, P.; Munro, K.; Taylor, C.; Richardson, A.; Schultz, M. Testing probiotic strain Escherichia coli Nissle 1917 (Mutaflor) for its ability to reduce carriage of multidrug-resistant E. coli by elderly residents in long-term care facilities. J. Med. Microbiol. 2011, 60, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 2008, 22, e1088. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Anderson, S.E.; Cohen, P.; Naumova, E.N.; Must, A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch. Pediatr. Adolesc. Med. 2006, 160, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rofey, D.L.; Kolko, R.P.; Iosif, A.M.; Silk, J.S.; Bost, J.E.; Feng, W.; Szigethy, E.M.; Noll, R.B.; Ryan, N.D.; Dahl, R.E. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum. Dev. 2009, 40, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, H.; An, J.J.; Houtz, J.; Tan, J.W.; Xu, H.; Liao, G.Y.; Xu, Z.X.; Xu, B. Activation of Anxiogenic Circuits Instigates Resistance to Diet-Induced Obesity via Increased Energy Expenditure. Cell Metab. 2019, 29, 917–931.e4. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef]
- Helaly, A.M.N.; El-Attar, Y.A.; Khalil, M.; Ahmed Ghorab, D.S.E.; El-Mansoury, A.M. Antibiotic Abuse Induced Histopathological and Neurobehavioral Disorders in Mice. Curr. Drug Saf. 2019, 14, 199–208. [Google Scholar] [CrossRef]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 15062. [Google Scholar] [CrossRef]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef]
- Schubert, A.M.; Sinani, H.; Schloss, P.D. Antibiotic-Induced Alterations of the Murine Gut Microbiota and Subsequent Effects on Colonization Resistance against Clostridium difficile. MBio 2015, 6, e00974. [Google Scholar] [CrossRef] [PubMed]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016, 1, 16140. [Google Scholar] [CrossRef] [PubMed]
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Nissle, A. Mutaflor and its medical significance. Z. Klin. Med. 1951, 2, 68. [Google Scholar]
- Schultz, M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 1012–1018. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukas, M.; Fixa, B.; Kascak, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef]
- Fabian, E.; Elmadfa, I. The effect of daily consumption of probiotic and conventional yoghurt on oxidant and anti-oxidant parameters in plasma of young healthy women. Int. J. Vitam. Nutr. Res. 2007, 77, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res 2008, 43, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef]
- Von Buenau, R.; Jaekel, L.; Schubotz, E.; Schwarz, S.; Stroff, T.; Krueger, M. Escherichia coli strain Nissle 1917: Significant reduction of neonatal calf diarrhea. J. Dairy Sci. 2005, 88, 317–323. [Google Scholar] [CrossRef]
- Cukrowska, B.; LodInova-ZadnIkova, R.; Enders, C.; Sonnenborn, U.; Schulze, J.; Tlaskalova-Hogenova, H. Specific proliferative and antibody responses of premature infants to intestinal colonization with nonpathogenic probiotic E. coli strain Nissle 1917. Scand. J. Immunol. 2002, 55, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, L.; Zhang, Y.; Walzem, R.L.; Pendergast, J.S.; Printz, R.L.; Morris, L.C.; Matafonova, E.; Stien, X.; Kang, L.; et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Investig. 2014, 124, 3391–3406. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Park, S.; Nagappan, A.; Ray, N.; Kim, J.; Yoon, S.; Moon, Y. Probiotic Escherichia coli Ameliorates Antibiotic-Associated Anxiety Responses in Mice. Nutrients 2021, 13, 811. https://doi.org/10.3390/nu13030811
Park K, Park S, Nagappan A, Ray N, Kim J, Yoon S, Moon Y. Probiotic Escherichia coli Ameliorates Antibiotic-Associated Anxiety Responses in Mice. Nutrients. 2021; 13(3):811. https://doi.org/10.3390/nu13030811
Chicago/Turabian StylePark, Kiwoong, Suhyeon Park, Arulkumar Nagappan, Navin Ray, Juil Kim, Sik Yoon, and Yuseok Moon. 2021. "Probiotic Escherichia coli Ameliorates Antibiotic-Associated Anxiety Responses in Mice" Nutrients 13, no. 3: 811. https://doi.org/10.3390/nu13030811
APA StylePark, K., Park, S., Nagappan, A., Ray, N., Kim, J., Yoon, S., & Moon, Y. (2021). Probiotic Escherichia coli Ameliorates Antibiotic-Associated Anxiety Responses in Mice. Nutrients, 13(3), 811. https://doi.org/10.3390/nu13030811






