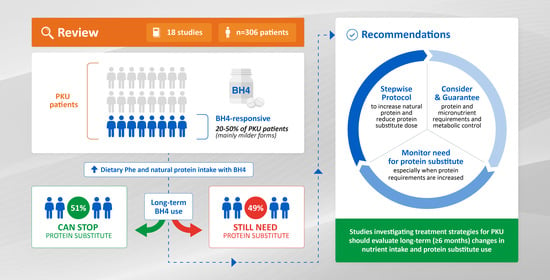
Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Terminology
2.2. Literature Search
2.3. Study Selection
2.4. Outcome Measures
2.5. Data Extraction
2.6. Quality Appraisal and Risk of Bias Assessment
2.7. Data Analysis
3. Results
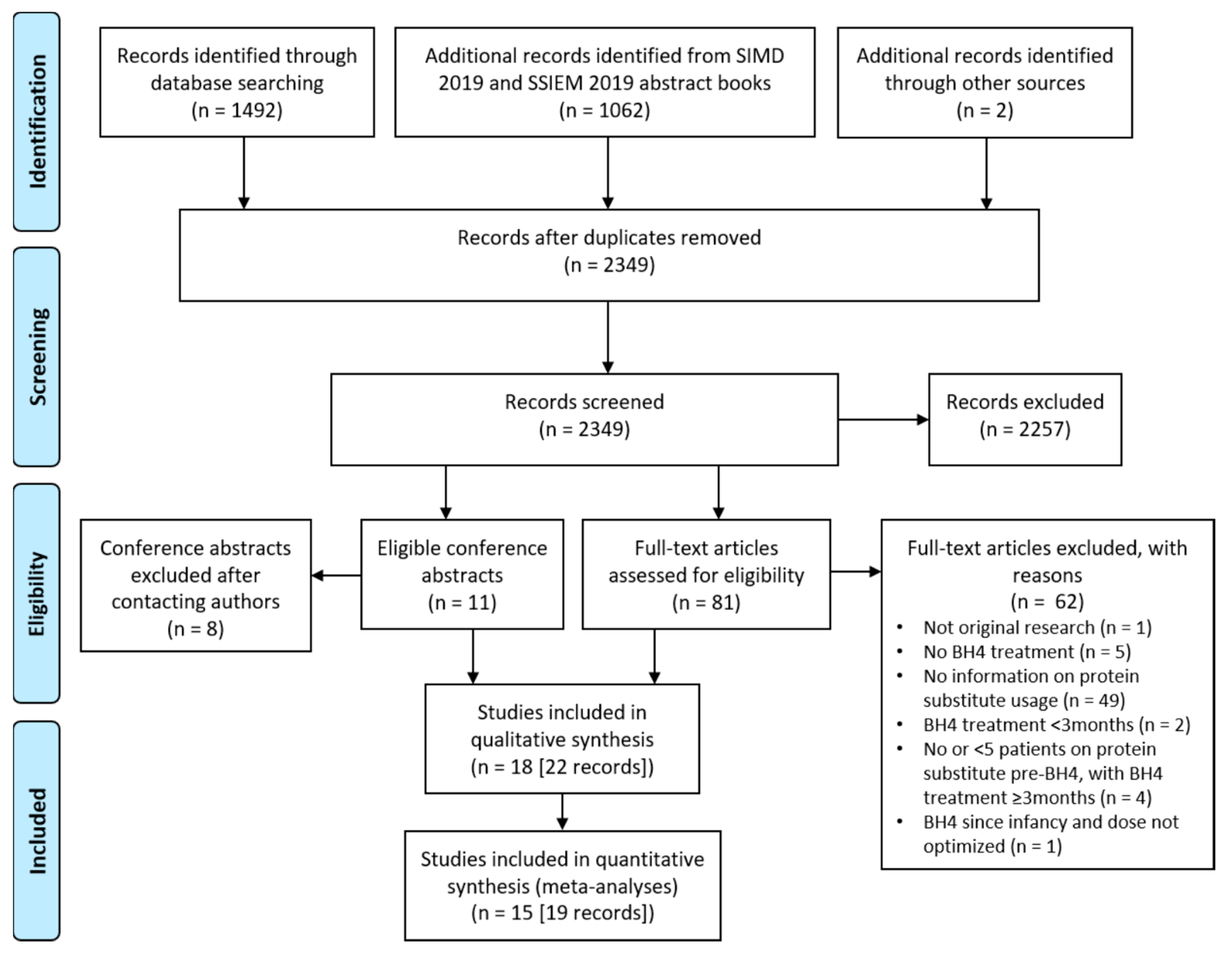
3.1. Study Selection
3.2. Study Characteristics
3.3. Systematic Review of Key Findings and Meta-Analyses
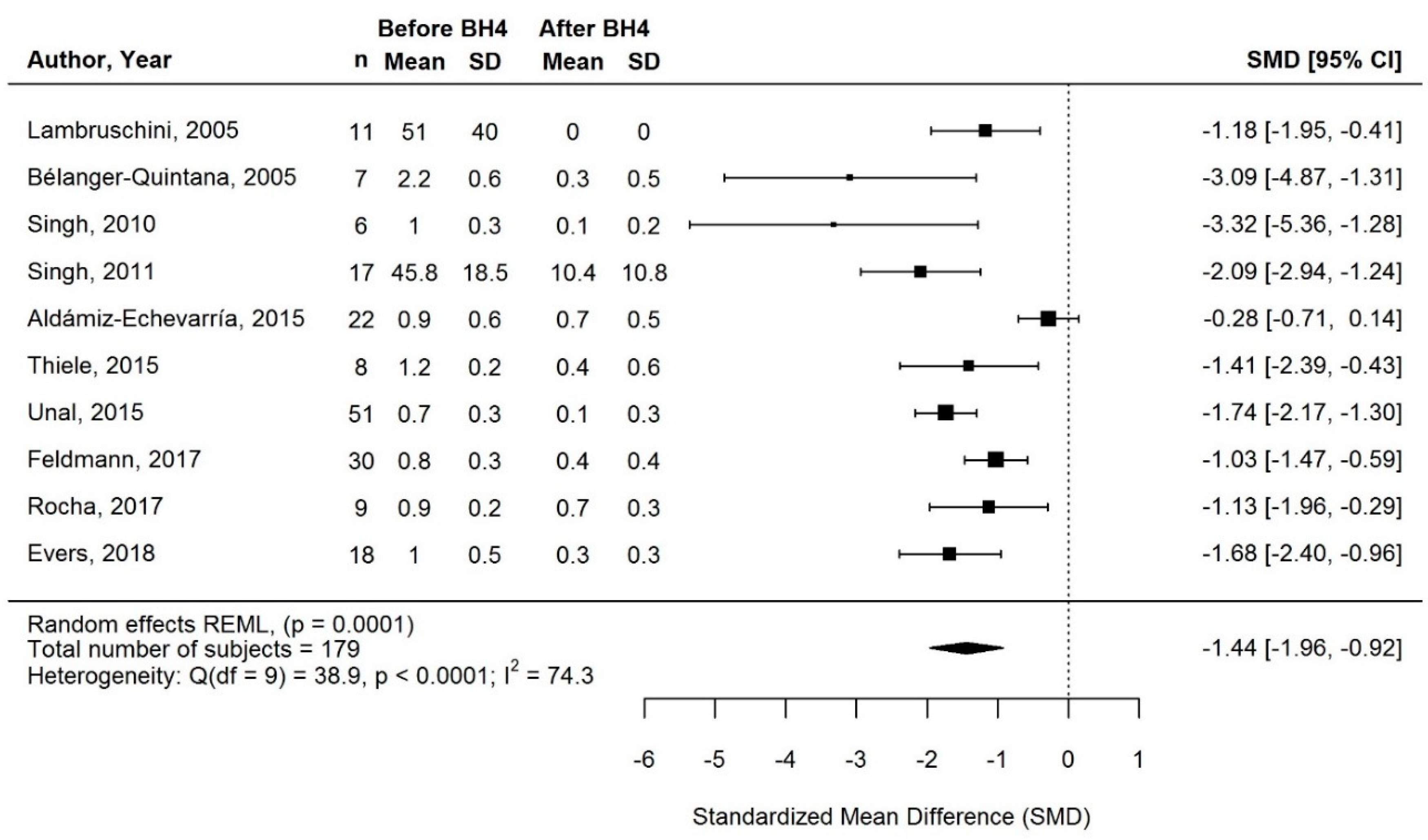
3.3.1. Change in Phe Intake with BH4 Treatment
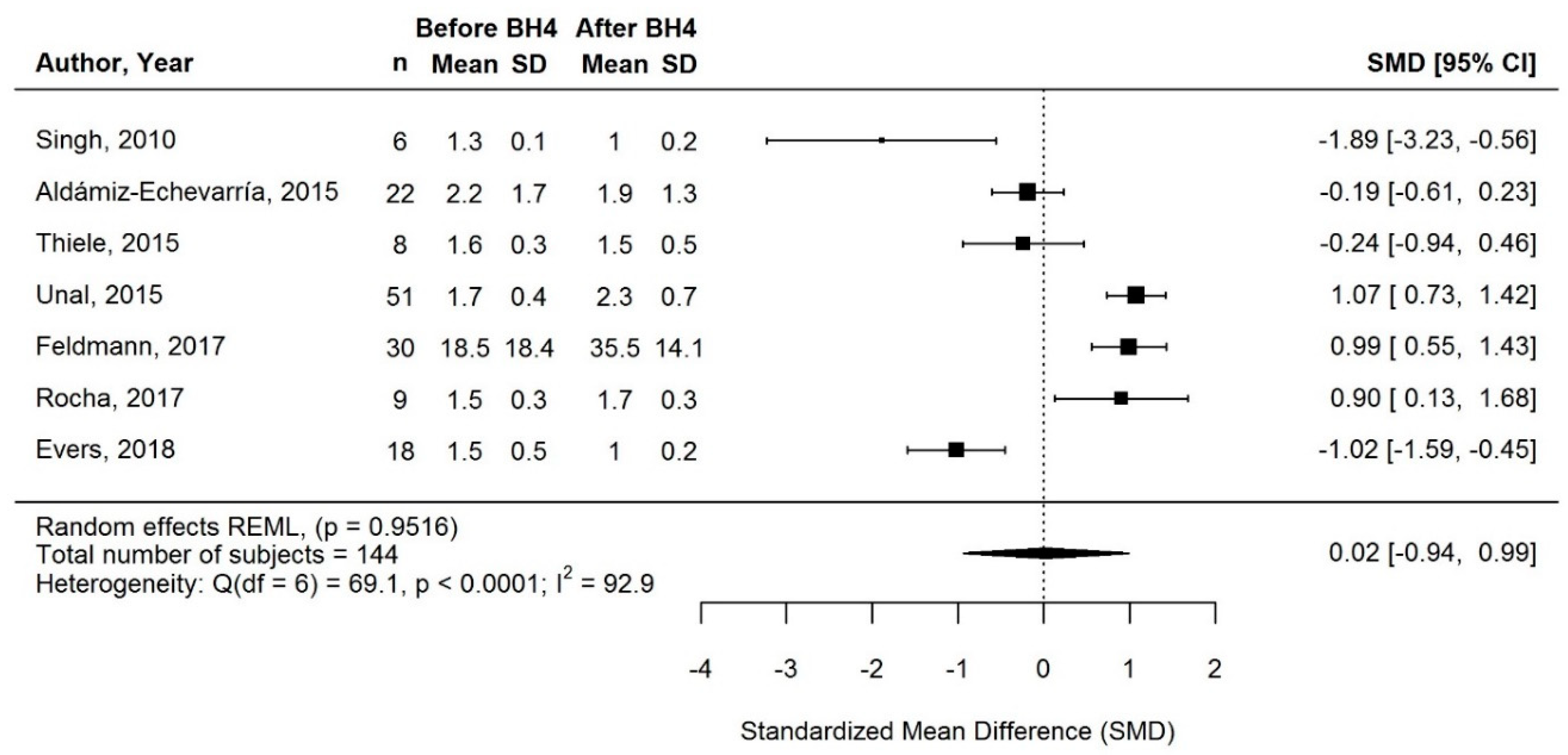
3.3.2. Change in Natural Protein Intake with BH4 Treatment
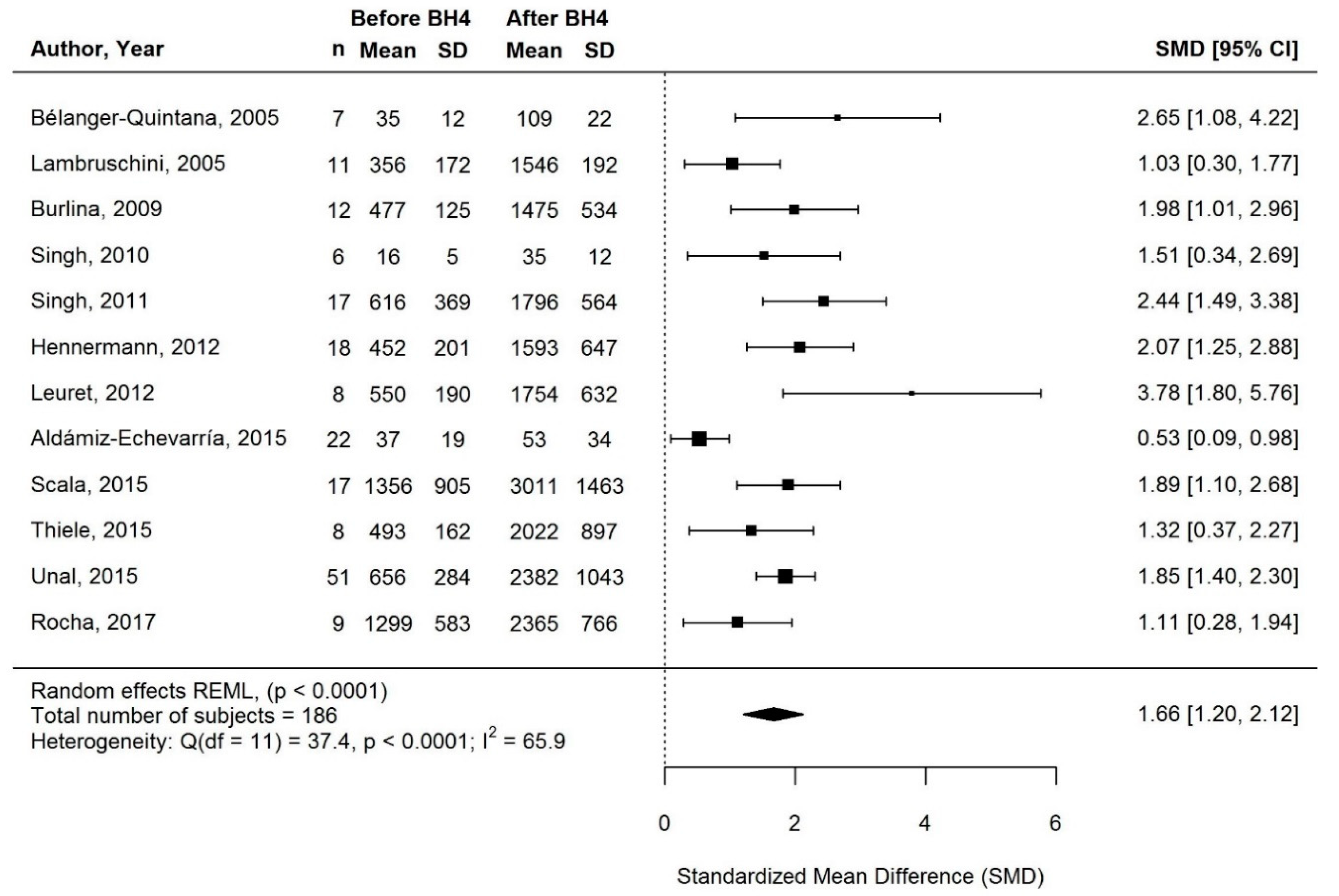
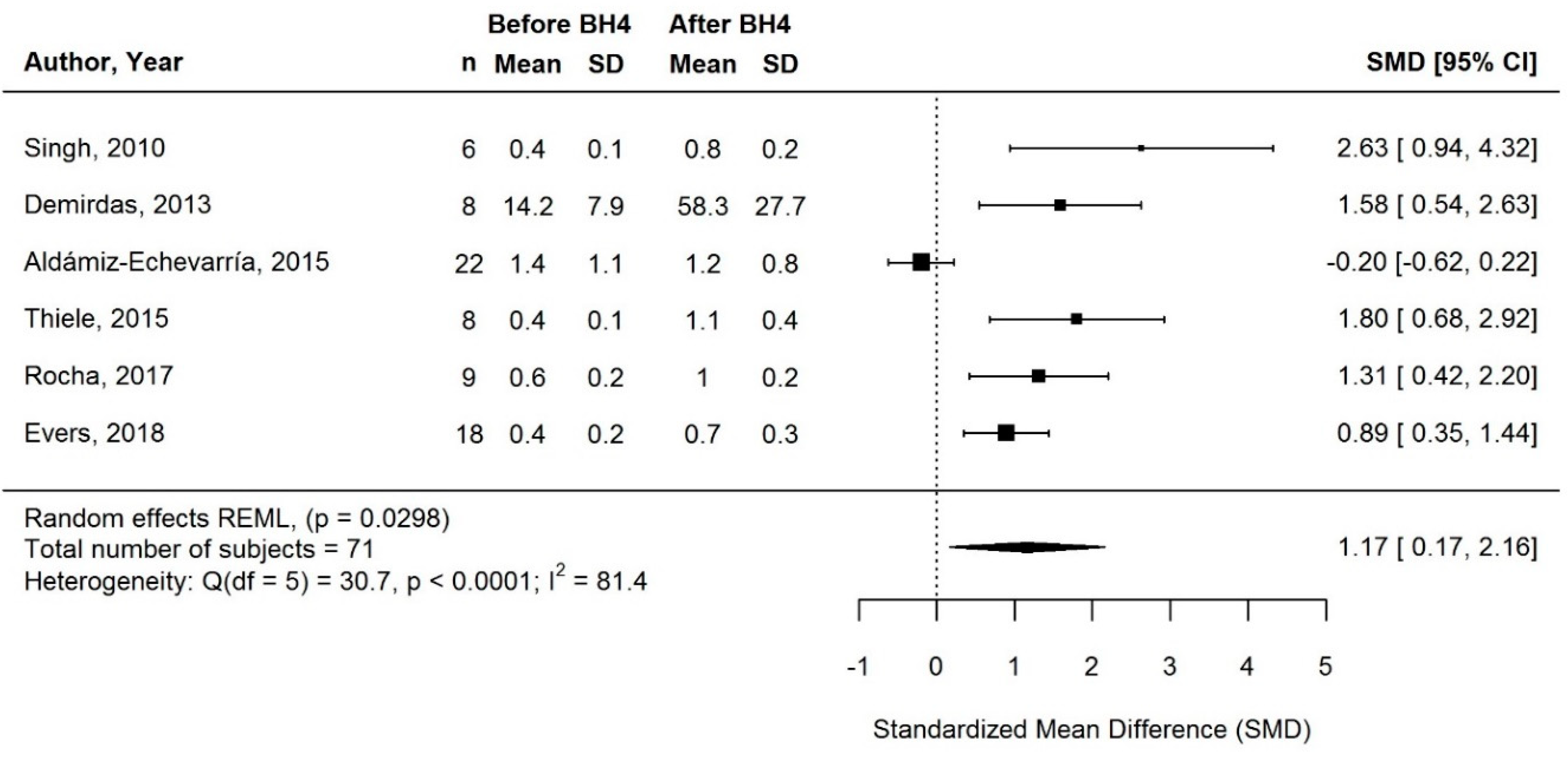
3.3.3. Change in Protein Equivalent Intake from Protein Substitute with BH4 Treatment
| Reference | Duration on BH4 (Mean or Range; Years) | Change in Phe Intake | Relative Change in Natural Protein Intake from Baseline 2 | Change in Protein Equivalent Intake from Protein Substitute | Relative Change in Total Protein Intake from Baseline 2 | ||
|---|---|---|---|---|---|---|---|
| Relative Change from Baseline 2 | No. of Responders with Increased Intake (%) | Relative Change from Baseline 2 | No. of Responders with Change in Dose (%) 3 | ||||
| Bélanger-Quintana 2005 [38] | 0.9 (range: 0.4–1.5) | 3.5-fold  (mean; mg/kg/day) 2.7-fold  (median; mg/kg/day) | 7/7 (100) | n/a | 90%  (mean; g/kg/day) 100%  (median; g/kg/day) | Decreased: 2/7 (29) Stopped: 5/7 (71) No change: - | n/a |
| Lambruschini 2005 [45] | 1.0 | 4.3-fold  * *(mean SR intake; mg/day) | 11/11 (100) | n/a | 100%  (mean and median; g/day) | Decreased: - Stopped: 11/11 (100) No change: - | n/a |
| Burlina 2009 [40] | 3.5 (range: 0.5–7.0) | 3.2-fold  (mean SR intake; mg/day) | 12/12 (100) | n/a | 100%  (mean and median; g/day) | Decreased: - Stopped: 12/12 (100) No change: - | n/a |
| Singh 2010 [49] | 2.0 | 3mo FU:2.2-fold  * *1y FU:2.3-fold  * *2y FU:2.2-fold  * *(mean SR intake; mg/kg/day) 3mo FU:3.4-fold  * *1y FU:3.4-fold  * *2y FU:3.1-fold  * *(mean prescription; mg/kg/day) | 6/6 (100) | 3mo FU:114%  * *1y FU:119%  * *2y FU:125%  * *(mean SR intake; g/kg/day) | 3mo FU:77%  * *1y FU:70%  * *2y FU:84%  * *(mean SR intake; g/kg/day) | 3mo FU: Decreased: 3/6 (50) Stopped: 3/6 (50) No change: - 2y FU: Decreased: 4/6 (67) Stopped: 2/6 (33) No change: - | 3mo FU:25% ns ns1y FU:18%  ns ns2y FU:27%  ns ns(mean SR intake; g/kg/day) |
| Vilaseca 2010 [51] | 5.7 (range: 5.3–6.0) | n/a | n/a | n/a | 100%  (mean and median; g/day) | Decreased: - Stopped: 10/10 (100) No change: - | n/a |
| Singh 2011 [48], Douglas 2013a [42], Douglas 2013b [43], Brantley 2018 [39] | 1.0 | 4mo FU: 2.7-fold * † * †(mean prescription; mg/day) 1y FU:2.9-fold  (mean prescription; mg/day) 1y FU:1.5-fold  ns ns(median SR intake; mg/day) | 4mo FU: 18/18 (100) 1y FU:17/17 (100) | n/a | 4mo FU: 83%  * *(mean prescription; g/day) 1y FU:77%  (mean prescription; g/day) 75 to 100%  (n = 6/17) (n = 6/17)50 to 75%  (n = 8/17) (n = 8/17)20 to 25%  (n = 1/17) (n = 1/17)<20%  (n = 2/17) (n = 2/17)(prescription; g/day) | 4mo FU: Decreased: 7/18 (39) Stopped: 9/18 (50) No change: 2/18 (11) 1y FU: Decreased: 10/17 (59) Stopped: 5/17 (29) No change: 2/17 (12) | n/a |
| Hennermann 2012 [17] | 4.0 (range: 0.7–8.8) | 3.8-fold  (mean; mg/day) 3.1-fold  (median; mg/day) | 18/18 (100) | n/a | n/a | Decreased/ No change: 10/18 (56) Stopped: 8/18 (44) | n/a |
| Leuret 2012 [46] | 1.9 § (range: 0.6–6.7) | 3.2-fold  * *(mean SR intake; mg/day) | 8/8 (100) | n/a | n/a | Decreased: - Stopped: 7/8 (87) No change: 1/8 (13) | n/a |
| Aldámiz-Echevarría 2013 [36] | 2.0 (cohort 1) # 5.0 (cohort 2) # | 2y FU:1.4-fold  (median SR intake; mg/kg/day) 5y FU:1.2-fold  (median SR intake; mg/kg/day) | 2y FU:28/36 (78) 5y FU:6/10 (60) | 2y FU:14%  (median SR intake; g/kg/day) 5y FU:13%  (median SR intake; g/kg/day) | 2y FU:44%  (median SR intake; g/kg/day) 5y FU:57%  (median SR intake; g/kg/day) | 2y FU: Decreased/ No change: 25/36 (69) Stopped: 11/36 (31) 5y FU: Decreased/ No change: 8/10 (80) Stopped: 2/10 (20) | 2y FU:17%  (median SR intake; g/kg/day) 5y FU:29%  (median SR intake; g/kg/day) |
| Demirdas 2013 [41] | range: 1.4–2.0 | n/a | 8/8 (100) | 311%  * *(mean SR intake; g/day) | 100%  (n = 3/8) (n = 3/8)>60%  (n = 3/8) (n = 3/8)<20%  (n = 2/8) (n = 2/8)(SR intake; g/day) | Decreased: 5/8 (63) Stopped: 3/8 (37) No change: - | n/a |
| Aldámiz-Echevarría 2015 [37] | 1.0 | 1.4-fold  * *(mean SR intake; mg/kg/day) | 20/22 (90) | 14%  ns ns(mean SR intake; g/kg/day) | 22%  ns ns(mean SR intake; g/kg/day) | Decreased/ No change: 20/22 (91) Stopped: 2/22 (9) | 14%  ns ns(mean SR intake; g/kg/day) |
| Scala 2015 [47] | 5.7 (range: 1.0–7.0) | 2.5-fold  * *(mean SR intake; mg/day) 2.7-fold  * *(median SR intake; mg/day) | 17/17 (100) | n/a | n/a | Decreased: 2/17 (12) Stopped: 9/17 (53) No change: 6/17 (35) | n/a |
| Thiele 2015 [29] | 2.0 | 3mo FU:4.5-fold  * *2y FU:4.1-fold  * *(mean SR intake; mg/day) | 8/8 (100) | 3mo FU: 307%  *(g/day) *(g/day)244%  *(g/kg/day) *(g/kg/day)(median SR intake) 2y FU: 244%  *(g/day) *(g/day)157%  *(g/kg/day) *(g/kg/day)(median SR intake) | 3mo FU: 100%  *(g/day) *(g/day)100%  *(g/kg/day) *(g/kg/day)(median SR intake) 2y FU: 84%  *(g/day) *(g/day)88%  *(g/kg/day) *(g/kg/day)(median SR intake) | Decreased: - Stopped: 4/8 (50) No change: 4/8 (50) | 3mo FU: 12%  ns (g/day) ns (g/day)4%  ns (g/kg/day) ns (g/kg/day)(median SR intake) 2y FU: 27%  ns (g/day) ns (g/day)2%  ns (g/kg/day) ns (g/kg/day)(median SR intake) |
| Ünal 2015 [50] Gökmen Özel 2014 [52] | 2.5 (range: 0.5–4.0) | 3.8-fold  (mg/day) (mg/day)2.9-fold  (mg/kg/day) (mg/kg/day)(mean SR intake) 3.7-fold  (mg/day) (mg/day)2.8-fold  (mg/kg/day) (mg/kg/day)(median SR intake) | 51/51 (100) | n/a | 87%  (g/day) (g/day)89%  (g/kg/day) (g/kg/day)(mean SR intake) 100%  (g/day) (g/day)100%  (g/kg/day) (g/kg/day)(median SR intake) | Decreased: 5/51 (10) Stopped: 43/51 (84) No change: 3/51 (6) | 79%  (g/day) (g/day)35%  (g/kg/day) (g/kg/day)(mean SR intake) 78%  (g/day) (g/day)33%  (g/kg/day) (g/kg/day)(median SR intake) |
| Feldmann 2017 [16] | 0.5 | n/a | n/a | n/a | 49%  (mean; g/kg/day) | Decreased/ No change: 23/30 (77) Stopped: 7/30 (23) | 92%  (mean; g/day) |
| Rocha 2017 [54] | 1.0 (range: 0.3–1.4) | 1.8-fold  (mg/day) (mg/day)1.5-fold  (mg/kg/day) (mg/kg/day)(median SR intake) | 8/9 (89) | 79%  (g/day) (g/day)51%  (g/kg/day) (g/kg/day)(median SR intake) | 16%  (g/day) (g/day)23%  (g/kg/day) (g/kg/day)(median SR intake) | Decreased: 4/9 (44) Stopped: - No change: 5/9 (56) | 19%  (g/day) (g/day)8%  (g/kg/day) (g/kg/day)(median SR intake) |
| Evers 2018 [44] | 5.0 (range: 4.5–5.5) | n/a | n/a | 59%  (mean prescription; g/kg/day) 100%  (median prescription; g/kg/day) | 69%  (mean prescription; g/kg/day) 61%  (median prescription; g/kg/day) | Decreased: 10/18 (56) Stopped: 8/18 (44) No change: - | 33%  (mean prescription; g/kg/day) |
| Paras 2018 [53] | ≥0.3 (range: ≥0.3–≥3.5) | n/a | 8/8 (100) | n/a | 100%  (mean and median; g/day) | Decreased: - Stopped: 8/8 (100) No change: - | n/a |
 : increase;
: increase;  : decrease. 1 Only long-term responders (follow-up ≥3 months) who were on a Phe-restricted diet and protein substitute before BH4 were included in the analyses. Long-term responsiveness as reported by the original authors, except for Rocha 2017 where 1 patient was considered long-term non-responder after discussing with the authors (lack of changes in Phe tolerance and natural protein intake, while Phe levels only decreased by 10%). 2 Superscripts indicate that a statistical analysis was performed by the original authors. *: statistically significant change; ns: change not statistically significant. Otherwise, no statistical analysis was performed with the exception of Ünal 2015, Rocha 2017, and Evers 2018, who performed statistical analyses with their original samples. However, statistical significance is not reported here because some patients included in the original analyses did not meet our inclusion criteria (i.e., long-term responders followed up ≥3 months who were on a Phe-restricted diet and protein substitute before BH4). 3 Change as reported by the original authors. If individual data were available (i.e., reported or provided upon request), change in protein substitute intake was considered a “decrease” only if the reduction was ≥25% compared with baseline, as this was deemed clinically meaningful. Reductions <25% of baseline were counted as “no change”. † Singh 2011: Change in Phe tolerance at 4mo FU included 1 patient never taking any protein substitute but who could not be removed from this analysis, and thus n = 19 instead of 18. One other patient was lost to follow-up between 4mo and 1y FU. § Leuret 2012: Median duration of BH4 treatment, not mean. Only 8/15 patients were on a Phe-restricted diet before BH4 and were therefore included in our analyses; however, duration of BH4 treatment was only available for the total sample of 15 patients. # Aldámiz-Echevarría 2013: Unclear if patients with a 5y follow-up were also described in the group of patients with a 2y follow-up. It was assumed that the 2 cohorts comprised different patients.
: decrease. 1 Only long-term responders (follow-up ≥3 months) who were on a Phe-restricted diet and protein substitute before BH4 were included in the analyses. Long-term responsiveness as reported by the original authors, except for Rocha 2017 where 1 patient was considered long-term non-responder after discussing with the authors (lack of changes in Phe tolerance and natural protein intake, while Phe levels only decreased by 10%). 2 Superscripts indicate that a statistical analysis was performed by the original authors. *: statistically significant change; ns: change not statistically significant. Otherwise, no statistical analysis was performed with the exception of Ünal 2015, Rocha 2017, and Evers 2018, who performed statistical analyses with their original samples. However, statistical significance is not reported here because some patients included in the original analyses did not meet our inclusion criteria (i.e., long-term responders followed up ≥3 months who were on a Phe-restricted diet and protein substitute before BH4). 3 Change as reported by the original authors. If individual data were available (i.e., reported or provided upon request), change in protein substitute intake was considered a “decrease” only if the reduction was ≥25% compared with baseline, as this was deemed clinically meaningful. Reductions <25% of baseline were counted as “no change”. † Singh 2011: Change in Phe tolerance at 4mo FU included 1 patient never taking any protein substitute but who could not be removed from this analysis, and thus n = 19 instead of 18. One other patient was lost to follow-up between 4mo and 1y FU. § Leuret 2012: Median duration of BH4 treatment, not mean. Only 8/15 patients were on a Phe-restricted diet before BH4 and were therefore included in our analyses; however, duration of BH4 treatment was only available for the total sample of 15 patients. # Aldámiz-Echevarría 2013: Unclear if patients with a 5y follow-up were also described in the group of patients with a 2y follow-up. It was assumed that the 2 cohorts comprised different patients.3.3.4. Change in Total Protein Intake after BH4 Treatment
3.3.5. Supplementary Sensitivity Meta-Analyses
3.4. Systematic Review of Findings Related to Secondary Outcomes
3.4.1. Change in Micronutrient Intakes and Serum Concentrations with BH4
3.4.2. Change in Growth with BH4
3.4.3. Change in Metabolic Control with BH4
3.5. Quality Appraisal and Risk of Bias Assessment
4. Discussion
Strengths and Limitations
5. Recommendations
5.1. BH4 Treatment Trial and Adjusting Phe Intake
- BH4 responsiveness requires careful assessment—the aim is to maintain blood Phe within target therapeutic range while maintaining normal growth but also (1) establish an increase in Phe tolerance, (2) reduce protein equivalent intake from protein substitute in alignment with any increase in natural protein intake, and (3) establish the maintenance dose of BH4.
- Once BH4 is administered, if three consecutive blood Phe levels are maintained within target therapeutic range, then Phe intake should be increased by at least 20%, and then this process should be repeated until natural protein tolerance is established. If the mean blood Phe level exceeds target therapeutic range, then the Phe intake should be reduced by approximately 10 to 30%, depending on the degree of elevation of the blood Phe levels (adapted from Muntau et al. [63]).
- With BH4 treatment, it is expected that the final Phe tolerance should be increased by ≥100% of baseline, provided natural protein intake is below safe levels of protein intake. If natural protein intake already exceeds safe levels of protein intake at baseline, an improvement in blood Phe control may be an appropriate alternative goal. Maintenance of blood Phe levels within target therapeutic range and an increase in Phe tolerance should be observed for at least 3 months to ascertain BH4 responsiveness.
5.2. Quality of Natural Protein Intake
- Natural protein intake should be sourced from different proteins, e.g., dairy and eggs, cereals, lentils, and protein-rich vegetables if tolerated. Food choices should be made according to national and international recommendations. Natural protein sources should provide micronutrients to minimize the need for extra micronutrient supplements. Continuous patient education and support about the need for a healthy diet with appropriate food choices will be necessary with BH4 treatment.
5.3. Adapting Protein Substitute Dose
- Protein equivalent from substitute intake should be reduced in parallel with any increase in natural protein intake. The more natural protein that is tolerated, the lower the requirement should be for protein substitute. For every increase in natural protein, the protein equivalent from protein substitute should be reduced accordingly.
- It is possible that the natural protein intake meets or exceeds safe levels of protein intake so that a protein substitute is not needed to meet protein requirements. However, some protein substitute might be necessary for micronutrient requirements to be met. Micronutrient supply should be monitored carefully, especially if patients cannot be allowed an unlimited Phe intake. Moreover, it may be better for patients to remain familiar with and accepting of the taste of protein substitute in case it needs to be reintroduced in illness, pre-conception, pregnancy, or lactation, or if BH4 therapy is discontinued. It is also good practice to give a small dose of protein substitute each day to infants who may appear fully responsive to BH4 and without immediate need for a protein restriction. It is possible protein restriction may be necessary at a later age when daily protein requirements increase.
5.4. Monitoring
- Once patients are established on BH4 therapy and the diet is stabilized, clinic visits and blood monitoring should occur at the same frequency as for other patients with PKU who are not on BH4 treatment. If there are any concerns about adherence with BH4 or diet, more frequent monitoring may be required.
- Continue to assess that at least 75% of blood Phe levels remain within target therapeutic range and that more than 100% of original prescription of Phe intake is maintained (unless patients are already meeting safe levels of protein intake). If more than 25% of blood Phe levels are outside target therapeutic range, consider adjusting BH4 dosage or reduce Phe intake. BH4 treatment continuation should be evaluated.
- Evaluate if protein substitute should be re-introduced, or prescription increased, in any event of increased protein requirements (rapid growth, illness, injury/trauma, pregnancy, lactation).
- Patient’s nutritional status including height/length, weight, and body mass index (BMI) should be conducted at least 6-monthly. It is important that patients are encouraged to maintain a healthy BMI.
- Assessment of patient’s nutritional biochemical markers such as plasma amino acids, homocysteine/or methyl malonic acid, hemoglobin, mean corpuscular volume, ferritin, zinc, calcium, selenium, vitamin D, vitamin B12, and folic acid should be completed annually for patients on BH4 therapy.
- Monitor nutritional intake adequacy by 3-day dietary assessments regularly, at least every 3 months in the first year of BH4 therapy. Vitamin and mineral supplements may be required if dietary assessment or patient’s nutritional biomarkers indicate they are necessary. Patients may be more vulnerable to nutritional deficiency if they have stopped or reduced protein substitute intake.
- The ongoing prescription for BH4 should be reassessed and adjusted as appropriate at each clinic visit.
5.5. Clinical Trials of (New) Treatments
- Any future studies investigating treatment strategies for PKU should evaluate long-term (at least 6 months) changes in nutrient intake, in particular natural protein, the need for protein substitute, and micronutrient supplementation. Data about prescribed as well as self-reported protein/Phe intakes should be collected and reported (both gram (or milligram) per day and gram (or milligram) per kilogram bodyweight per day). In published studies, individual data should be provided rather than only summary statistics such as means or medians.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Van Wegberg, A.M.J.; Macdonald, A.; Ahring, K.; BãLanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 1–56. [Google Scholar] [CrossRef]
- Mitchell, J.J.; Trakadis, Y.J.; Scriver, C.R. Phenylalanine hydroxylase deficiency. Genet. Med. 2011, 13, 697–707. [Google Scholar] [CrossRef]
- Macdonald, A.; Van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J. Rare Dis. 2020, 15, 1–21. [Google Scholar] [CrossRef]
- MacDonald, A.; White, F. Amino Acid Disorders. In Clinical Paediatric Dietetics; John Wiley & Sons: Chichester, UK, 2015; pp. 391–455. [Google Scholar] [CrossRef]
- Ford, S.; O’Driscoll, M.; MacDonald, A. Living with Phenylketonuria: Lessons from the PKU community. Mol. Genet. Metab. Rep. 2018, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Acosta, P.B.; Yannicelli, S. Protein intake affects phenylalanine requirements and growth of infants with phenylketonuria. Acta Paediatr. 1994, 83, 66–67. [Google Scholar] [CrossRef]
- Kindt, E.; Motzfeldt, K.; Halvorsen, S.; Lie, O.S. Protein requirements in infants and children: A longitudinal study of children treated for phenylketonuria. Am. J. Clin. Nutr. 1983, 37, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Rylance, G.; Davies, P.; Asplin, D.; Hall, S.K.; Booth, I.W. Administration of protein substitute and quality of control in phenylketonuria: A randomized study. J. Inherit. Metab. Dis. 2003, 26, 319–326. [Google Scholar] [CrossRef]
- Brown, C.S.; Lichter-Konecki, U. Phenylketonuria (PKU): A problem solved? Mol. Genet. Metab. Rep. 2016, 6, 8–12. [Google Scholar] [CrossRef]
- Burnett, J.R. Sapropterin dihydrochloride (Kuvan/phenoptin), an orally active synthetic form of BH4 for the treatment of phenylketonuria. IDrugs Investig. Drugs J. 2007, 10, 805–813. [Google Scholar]
- Hydery, T.; Coppenrath, V.A. A Comprehensive Review of Pegvaliase, an Enzyme Substitution Therapy for the Treatment of Phenylketonuria. Drug Target Insights 2019, 13, 1177392819857089. [Google Scholar] [CrossRef]
- Kure, S.; Hou, D.-C.; Ohura, T.; Iwamoto, H.; Suzuki, S.; Sugiyama, N.; Sakamoto, O.; Fujii, K.; Matsubara, Y.; Narisawa, K. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J. Pediatr. 1999, 135, 375–378. [Google Scholar] [CrossRef]
- Burton, B.K.; Grange, D.K.; Milanowski, A.; Vockley, G.; Feillet, F.; Crombez, E.A.; Abadie, V.; Harding, C.O.; Cederbaum, S.; Dobbelaere, D.; et al. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): A phase II, multicentre, open-label, screening study. J. Inherit. Metab. Dis. 2007, 30, 700–707. [Google Scholar] [CrossRef]
- Feldmann, R.; Wolfgart, E.; Weglage, J.; Rutsch, F. Sapropterin treatment does not enhance the health-related quality of life of patients with phenylketonuria and their parents. Acta Paediatr. 2017, 106, 953–959. [Google Scholar] [CrossRef]
- Hennermann, J.B.; Roloff, S.; Gebauer, C.; Vetter, B.; Von Arnim-Baas, A.; Mönch, E. Long-term treatment with tetrahydrobiopterin in phenylketonuria: Treatment strategies and prediction of long-term responders. Mol. Genet. Metab. 2012, 107, 294–301. [Google Scholar] [CrossRef]
- Trefz, F.K.; Burton, B.K.; Longo, N.; Casanova, M.M.-P.; Gruskin, D.J.; Dorenbaum, A.; Kakkis, E.D.; Crombez, E.A.; Grange, D.K.; Harmatz, P.; et al. Efficacy of Sapropterin Dihydrochloride in Increasing Phenylalanine Tolerance in Children with Phenylketonuria: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J. Pediatr. 2009, 154, 700–707.e1. [Google Scholar] [CrossRef] [PubMed]
- Utz, J.R.J.; Lorentz, C.P.; Markowitz, D.; Rudser, K.D.; Diethelm-Okita, B.; Erickson, D.; Whitley, C.B. START, a double blind, placebo-controlled pharmacogenetic test of responsiveness to sapropterin dihydrochloride in phenylketonuria patients. Mol. Genet. Metab. 2012, 105, 193–197. [Google Scholar] [CrossRef]
- Blau, N.; Erlandsen, H. The metabolic and molecular bases of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol. Genet. Metab. 2004, 82, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kure, S.; Sato, K.; Fujii, K.; Aoki, Y.; Suzuki, Y.; Kato, S.; Matsubara, Y. Wild-type phenylalanine hydroxylase activity is enhanced by tetrahydrobiopterin supplementation in vivo: An implication for therapeutic basis of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol. Genet. Metab. 2004, 83, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Erlandsen, H.; Pey, A.L.; Gámez, A.; Pérez, B.; Desviat, L.R.; Aguado, C.; Koch, R.; Surendran, S.; Tyring, S.; Matalon, R.; et al. From the Cover: Correction of kinetic and stability defects by tetrahydrobiopterin in phenylketonuria patients with certain phenylalanine hydroxylase mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 16903–16908. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, S.F.; Pey, A.L.; Koch, R.; Levy, H.; Ellingson, C.C.; Naylor, E.W.; Martinez, A. Biochemical characterization of mutant phenylalanine hydroxylase enzymes and correlation with clinical presentation in hyperphenylalaninaemic patients. J. Inherit. Metab. Dis. 2008, 32, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Anjema, K.; Van Rijn, M.; Hofstede, F.C.; Bosch, A.M.; Hollak, C.E.; Rubio-Gozalbo, E.; De Vries, M.C.; Janssen, M.C.; Boelen, C.C.; Burgerhof, J.G.; et al. Tetrahydrobiopterin responsiveness in phenylketonuria: Prediction with the 48-hour loading test and genotype. Orphanet J. Rare Dis. 2013, 8, 103. [Google Scholar] [CrossRef]
- Blau, N. Genetics of Phenylketonuria: Then and Now. Hum. Mutat. 2016, 37, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Karačić, I.; Meili, D.; Sarnavka, V.; Heintz, C.; Thöny, B.; Ramadža, D.P.; Fumić, K.; Mardešic, D.; Baric, I.; Blau, N. Genotype-predicted tetrahydrobiopterin (BH4)-responsiveness and molecular genetics in Croatian patients with phenylalanine hydroxylase (PAH) deficiency. Mol. Genet. Metab. 2009, 97, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, A.; Rocha, J.C.; Van Rijn, M.; Feillet, F. Nutrition in phenylketonuria. Mol. Genet. Metab. 2011, 104, S10–S18. [Google Scholar] [CrossRef]
- Pinto, A.; Almeida, M.F.; Macdonald, A.; Ramos, P.C.; Rocha Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; Jackson, R.; van Spronsen, F.; et al. Over Restriction of Dietary Protein Allowance: The Importance of Ongoing Reassessment of Natural Protein Tolerance in Phenylketonuria. Nutrients 2019, 11, 995. [Google Scholar] [CrossRef]
- Thiele, A.G.; Rohde, C.; Mütze, U.; Arelin, M.; Ceglarek, U.; Thiery, J.; Baerwald, C.; Kiess, W.; Beblo, S. The challenge of long-term tetrahydrobiopterin (BH4) therapy in phenylketonuria: Effects on metabolic control, nutritional habits and nutrient supply. Mol. Genet. Metab. Rep. 2015, 4, 62–67. [Google Scholar] [CrossRef]
- Thiele, A.G.; Weigel, J.F.; Ziesch, B.; Rohde, C.; Mütze, U.; Ceglarek, U.; Thiery, J.; Müller, A.S.; Kiess, W.; Beblo, S. Nutritional Changes and Micronutrient Supply in Patients with Phenylketonuria Under Therapy with Tetrahydrobiopterin (BH4). JIMD Rep. 2012, 9, 31–40. [Google Scholar] [CrossRef]
- Macdonald, A.; Ahring, K.; Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; Motzfeldt, K.; Robert, M.; Rocha, J.C.; Van Rijn, M.; Bélanger-Quintana, A. Adjusting diet with sapropterin in phenylketonuria: What factors should be considered? Br. J. Nutr. 2011, 106, 175–182. [Google Scholar] [CrossRef][Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute (NHLBI). Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 September 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Andrade, F.; Llarena, M.; Blasco-Alonso, J.; Alcalde, C.; et al. Tetrahydrobiopterin therapy vs phenylalanine-restricted diet: Impact on growth in PKU. Mol. Genet. Metab. 2013, 109, 331–338. [Google Scholar] [CrossRef]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Llarena, M.; Andrade, F.; Blasco-Alonso, J.; Alcalde, C.; et al. 6R-tetrahydrobiopterin treated PKU patients below 4years of age: Physical outcomes, nutrition and genotype. Mol. Genet. Metab. 2015, 115, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bélanger-Quintana, A.; García, M.J.; Castro, M.; Desviat, L.R.; Pérez, B.; Mejía, B.; Ugarte, M.; Martínez-Pardo, M. Spanish BH4-responsive phenylalanine hydroxylase-deficient patients: Evolution of seven patients on long-term treatment with tetrahydrobiopterin. Mol. Genet. Metab. 2005, 86, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Brantley, K.D.; Douglas, T.D.; Singh, R.H. One-year follow-up of B vitamin and Iron status in patients with phenylketonuria provided tetrahydrobiopterin (BH4). Orphanet J. Rare Dis. 2018, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.; Blau, N. Effect of BH4 supplementation on phenylalanine tolerance. J. Inherit. Metab. Dis. 2008, 32, 40–45. [Google Scholar] [CrossRef]
- Demirdas, S.; Maurice-Stam, H.; Boelen, C.C.; Hofstede, F.C.; Janssen, M.C.; Langendonk, J.G.; Mulder, M.F.; Rubio-Gozalbo, M.E.; Van Spronsen, F.J.; De Vries, M.; et al. Evaluation of quality of life in PKU before and after introducing tetrahydrobiopterin (BH4); a prospective multi-center cohort study. Mol. Genet. Metab. 2013, 110, S49–S56. [Google Scholar] [CrossRef]
- Douglas, T.D.; Jinnah, H.A.; Bernhard, D.; Singh, R.H. The effects of sapropterin on urinary monoamine metabolites in phenylketonuria. Mol. Genet. Metab. 2013, 109, 243–250. [Google Scholar] [CrossRef]
- Douglas, T.D.; Ramakrishnan, U.; Kable, A.J.; Singh, R.H. Longitudinal quality of life analysis in a phenylketonuria cohort provided sapropterin dihydrochloride. Health Qual. Life Outcomes 2013, 11, 218. [Google Scholar] [CrossRef]
- Evers, R.A.; Van Wegberg, A.M.; Van Dam, E.; De Vries, M.C.; Janssen, M.C.; Van Spronsen, F.J. Anthropomorphic measurements and nutritional biomarkers after 5 years of BH 4 treatment in phenylketonuria patients. Mol. Genet. Metab. 2018, 124, 238–242. [Google Scholar] [CrossRef]
- Lambruschini, N.; Pérez-Dueñas, B.; Vilaseca, M.A.; Mas, A.; Artuch, R.; Gassió, R.; Gómez, L.; Gutiérrez, A.; Campistol, J. Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol. Genet. Metab. 2005, 86, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Leuret, O.; Barth, M.; Kuster, A.; Eyer, D.; De Parscau, L.; Odent, S.; Gilbert-Dussardier, B.; Feillet, F.; Labarthe, F. Efficacy and safety of BH4 before the age of 4 years in patients with mild phenylketonuria. J. Inherit. Metab. Dis. 2012, 35, 975–981. [Google Scholar] [CrossRef]
- Scala, I.; Concolino, D.; Della Casa, R.; Nastasi, A.; Ungaro, C.; Paladino, S.; Capaldo, B.; Ruoppolo, M.; Daniele, A.; Bonapace, G.; et al. Long-term follow-up of patients with phenylketonuria treated with tetrahydrobiopterin: A seven years experience. Orphanet J. Rare Dis. 2015, 10, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.H.; Quirk, M.E. Using change in plasma phenylalanine concentrations and ability to liberalize diet to classify responsiveness to tetrahydrobiopterin therapy in patients with phenylketonuria. Mol. Genet. Metab. 2011, 104, 485–491. [Google Scholar] [CrossRef]
- Singh, R.H.; Quirk, M.E.; Douglas, T.D.; Brauchla, M.C. BH4 therapy impacts the nutrition status and intake in children with phenylketonuria: 2-year follow-up. J. Inherit. Metab. Dis. 2010, 33, 689–695. [Google Scholar] [CrossRef]
- Ünal, Ö.; Gökmen-Özel, H.; Coşkun, T.; Özgül, R.K.; Yücel, D.; Hişmi, B.; Tokatlı, A.; Dursun, A.; Sivri, H.S. Sapropterin dihydrochloride treatment in Turkish hyperphenylalaninemic patients under age four. Turk. J. Pediatr. 2015, 57, 213–218. [Google Scholar] [PubMed]
- Vilaseca, M.A.; Lambruschini, N.; Gomez-Lopez, L.; Gutiérrez, A.; Moreno, J.; Tondo, M.; Artuch, R.; Campistol, J. Long-chain polyunsaturated fatty acid status in phenylketonuric patients treated with tetrahydrobiopterin. Clin. Biochem. 2010, 43, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Gökmen Özel, H.; Ünal, C.; Dursun, A.; Tokatli, A.; Çoşkun, T.; Sivri, H.S. Does diet liberalization impact anthropometric measures in PKU patients on relatively long-term BH4 treatment. J. Inherit. Metab. Dis. 2014, 37, 27–185. [Google Scholar] [CrossRef]
- Paras, A.; Bausell, H.; Arduini, K.; Johnson, A.; Kalb, F.; Widera, S.; Burton, B. Sapropterin Dihydrochloride As Sole Treatment In Subset of Patients With Non-Classical Phenylketonuria. In Proceedings of the American College of Medical Genetics Annual Clinical Genetics Meeting 2018, Charlotte, NC, USA, 10 April 2018. [Google Scholar]
- Rocha, J.C.; Almeida, M.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; Borges, N.; Macdonald, A.; Van Spronsen, F. Nutritional status in BH4 treated patients with phenylketonuria: Preliminary data from TNSPKU project. J. Inborn Errors Metab. Screen. 2017, 5, 90–91. [Google Scholar] [CrossRef][Green Version]
- Energy and Protein Requirements. In Report of a joint FAO/WHO/UNU Expert Consultation; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 1985; Volume 724, pp. 1–206.
- Lindegren, M.L.; Krishnaswami, S.; Reimschisel, T.; Fonnesbeck, C.; Sathe, N.A.; McPheeters, M.L. A Systematic Review of BH4 (Sapropterin) for the Adjuvant Treatment of Phenylketonuria. JIMD Rep. 2012, 8, 109–119. [Google Scholar] [CrossRef]
- Somaraju, U.R.; Merrin, M. Sapropterin dihydrochloride for phenylketonuria. Cochrane Database Syst. Rev. 2015, 2015, CD008005. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yang, T.; Wang, E.; Li, M.; Chen, C.; Ma, L.; Zhou, Y.; Cui, Y. Efficacy and safety of sapropterin dihydrochloride in patients with phenylketonuria: A meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2019, 85, 893–899. [Google Scholar] [CrossRef]
- Bueno, M.A.; Lage, S.; Delgado, C.; Andrade, F.; Couce, M.L.; González-Lamuño, M.; Pérez, M.; Aldámiz-Echevarría, L. New evidence for assessing tetrahydrobiopterin (BH4) responsiveness. Metabolism 2012, 61, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Van Wegberg, A.M.; Evers, R.A.; Van Dam, E.; De Vries, M.C.; Janssen, M.C.; Heiner-Fokkema, M.R.; Van Spronsen, F.J. Does the 48-hour BH4 loading test miss responsive PKU patients? Mol. Genet. Metab. 2020, 129, 186–192. [Google Scholar] [CrossRef]
- Ozel, H.G.; Lammardo, A.; Motzfeldt, K.; Robert, M.; Rocha, J.C.; Van Rijn, M.; Ahring, K.; Belanger-Quintana, A.; Macdonald, A.; Dokoupil, K. Use of sapropterin in the management of phenylketonuria: Seven case reports. Mol. Genet. Metab. 2013, 108, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Trefz, F.K.; Scheible, D.; Frauendienst-Egger, G.; Korall, H.; Blau, N. Long-term treatment of patients with mild and classical phenylketonuria by tetrahydrobiopterin. Mol. Genet. Metab. 2005, 86, 75–80. [Google Scholar] [CrossRef]
- Muntau, A.C.; Du Moulin, M.; Feillet, F. Diagnostic and therapeutic recommendations for the treatment of hyperphenylalaninemia in patients 0–4 years of age. Orphanet J. Rare Dis. 2018, 13, 1–9. [Google Scholar] [CrossRef]
| Reference | Country | Study Design | No. of Patients Tested/No. of Long-Term Responders a | Gender of Long-Term Responders (M/F) | Duration of BH4 Loading Test | BH4 Dose (Mean or Range; mg/kg/day) | Age at Initiation of BH4 (Mean or Range; Years) | Duration of Follow-up (Mean or Range; Years) | |
|---|---|---|---|---|---|---|---|---|---|
| Bélanger-Quintana 2005 [38] | Spain | Retrospective longitudinal single-center study | Total: mHPA: mPKU: mo/cPKU: | 50/7 b 7/- 22/7 21/- | n/a | 24 h | 5–20 † | 7.8 (range: 0.7–18) | 0.9 (range: 0.4–1.5) |
| Lambruschini 2005 [45] | Spain | Prospective longitudinal single-center study | Total: mHPA: mPKU: moPKU: cPKU: | 73/11 c - -/9 -/2 - | 4/7 | 24 h d | 5–10 † | 5.0 (range: 0.2–12.2) | 1.0 |
| Burlina 2009 [40] | Italy | Retrospective longitudinal single-center study | Total: | 30/12 e | n/a | 24 h | 10 † | 5.5 (range: 2.0–16.0) | 3.5 (range: 0.5–7.0) |
| Singh 2010 [49] | USA | Prospective longitudinal single-center study | Total: | 10/6 f | 6/0 | 1 week | 20 ‡ | 8.7 (range: 5–12) | 2.0 |
| Vilaseca 2010 [51] | Spain | Cross-sectional single-center study | Total: mHPA: mPKU: moPKU: cPKU: | 61/10 g - 5/3 21/7 35/- | n/a | 21 h | 5–15 † | 7.4 (range: 1.0–16.0) | 5.7 (range: 5.3–6.0) |
| Singh 2011 [48] Douglas 2013a [42] Douglas 2013b [43] Brantley 2018 [39] | USA | Prospective longitudinal single-center study | Total: | 57/17 h | 10/7 | 4 months | 20 ‡ | 16.6 (range: 6.1–36.8) | 1.0 |
| Hennermann 2012 [17] | Germany | Prospective longitudinal single-center study | Total: | 84/18 i | n/a | 24 h (n = 56) 8 h (n = 26) | 8–19 § | n/a | 4.0 (range: 0.7–8.8) |
| Leuret 2012 [46] | France | Retrospective longitudinal multicenter study | Total: mHPA: mPKU: moPKU: cPKU: | -/8 j - -/8 - - | n/a | 24 h | 8–24 § | 1.1 (range: 0.4–2.9) | 1.9 j (range: 0.6–6.7) |
| Aldámiz-Echevarría 2013 [36] | Spain | Retrospective longitudinal multicenter study | Cohort 1: Patients with 2 y follow-upk | ||||||
| Total: mHPA: mPKU: moPKU: cPKU: | -/36 - -/7 -/24 -/5 | 18/18 | 24 h (24 h or 1 week at one hospital after 2005) | 5–20 § | 5.0 | 2.0 | |||
| Cohort 2: Patients with 5 y follow-upk | |||||||||
| Total: mHPA: mPKU: moPKU: cPKU: | -/10 - -/1 -/9 - | 6/4 | 24 h (24 h or 1 week at one hospital after 2005) | 5–20 § | 5.2 | 5.0 | |||
| Demirdas 2013 [41] | The Netherlands | Prospective multicenter cohort study | Total: | 45/8 l | n/a | 48 h | n/a ‡ | n/a | range: 1.4–2.0 |
| Aldámiz-Echevarría 2015 [37] | Spain | Retrospective longitudinal multicenter study | Total: mHPA: mPKU: moPKU: cPKU: | -/22 - -/5 -/14 -/3 | 12/10 | 8 h or 12 h; (24 h or 1 week at one hospital after 2005) | 5–20 § | 1.4 (neonatal in n = 4) | 1.0 |
| Scala 2015 [47] | Italy | Prospective longitudinal multicenter study | Total: mHPA: mPKU: moPKU: cPKU: | 43/17 m -/3 -/8 -/4 -/2 | 11/6 | 48 h | 10 § | 15.1 (range: 7.0–22.0) | 5.7 (range: 1.0–7.0) |
| Thiele 2015 [29] | Germany | Retrospective longitudinal single-center study | Total: mHPA: mPKU: moPKU: cPKU: | -/8 -/3 -/3 -/1 -/1 | 5/3 | 6 weeks | 10–19 ‡ | 8.8 (range: 5.0–15.0) | 2.0 |
| Ünal 2015 [50] Gökmen Özel 2014 [52] | Turkey | Cross-sectional single-center study | Total: mHPA: mPKU: moPKU: cPKU: | -/51 n -/18 -/23 -/6 -/3 | 27/24 | 48 h | 20 ‡ | 5.4 (range: 0.5–14.0) | 2.5 (range: 0.5–4.0) |
| Feldmann 2017 [16] | Germany | Prospective longitudinal single-center study | Total: | 112/30 o | n/a | 2 weeks | 20 ‡ | n/a | 0.5 |
| Rocha 2017 [54] | Portugal | Retrospective single-center cohort study | Total: mHPA: mPKU: moPKU: cPKU: | -/9 p - - -/8 -/1 | 3/6 | 48 h | n/a ‡ | 16.6 (range: 9.0–28.0) | 1.0 (range: 0.3–1.4) |
| Evers 2018 [44] | The Netherlands | Retrospective multicenter cohort study | Total: | -/18 q | 5/13 | 48 h | 10–20 ‡ | 12.0 (range: 4.0–19.0) | 5.0 (range: 4.5–5.5) |
| Paras 2018 [53] | USA | Retrospective longitudinal single-center study | Total: | -/8 r | n/a | n/a | 20 ‡ | 5.8 (range: 0.4–18.0) | ≥0.3 |
| Study (Author, Year) | Items of “Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group” | Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Bélanger-Quintana 2005 [38] | x | + | ? | ? | + | + | + | NA | ? | x | + | NA | Fair |
| Lambruschini 2005 [45] | + | + | ? | + | + | + | + | NA | + | + | + | NA | Fair |
| Burlina 2009 [40] | + | + | ? | ? | + | + | + | NA | ? | x | + | NA | Fair |
| Singh 2010 [49] | + | + | ? | x | + | + | + | NA | + | + | + | NA | Fair |
| Vilaseca 2010 [51] | + | + | ? | ? | + | + | + | NA | ? | x | + | NA | Fair |
| Singh 2011 [48] Douglas 2013a [42] Douglas 2013b [43] Brantley 2018 [39] | + | + | ? | + | + | + | + | NA | + | + | + | NA | Good |
| Hennermann 2012 [17] | + | + | ? | x | ? | + | + | NA | + | x | + | NA | Fair |
| Leuret 2012 [46] | x | + | ? | ? | + | ? | + | NA | + | + | ? | NA | Fair |
| Aldámiz-Echevarría 2013 [36] | + | + | ? | ? | ? | + | + | NA | ? | x | + | NA | Fair |
| Demirdas 2013 [41] | + | + | ? | x | ? | ? | x | NA | ? | + | ? | NA | Poor |
| Aldámiz-Echevarría 2015 [37] | + | + | ? | ? | ? | + | + | NA | ? | + | + | NA | Fair |
| Scala 2015 [47] | x | + | ? | x | ? | + | + | NA | + | + | + | NA | Fair |
| Thiele 2015 [29] | + | + | ? | ? | + | + | + | NA | ? | + | + | NA | Fair |
| Ünal 2015 [50] Gökmen Özel 2014 [52] | + | + | + | ? | + | + | + | NA | ? | + | + | NA | Good |
| Feldmann 2017 [16] | + | + | ? | x | ? | + | + | NA | x | x | + | NA | Fair |
| Rocha 2017 [54] | + | x | ? | ? | + | ? | + | NA | ? | + | ? | NA | Fair |
| Evers 2018 [44] | + | + | ? | + | + | + | + | NA | ? | + | + | NA | Good |
| Paras 2018 [53] | + | x | x | ? | ? | x | + | NA | ? | x | ? | NA | Poor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilgaz, F.; Marsaux, C.; Pinto, A.; Singh, R.; Rohde, C.; Karabulut, E.; Gökmen-Özel, H.; Kuhn, M.; MacDonald, A. Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1040. https://doi.org/10.3390/nu13031040
Ilgaz F, Marsaux C, Pinto A, Singh R, Rohde C, Karabulut E, Gökmen-Özel H, Kuhn M, MacDonald A. Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(3):1040. https://doi.org/10.3390/nu13031040
Chicago/Turabian StyleIlgaz, Fatma, Cyril Marsaux, Alex Pinto, Rani Singh, Carmen Rohde, Erdem Karabulut, Hülya Gökmen-Özel, Mirjam Kuhn, and Anita MacDonald. 2021. "Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis" Nutrients 13, no. 3: 1040. https://doi.org/10.3390/nu13031040
APA StyleIlgaz, F., Marsaux, C., Pinto, A., Singh, R., Rohde, C., Karabulut, E., Gökmen-Özel, H., Kuhn, M., & MacDonald, A. (2021). Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis. Nutrients, 13(3), 1040. https://doi.org/10.3390/nu13031040