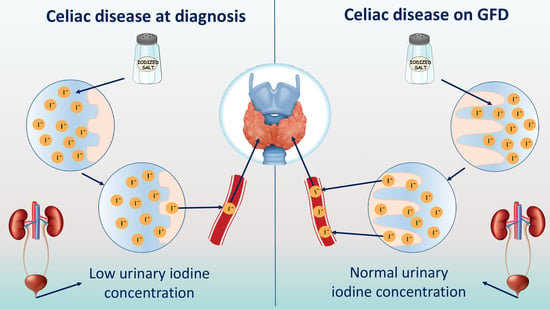
Iodine Absorption in Celiac Children: A Longitudinal Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Methods
2.4. Statistical Analysis
3. Results
3.1. Patients Features
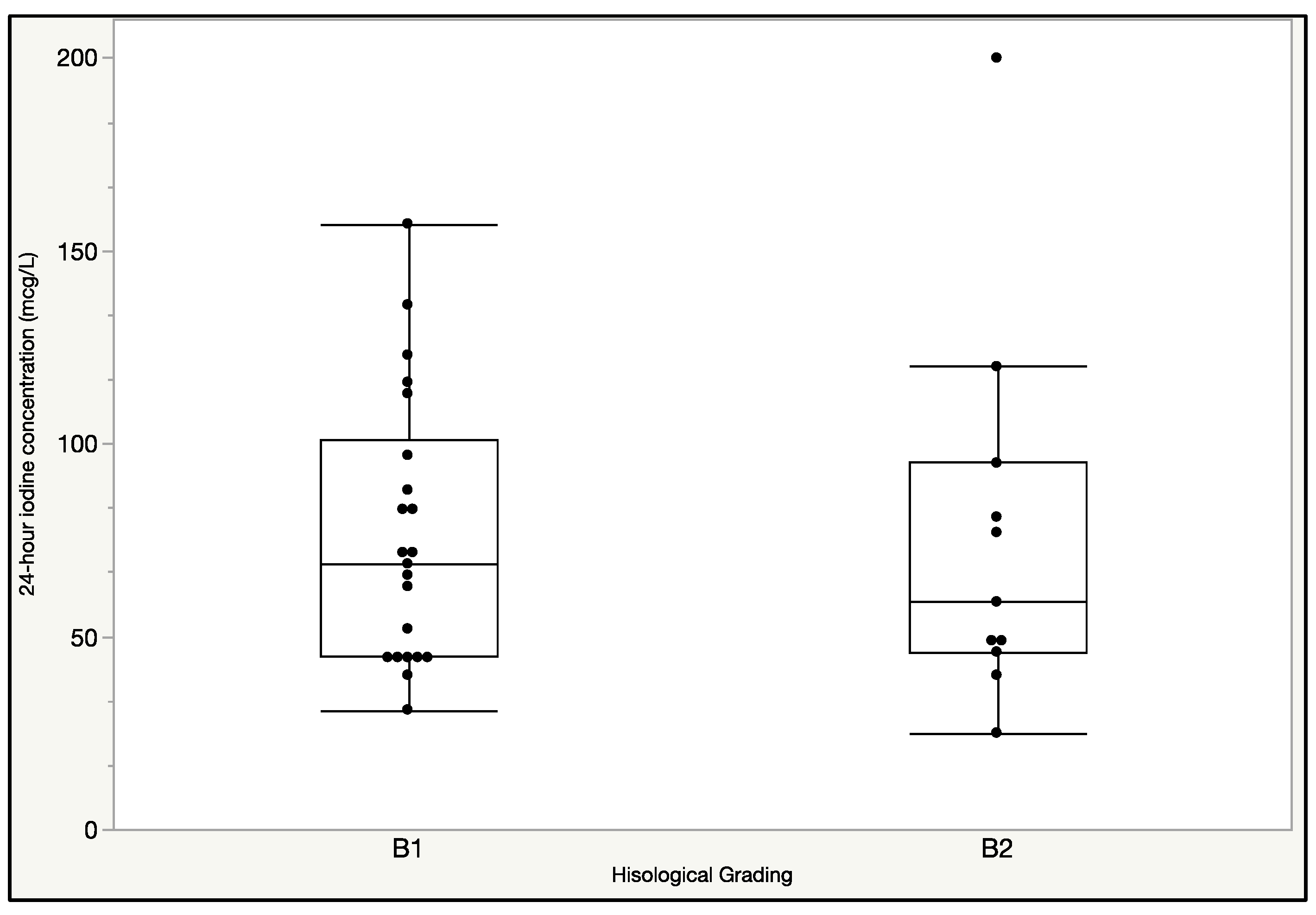
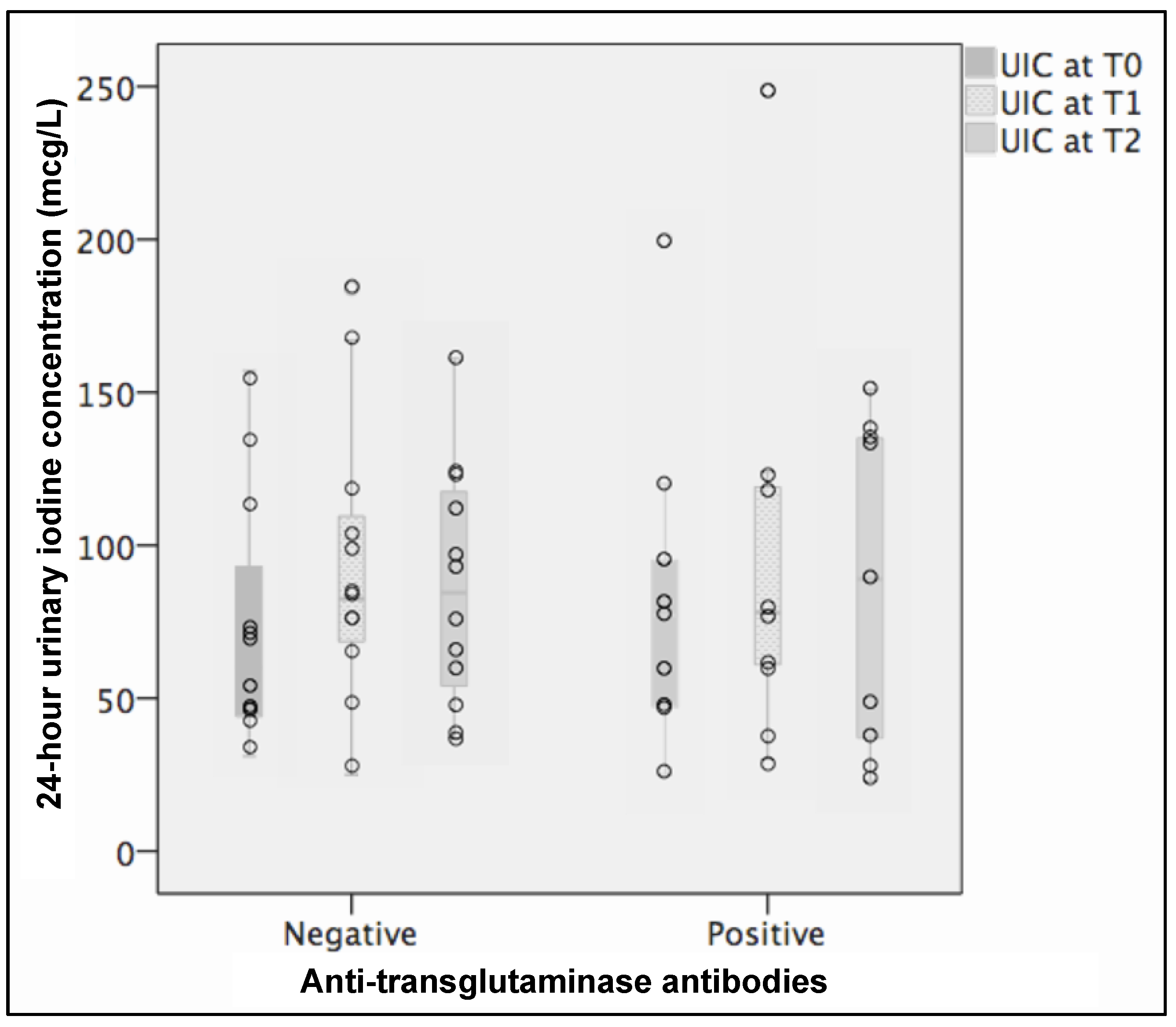
3.2. Findings at T0
3.3. Findings at T1
3.4. Findings at T2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fasano, A.; Catassi, C. Coeliac disease in children. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 467–478. [Google Scholar] [CrossRef]
- Green, P.H.; Cellier, C. Medical progress: Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef]
- Kowalska, E.; Wasowska-Krolikowska, K.; Toporowska-Kowalska, E. Estimation of antithyroid antibodies occurrence in children with coeliac disease. Med. Sci. Monit. 2000, 6, 719–721. [Google Scholar] [PubMed]
- Oderda, G.; Rapa, A.; Zavallone, A.; Strigini, L.; Bona, G. Thyroid autoimmunity in childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 704–705. [Google Scholar] [CrossRef]
- Ansaldi, N.; Palmas, T.; Corrias, A.; Barbato, M.; D’Altiglia, M.R.; Campanozzi, A.; Baldassarre, M.; Rea, F.; Pluvio, R.; Bonamico, M.; et al. Autoimmune thyroid disease and celiac disease in children. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Cassio, A.; Ricci, G.; Baronio, F.; Miniaci, A.; Bal, M.; Bigucci, B.; Conti, V.; Cicognani, A. Long-term Clinical Significance of Thyroid Autoimmunity in Children with Celiac Disease. J. Pediatr. 2010, 156, 292–295. [Google Scholar] [CrossRef]
- Sategna-Guidetti, C.; Volta, U.; Ciacci, C.; Usai, P.; Carlino, A.; Franceschi, L.; Camera, A.; Pelli, A.; Brossa, C. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: An Italian multicenter study. Am. J. Gastroenterol. 2001, 96, 751–757. [Google Scholar] [CrossRef]
- Elfström, P.; Montgomery, S.M.; Kämpe, O.; Ekbom, A.; Ludvigsson, J.F. Risk of thyroid disease in individuals with celiac disease. J. Clin. Endocrinol. Metab. 2008, 93, 3915–3921. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, L.; Yang, R.; Li, Y.; Shan, L.; Wang, Y. Increased incidence of thyroid disease in patients with celiac disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0168708. [Google Scholar] [CrossRef]
- Carter, J.N.; Corcoran, J.M.; Eastman, C.J.; Lazarus, L. Effect of severe, chronic illness on thyroid function. Lancet 1974, 304, 971–974. [Google Scholar] [CrossRef]
- Farthing, M.J.; Rees, L.; Edward, C.R.; Byfield, P.G.; Himsworth, R.L.; Dawson, A.M. Thyroid hormones and the regulation of thyroid function in men with coeliac disease. Clin. Endocrinol. 1982, 16, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Nicola, J.P.; Basquin, C.; Portulano, C.; Reyna-Neyra, A.; Paroder, M.; Carrasco, N. The Na+/I− symporter mediates active iodide uptake in the intestine. Am. J. Physiol. Cell Physiol. 2009, 296, C654–C662. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Buyse, I.; Decorte, R.; Baens, M.; Cuppens, H.; Semana, G.; Emonds, M.; Marynen, P.; Cassiman, J. Rapid DNA typing of class II HLA antigens using the polymerase chain reaction and reverse dot blot hybridization. Tissue Antigens 1993, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Villanacci, V. Coeliac disease. J. Clin. Pathol. 2005, 58, 573–574. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessment of the Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007; pp. 1–107. [Google Scholar]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Bianchi, P.I.; Marchese, A.; Trotta, L.; Vattiato, C.; Balduzzi, D.; Brusco, G.; Andrealli, A.; Cisaro, F.; Astegiano, M.; et al. A score that verifies adherence to a gluten-free diet: A cross-sectional, multicentre validation in real clinical life. Br. J. Nutr. 2012, 108, 1884–1888. [Google Scholar] [CrossRef]
- Olivieri, A.; Trimarchi, F.; Vitti, P. Global iodine nutrition 2020: Italy is an iodine sufficient country. J. Endocrinol. Investig. 2020, 43, 1671–1672. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef]
- Cavalieri, R.R. Iodine metabolism and thyroid physiology: Current concepts. Thyroid 1997, 7, 177–181. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: Past, present, and future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef]
- Vejbjerg, P.; Knudsen, N.; Perrild, H.; Laurberg, P.; Andersen, S.; Rasmussen, L.B.; Ovesen, L.; Jørgensen, T. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009, 19, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Bethancourt, G.; Johner, S.A.; Stehle, P.; Neubert, A.; Remer, T. Iodine status assessment in children: Spot urine iodine concentration reasonably reflects true twenty-four-hour iodine excretion only when scaled to creatinine. Thyroid 2015, 25, 688–697. [Google Scholar] [CrossRef]
- Meloni, A.; Mandas, C.; Jores, R.D.; Congia, M. Prevalence of Autoimmune Thyroiditis in Children with Celiac Disease and Effect of Gluten Withdrawal. J. Pediatr. 2009, 155, 51–55. [Google Scholar] [CrossRef]
- Norström, F.; Van Der Pals, M.; Myléus, A.; Hammarroth, S.; Högberg, L.; Isaksson, A.; Ivarsson, A.; Carlsson, A. Impact of Thyroid Autoimmunity on Thyroid Function in 12-year-old Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, M.; De Bellis, A.; Francavilla, R.; Rutigliano, V.; Predieri, B.; Indrio, F.; De Venuto, D.; Sinisi, A.A.; Bizzarro, A.; Bellastella, A.; et al. Anti-pituitary antibodies in children with newly diagnosed celiac disease: A novel finding contributing to linear-growth impairment. Am. J. Gastroenterol. 2010, 105, 691–696. [Google Scholar] [CrossRef]
- Murray, J.A.; Rubio-tapia, A.; Van Dyke, C.T.; Brogan, D.L.; Knipschield, M.A.; Lahr, B.; Rumalla, A.; Zinsmeister, A.R.; Gostout, C.J. Mucosal Atrophy in Celiac Disease: Extent of Involvement, Correlation with Clinical Presentation, and Response to Treatment. Clin. Gastroenterol. Hepatol. 2008, 6, 186–193. [Google Scholar] [CrossRef]
- Rondonotti, E.; Spada, C.; Cave, D.; Pennazio, M.; Riccioni, M.E.; De Vitis, I.; Schneider, D.; Sprujevnik, T.; Villa, F.; Langelier, J.; et al. Video capsule enteroscopy in the diagnosis of celiac disease: A multicenter study. Am. J. Gastroenterol. 2007, 102, 1624–1631. [Google Scholar] [CrossRef]
- Virili, C.; Centanni, M. “With a little help from my friends”—The role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol. Cell. Endocrinol. 2017, 458, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Rizzello, C.G.; Gagliardi, F.; Ricciuti, P.; Ndagijimana, M.; Francavilla, R.; Guerzoni, M.E.; Crecchio, C.; Gobbetti, M.; De Angelis, M. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 2009, 75, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Olivares, M.; Codoñer-Franch, P.; Sanz, Y. Intestinal microbiota and celiac disease: Cause, consequence or co-evolution? Nutrients 2015, 7, 6900–6923. [Google Scholar] [CrossRef] [PubMed]
| Study Group | |||
|---|---|---|---|
| T0 (36 Patients) | T1 (28 Patients) | T2 (23 Patients) | |
| Age (years) | 7.4 (4.6/9.8) | 7.6 (5/9.8) | 8.5 (5.6/11.6) |
| Gender | 10 M, 26 F | 8 M, 20 F | 6 M, 17 F |
| UIC (μg/L) | 64 (45/93.25) | 76 (60.25/105) | 89 (48/124) |
| TSH (μg/L) | 2.31 (1.85/3.06) | 2.19 (1.70/3.46) | 2.03 (1.52/3.50) |
| fT4 (ng/dL) | 1.03 (0.99/1.13) | 1.05 (0.97/1.14) | 1.00 (0.95/1.19) |
| fT3 (pg/mL) | 4.18 (3.61/4.22) | 4.23 (3.60/4.66) | 4.08 (3.72/4.38) |
| tTG-IgA (titer range, AU) | Positive in all patients (31.9/>200) | Positive in 14 patients (50%) (10.1/>200) | Positive in 10 patients (43.5%) (12.1/>200) |
| EMA-IgA | Positive in all patients | n.a. | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delvecchio, M.; Bizzoco, F.; Lapolla, R.; Gentile, A.; Carrozza, C.; Barone, M.; Simonetti, S.; Giordano, P.; Dargenio, V.N.; Cristofori, F.; et al. Iodine Absorption in Celiac Children: A Longitudinal Pilot Study. Nutrients 2021, 13, 808. https://doi.org/10.3390/nu13030808
Delvecchio M, Bizzoco F, Lapolla R, Gentile A, Carrozza C, Barone M, Simonetti S, Giordano P, Dargenio VN, Cristofori F, et al. Iodine Absorption in Celiac Children: A Longitudinal Pilot Study. Nutrients. 2021; 13(3):808. https://doi.org/10.3390/nu13030808
Chicago/Turabian StyleDelvecchio, Maurizio, Francesca Bizzoco, Rosa Lapolla, Antonia Gentile, Cinzia Carrozza, Michele Barone, Simonetta Simonetti, Paola Giordano, Vanessa Nadia Dargenio, Fernanda Cristofori, and et al. 2021. "Iodine Absorption in Celiac Children: A Longitudinal Pilot Study" Nutrients 13, no. 3: 808. https://doi.org/10.3390/nu13030808
APA StyleDelvecchio, M., Bizzoco, F., Lapolla, R., Gentile, A., Carrozza, C., Barone, M., Simonetti, S., Giordano, P., Dargenio, V. N., Cristofori, F., & Francavilla, R. (2021). Iodine Absorption in Celiac Children: A Longitudinal Pilot Study. Nutrients, 13(3), 808. https://doi.org/10.3390/nu13030808