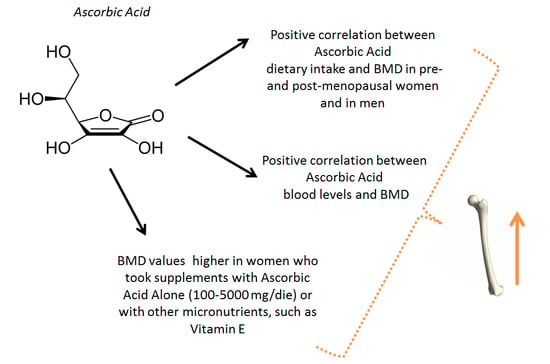
Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density
Abstract
1. Introduction
AA and Bone: In Vitro and Animal Model Studies
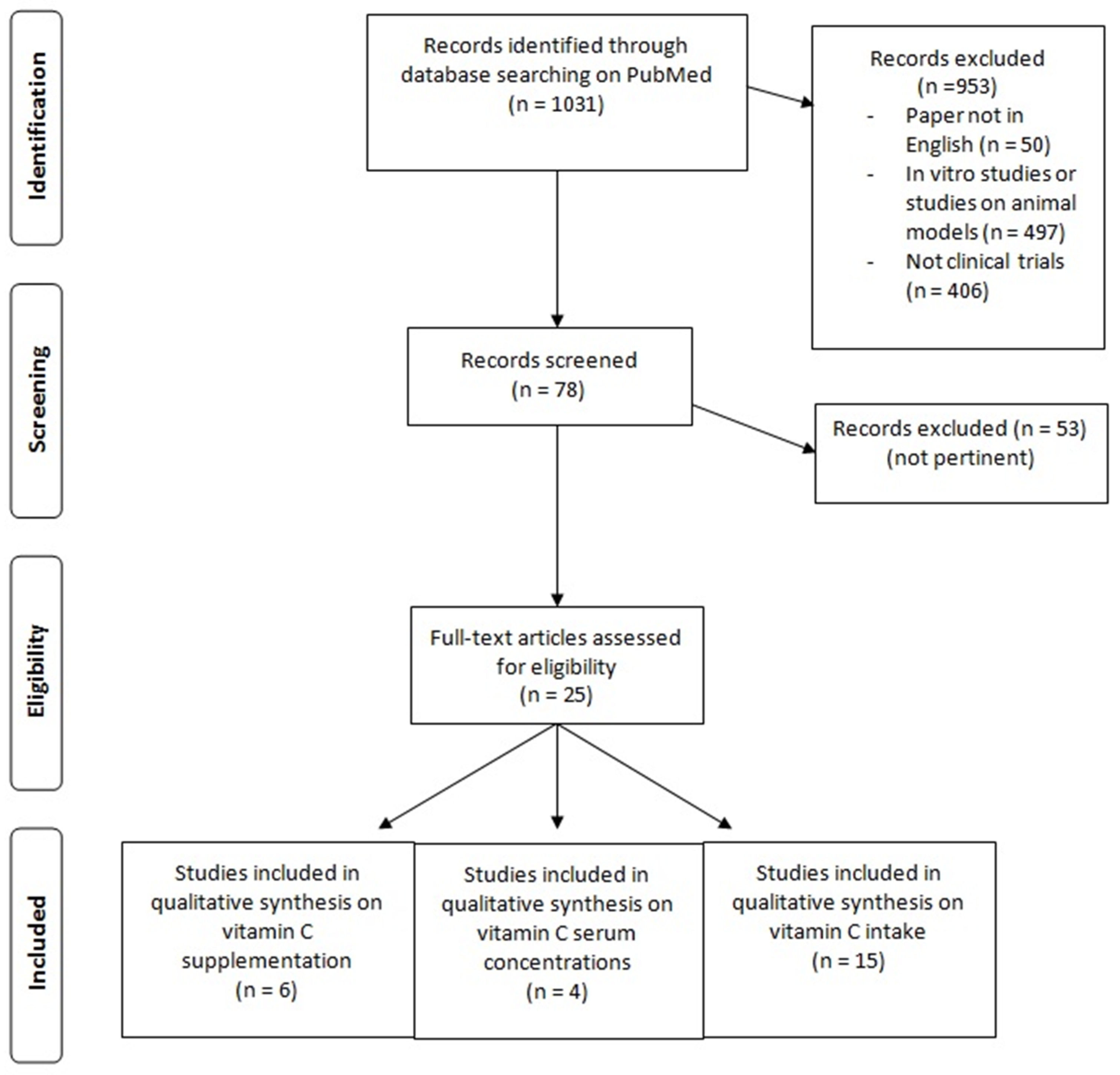
2. Materials and Methods
3. Results
3.1. Hematic Values of AA and Bone Metabolism
3.2. Dietary Intake of AA and Bone Metabolism
3.3. Supplementation with AA and Bone Metabolism
4. Discussion
4.1. Plasma Levels of AA and Bone Metabolism
4.2. Intake of AA and Effects on Bone Metabolism in Humans
4.3. Effects of AA on Bone Metabolism in Humans: Intervention Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haworth, W.; Hirst, E. Synthesis of ascorbic acid. J. Soc. Chem. Ind. 1933, 52, 645–647. [Google Scholar] [CrossRef]
- Carnovale, E.; Marletta, L. Tabelle di Composizione Degli Alimenti; Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione: Rome, Italy, 2013. [Google Scholar]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA. 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R. Assessment of human vitamin C status. J. Nutr. 1990, 120, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef]
- Kipp, D.E.; McElvain, M.; Kimmel, D.B.; Akhter, M.P.; Robinson, R.G.; Lukert, B.P. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone 1996, 18, 281–288. [Google Scholar] [CrossRef]
- Tsunenari, T.; Fukase, M.; Fujita, T. Bone histomorphometric analysis for the cause of osteopenia in vitamin C-deficient rat (ODS rat). Calcif. Tissue Int. 1991, 48, 18–27. [Google Scholar] [CrossRef]
- Bates, C. Vitamin C deficiency in guinea pigs: Variable sensitivity of collagen at different sites. Int. J. Vitam. Nutr. Res. 1979, 77–86. [Google Scholar]
- Tsuchiya, H.; Bates, C.J. Ascorbic acid deficiency in guinea pigs: Contrasting effects of tissue ascorbic acid depletion and of associated inanition on status indices related to collagen and vitamin D. Br. J. Nutr. 1994, 72, 745–752. [Google Scholar] [CrossRef]
- Leboy, P.; Vaias, L.; Uschmann, B.; Golub, E.; Adams, S.; Pacifici, M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J. Biol. Chem. 1989, 264, 17281–17286. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Temu, T.M.; Wu, K.Y.; Gruppuso, P.A.; Phornphutkul, C. The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E325–E334. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Miner. Res. 1992, 7, 235–246. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Iyer, B.S.; Cui, Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res. 1994, 9, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Tsuneto, M.; Yamazaki, H.; Yoshino, M.; Yamada, T.; Hayashi, S.I. Ascorbic acid promotes osteoclastogenesis from embryonic stem cells. Biochem. Biophys. Res. Commun. 2005, 335, 1239–1246. [Google Scholar] [CrossRef]
- Xiao, X.H.; Liao, E.Y.; Zhou, H.D.; Dai, R.C.; Yuan, L.Q.; Wu, X.P. Ascorbic acid inhibits osteoclastogenesis of RAW264.7 cells induced by receptor activated nuclear factor kappaB ligand (RANKL) in vitro. J. Endocrinol. Investig. 2005, 28, 253–260. [Google Scholar] [CrossRef]
- Le Nihouannen, D.; Barralet, J.E.; Fong, J.E.; Komarova, S.V. Ascorbic acid accelerates osteoclast formation and death. Bone 2010, 46, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care: Meta-Analysis in Context; BMJ Books: London, UK, 2001; ISBN 9780727914880. [Google Scholar]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Falch, J.A.; Mowé, M.; Bøhmer, T. Low levels of serum ascorbic acid in elderly patients with hip fracture. Scand. J. Clin. Lab. Investig. 1998, 58, 225–228. [Google Scholar] [CrossRef]
- Martínez-Ramírez, M.J.; Pérez, S.P.; Delgado-Martínez, A.D.; Martínez-González, M.Á.; De La Fuente Arrillaga, C.; Delgado-Rodríguez, M. Vitamin C, vitamin B12, folate and the risk of osteoporotic fractures. A case-control study. Int. J. Vitam. Nutr. Res. 2007, 77, 359–368. [Google Scholar] [CrossRef]
- Lumbers, M.; New, S.A.; Gibson, S.; Murphy, M.C. Nutritional status in elderly female hip fracture patients: Comparison with an age-matched home living group attending day centres. Br. J. Nutr. 2001, 85, 733–740. [Google Scholar] [CrossRef]
- Hall, S.L.; Greendale, G.A. The relation of dietary vitamin C intake to bone mineral density: Results from the PEPI study. Calcif. Tissue Int. 1998, 63, 183–189. [Google Scholar] [CrossRef]
- Kaptoge, S.; Welch, A.; McTaggart, A.; Mulligan, A.; Dalzell, N.; Day, N.E.; Bingham, S.; Khaw, K.T.; Reeve, J. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporos. Int. 2003, 14, 418–428. [Google Scholar] [PubMed]
- Leveille, S.G.; LaCroix, A.Z.; Koepsell, T.D.; Beresford, S.A.; Van Belle, G.; Buchner, D.M. Dietary vitamin C and bone mineral density in postmenopausal women in Washington State, USA. J. Epidemiol. Community Health 1997, 51, 479–485. [Google Scholar] [CrossRef]
- Pasco, J.A.; Henry, M.J.; Wilkinson, L.K.; Nicholson, G.C.; Schneider, H.G.; Kotowicz, M.A. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J. Women’s Health 2006, 15, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Hannan, M.T.; Gagnon, D.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. High vitamin C intake is associated with lower 4-year bone loss in elderly men. J. Nutr. 2008, 138, 1931–1938. [Google Scholar] [CrossRef]
- Sahni, S.; Hannan, M.T.; Gagnon, D.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture-a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos. Int. 2009, 20, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Brownbill, R.A.; Tamborini, L. Bone and nutrition in elderly women: Protein, energy, and calcium as main determinants of bone mineral density. Eur. J. Clin. Nutr. 2003, 57, 554–565. [Google Scholar] [CrossRef]
- New, S.A.; Bolton-Smith, C.; Grubb, D.A.; Reid, D.M. Nutritional influences on bone mineral density: A cross-sectional study in premenopausal women. Am. J. Clin. Nutr. 1997, 65, 1831–1839. [Google Scholar] [CrossRef]
- New, S.A.; Robins, S.P.; Campbell, M.K.; Martin, J.C.; Garton, M.J.; Bolton-Smith, C.; Grubb, D.A.; Lee, S.J.; Reid, D.M. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am. J. Clin. Nutr. 2000, 71, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.L.; Cauley, J.A.; Pettinger, M.; Jackson, R.; Lacroix, A.; Leboff, M.S.; Lewis, C.E.; Nevitt, M.C.; Simon, J.A.; Stone, K.L.; et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the Women’s Health Initiative. Am. J. Clin. Nutr. 2005, 82, 581–588. [Google Scholar] [CrossRef]
- Prynne, C.J.; Mishra, G.D.; O’Connell, M.A.; Muniz, G.; Laskey, M.A.; Yan, L.; Prentice, A.; Ginty, F. Fruit and vegetable intakes and bone mineral status: A cross sectional study in 5 age and sex cohorts. Am. J. Clin. Nutr. 2006, 83, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Hudes, E. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am. J. Epidemiol. 2001, 154, 427–433. [Google Scholar] [CrossRef]
- Park, H.M.; Heo, J.; Park, Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women. Nutr. Res. 2011, 31, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, H.J. Osteoporosis, vitamin c intake, and physical activity in korean adults aged 50 years and over. J. Phys. Ther. Sci. 2016, 28, 725–730. [Google Scholar] [CrossRef]
- Morton, D.J.; Barrett-Connor, E.L.; Schneider, D.L. Vitamin C supplement use and bone mineral density in postmenopausal women. J. Bone Miner. Res. 2001, 16, 135–140. [Google Scholar] [CrossRef]
- Chuin, A.; Labonté, M.; Tessier, D.; Khalil, A.; Bobeuf, F.; Doyon, C.Y.; Rieth, N.; Dionne, I.J. Effect of antioxidants combined to resistance training on BMD in elderly women: A pilot study. Osteoporos. Int. 2009, 20, 1253–1258. [Google Scholar] [CrossRef]
- Ruiz-Ramos, M.; Alberto Vargas, L.; Fortoul Van Der Goes, T.I.; Cervantes-Sandoval, A.; Mendoza-Núñez, V.M. Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J. Nutr. Health Aging 2010, 14, 467–472. [Google Scholar] [CrossRef]
- Chavan, S.N.; More, U.; Mulgund, S.; Saxena, V.; Sontakke, A.N. Effect of supplementation of vitamin C and E on oxidative stress in osteoporosis. Indian J. Clin. Biochem. 2007, 22, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Maïmoun, L.; Simar, D.; Caillaud, C.; Peruchon, E.; Sultan, C.; Rossi, M.; Mariano-Goulart, D. Effect of antioxidants and exercise on bone metabolism. J. Sports Sci. 2008, 26, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Stunes, A.K.; Syversen, U.; Berntsen, S.; Paulsen, G.; Stea, T.H.; Hetlelid, K.J.; Lohne-Seiler, H.; Mosti, M.P.; Bjørnsen, T.; Raastad, T.; et al. High doses of vitamin C plus E reduce strength training-induced improvements in areal bone mineral density in elderly men. Eur. J. Appl. Physiol. 2017, 117, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year [Reference] | Type of Study | Results |
|---|---|---|
| Aghajanian P., 2015 [7] | Animal model (rats and mice) | Vertebrate organisms deficient in AA develop bone disorders |
| Kipp D.E., 1996 [8] | Animal model (guinea pigs) | Scurvy but not food restriction, per se, results in alterations in bone mass and tissue collagen synthesis |
| Tsunenari T., 1991 [9] | Animal model (AA deficent-rats) | Osteopathy could be due to AA deficiency itself rather than malnutrition |
| Bates C.J., 1979 [10] | Animal model (guinea pigs) | Differential concentration of AA by different tissues seems more likely to be the critical factor |
| Tsuchiya, H., 1994 [11] | Animal model (guinea pigs) | Low tissue AA levels in guinea pigs alter the connective tissue composition of bones |
| Leboy P.S., 1989 [12] | In vitro | AA play a role in endochondral bone |
| Daniel, J.C., 1984 [13] | In vitro | AA facilitates the formation of an extracellular matrix in chondrocyte cultures |
| Temu, T.M., 2010 [14] | In vitro | AA promote the differentiation of ATDC5 cells |
| Franceschi, R.T. 1992 [15] | In vitro | Actions of AA on osteoblast marker gene expression are mediated by increases in collagen synthesis |
| Franceschi, R.T. 1994 [16] | In vitro | AA addition that allows subsequent induction of osteoblast-related genes |
| Tsuneto, M., 2005 [17] | In vitro | AA promote osteoclastogenesis of ES cells |
| Xiao, X.H., 2005 [18] | In vitro | AA inhibits receptor-activated nuclear factor kappaB ligand (RANKL)-induced differentiation of osteoclast |
| Le Nihouannen, D., 2010 [19] | In vitro | During osteoclastogenesis AA acts as an oxidant, first stimulating osteoclast formation, but later limiting osteoclast lifespan |
| First Author, Year [Reference] | Study Design | Setting | Number of Subjects (M-F) Mean Age | Primary Outcomes | Micronutrient Serum Concentration Osteoporosis | Micronutrient Serum Concentration Osteopenia | Micronutrient Serum Concentration Normal | Micronutrient Serum Reference Value | % Subjects < Reference Value | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Maggio D., 2003 [21] | Cross-sectional, case-control study | Free-living subjects | 150 F 75 osteoporosis (70.4 ± 8.5 y) 75 controls (68.8 ± 3.5 y) | Plasma AA, vitamin E, and A; uric acid; superoxide dismutase, glutathione peroxidase | Plasma AA: 30 ± 3.7 μmoL/L | // | Plasma AA: 55.5 ± 13.1 μmoL/L | // | None of the subjects belonging to the two groups had levels below the normal AA range. | Dietary and endogenous antioxidants consistently lower in osteoporotic than in control subjects |
| Falch J.A., 1998 [22] | Case-control study | Cases admitted to the hospital and home-living controls | 40 81 ± 5 y (20 cases with a recent hip fracture + 20 controls) | Serum AA concentration | Patients with a recent hip fracture: 34 ± 19 μmoL/L | // | Controls: 54 ± 30 μmoL/L | // | // | Serum AA concentration significantly lower in the hip fracture patients. |
| Martínez-Ramírez M.J., 2007 [23] | Hospital-based case-control study | Hospital of Jaén, Spain | 334 (167 cases + 167 controls) (80% F) | Osteoporotic fractures | In cases: 19.31 μmoL/L | // | In controls: 23.28 μmoL/L | // | // | Statistically significant difference between cases and controls for AA blood levels. Association between serum AA and fracture risk, with a significantly reduced risk for the upper quartile. |
| Lumbers M., 2001 [24] | Case-control study | Cases: hospital patients admitted for emergency surgery for fractured neck of femur. Controls: independent-living females | 125 F 75 cases (80.5 y) 50 controls (79.8 y) | Levels of plasma albumin, transferrin, C-reactive protein (CRP), cholesterol, AA, Se, Zn, total antioxidant status, Se-dependent glutathione peroxidase activity. | Hip fracture patients Plasma AA: 42.7 ± 21.4 μmoL/L | // | Controls Plasma AA: 20.8 ± 14.2 μmoL/L | // | // | Fracture patients: higher plasma AA levels. |
| First Author, Year [Reference] | Study Design | Setting | Number of Subjects (M-F) Mean Age | Lowest Quintile Intake/RDA or EAR | % Subject in Lowest Quintile Intake/% Subject < RDA or EAR | Highest Quintile Intake | % Subject in Highest Quintile Intake | Primary Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|
| Hall SL, 1998 [25] | randomized, double-blinded, placebo-controlled study | Data from PEPI study | 775 F aged 45–64 y | // | // | // | // | Cross-sectional relation between dietary AA intake and BMD. | Positive association between AA and BMD. 0.018 g/cm2 BMD increment for each AA additional 100 mg intake |
| Kaptoge S, 2002 [26] | Longitudinal study | Data from EPIC Norfolk study | 470 M–474 F aged 67–79 y | lowest tertile (7–57 mg/day) | // | upper tertile (99–363 mg/day) | // | Nutritional determinants of BMD loss from the hip in a community-based sample | Women in the lowest AA tertile intake lost BMD at an average rate of −0.65%, significantly faster compared to loss rates in the middle (−0.31%) and upper intake tertiles (−0.30%) |
| Leveille SG, 1997 [27] | Cross sectional study | Seattle area of Washington State | 1892 F 55–80 y | // | // | // | // | Relationship between dietary AA and hip BMD in postmenopausal women | No BMD differences according to diet-only AA intake or combined dietary AA and supplementation. Women who used AA supplements for >10 y had a higher BMD than non-users |
| Martínez-Ramírez MJ, 2007 [23] | Case-control study | Hospital of Jaén | 167 cases–167 controls | // | // | // | // | Influence of water-soluble vitamins on adequate bone tissue structure development | The AA intake has not been related to fracture risk |
| Pasco JA, 2006 [28] | Observational study | Barwon Statistical Division surrounding Geelong in southeastern Australia | 533 F | // | // | // | // | Associations among use of antioxidants, AA and vitamin E, serum bone turnover markers and BMD | Antioxidants, vitamin E or AA supplements may suppress bone resorption |
| Sahni S, 2008 [29] | Population-based cohort study | Data from Framingham Osteoporosis Study | 334 M–540 F 70–80 y | Lowest tertile: 80–160 mg/day | // | Highest tertile: 242–314 mg/day | // | Associations of supplemental/dietary AA intake with BMD at varius sites and 4-y BMD change | Higher dietary AA intake tended to be associated with lower femoral neck-BMD loss |
| Sahni S, 2009 [30] | Population-based cohort study | Data from Framingham Osteoporosis Study | 366 M–592 F 70–80 y | Lowest tertile (median: 94 mg/day) | // | Highest tertile (median: 313 mg/day) | // | Possible protective effect of AA on bone health | Subjects in the highest tertile of total AA intake had significantly fewer hip and non-vertebral fractures |
| Ilich JZ, 2003 [31] | Cross-sectional study | Eastern part of Connecticut | 136 F 68.7 ± 7.1 y | // | // | // | // | Relationship between various nutrients and BMD of several skeletal sites | AA has been significantly related to BMD of several skeletal sites. |
| New SA, 1997 [32] | Cross-sectional study | Data from Osteoporosis Screening Program—Aberdeen (Scotland) | 994 F, 45–49 y | 40 mg/day (EAR) | // | // | // | Association between dietary intake and BMD | The BMD across the quartiles of AA intake was nonlinear. BMD was higher in the third quartile and significantly different from the lowest quartile at all four sites even after adjustment for the confounding factors |
| New SA, 2000 [33] | Cross-sectional study | Data from Osteoporosis Screening Program—Aberdeen (Scotland) | 62 F, 45–55 y | 40 mg/day (EAR)/ | // | // | // | Association between micronutrients identified as important to BMD and bone heath indexes | A nonsignificant trend was seen for AA intake and deoxypyridinoline excretion. Low intakes of AA were associated with increased bone resorption |
| Wolf RL, 2005 [34] | Population-based cohort study | 3 clinic sites (Pittsburgh, PA; Birmingham, AL; Tucson, AZ) of Women’s Health Initiative (WHI) | 11,068 F, 50–79 y | // | // | // | // | Association between higher/total intakes, and serum antioxidants with higher BMD | A significant interaction effect has been demonstrated between intake of total AA and use of hormone therapy |
| Prynne CJ, 2006 [35] | Cross-sectional study | Cambridge (United Kingdom) | 436 (M-F) 16–83 y | Lowest tertile: 46–80 mg (boys) and 46–68 mg (girls) | Highest tertile: 134–181 mg (boys) and 124–169 mg (girls) | Associations between BMD and actual fruit and vegetable intakes, as estimated from 7-d food diaries | In boys only, femoral neck size-adjusted bone mineral content was significantly and positively associated with the intakes of both fruit and dietary AA | ||
| Simon J, 2001 [36] | Population-based cohort study | Data from NHANES III during 1988–1994 | 13080 (3778 premenopausal women, 3165 postmenopausal women, and 6137 men) 26–75 y | // | // | // | // | Relation of AA to BMD and the prevalence of self-reported fractures | Dietary AA intake was independently associated with BMD among premenopausal women. Among men dietary AA intake was associated in a nonlinear mode with self-reported fracture tucker |
| Park HM, 2011 [37] | Case-control study | 10 different hospitals in Seoul | 144 F (72 cases, 59.76 ± 0.5 y—72 controls 58.03 ± 0.66 y) | ≤ 91.54 mg/day (lowest quartile) | 32 cases—18 controls | 9 cases–18 controls | > 176.30 mg/day (highest quartile) | Examine the hypothesis that calcium from vegetable sources is associated with osteoporosis risk and BMD | Intake of vegetables and some nutrients as AA was associated with significantly reduced risk of osteoporosis |
| Kim MH, 2016 [38] | Case-control study | Data from the 2008, 2009, 2010, and 2011 Korean National Health and Nutritional Examination Survey (KNHANES) | 3047 (M-F) 64.6 ± 0.3 y | 0.0–45.0 mg/day (case group) | 452 subjects (case group) | 128.1–801.5 mg/day (case group) | 202 subjects (case group) | Associations between AA intake, physical activity, and osteoporosis | Higher AA intake levels have been associated with a lower risk of osteoporosis in Korean adults aged over 50 with low levels of physical activity |
| First Author, Year [Reference] | Study Design | Setting | Intervention | Parallel Treatments | Number of Subjects (M-F) | Duration of the Intervention | Primary Outcomes | Secondary Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|
| Morton D.J., 2001 [39] | Population-cohort study | Free-living subjects | Use of daily AA supplements: from 70 to 5000 mg/day (mean 745 mg/day) | Non use of AA supplements | 994 F 50–98 y (mean age 72 y) | Mean duration 12.4 y | BMD at the ultradistal radius and midshaft radius of the nondominant arm using single photon absorptiometry; femoral neck, total hip, and lumbar spine BMD by DXA. | // | In AA users BMD levels approximately 3% higher at the midshaft radius, femoral neck, and total hip. |
| Chuin A., 2009 [40] | Pilot randomized, controlled study | Free-living subjects | Antioxidants (n = 8) (600 mg/day vit E + 1000 mg/day AA) | Placebo (n = 7); resistance exercise (supervised 60-min sessions 3 times/week on alternating days) and placebo (n = 11); resistance exercise and antioxidants (n = 8). | 34 F 61–73 y (mean age 66.1 ± 3.3 y) | 6 months | Femoral neck and lumbar spine BMD (DXA) | // | Significant decrease in the placebo group for lumbar spine BMD, stable in all other groups. No changes for femoral neck BMD. |
| Ruiz-Ramos M., 2010 [41] | Randomized, double-blind, controlled clinical trial | Free-living subjects | Group Tx1 (n = 30): 500 mg AA + 400 IU vit E/day; group Tx2 (n = 30): 1000 mg AA + 400 IU vit E/day | Placebo (group Tx0, n = 30) | 90 (25 M-65 F) | 12 months | Thiobarbituric acid reactive substances, total antioxidant status, superoxide dismutase, glutation peroxidase; BMD of hip and spine (DXA). | // | Statistically significant positive correlation between hip-BMD and SOD activity and of GPx. In terms of BMD, less bone loss at the hip level in group Tx2 vs. group Tx0. |
| Chavan S.N., 2007 [42] | Randomized controlled study | Free-living subjects | 500 mg/day AA (group B, n = 25) | 400 mg/day vit E (group A, n = 25); 500 mg/day vit C + 400 mg/day vit E (group C, n = 25). | 75 | 90 days | Serum alkaline phosphatase, free or ionic calcium, inorganic phosphorus; tartrate resistant acid phosphatase, malondialdehyde; superoxide dismutase and erythrocyte reduced glutathione. | // | In group A: significant serum MDA and TrACP decrease after 45 and 90 days, non significant serum ALP decrease. In group B: significant decrease of serum MDA and TrACP after 45 days and 90 days, significant decrease of serum ALP only at 90 days. |
| Maïmoun L., 2008 [43] | Observational study | Free-living subjects | AA (500 mg) and vit E (100 mg) daily + supervised progressive aerobic training programme | // | 13 (mean age 73.9 ± 3.8 y) (4 M-9 F) | 8 weeks | Calcium homeostasis, bone cell activity (peripheral bone biochemical markers), bone related hormones. | // | 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations significantly increased by 42.8% and 26.8% respectively; parathyroid hormone concentration decreased by 17.5%. Bone alkaline phosphatase decreased by 14.6%. |
| Stunes A.K., 2017 [44] | Double-blinded, randomized, placebo-controlled study | Free-living subjects | 1000 mg AA+ 235 mg vit E daily (antioxidant group, AO, n = 16) + supervised strength training | Placebo (control group, CG, n = 17) + supervised strength training | 33 M | 12 weeks | Areal BMD at whole body, lumbar spine, total hip, and femoral neck (DXA), muscle strength by 1 RM. | Serum analyses of bone-related factors and adipokines. | In the controls: total hip aBMD increased by 1.0% versus pretest, and lumbar spine aBMD increased by 0.9% compared to the supplemented group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Peroni, G.; Fossari, F.; Vecchio, V.; Faliva, M.A.; Naso, M.; Perna, S.; Di Paolo, E.; Riva, A.; Petrangolini, G.; et al. Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density. Nutrients 2021, 13, 1012. https://doi.org/10.3390/nu13031012
Rondanelli M, Peroni G, Fossari F, Vecchio V, Faliva MA, Naso M, Perna S, Di Paolo E, Riva A, Petrangolini G, et al. Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density. Nutrients. 2021; 13(3):1012. https://doi.org/10.3390/nu13031012
Chicago/Turabian StyleRondanelli, Mariangela, Gabriella Peroni, Federica Fossari, Viviana Vecchio, Milena Anna Faliva, Maurizio Naso, Simone Perna, Enrica Di Paolo, Antonella Riva, Giovanna Petrangolini, and et al. 2021. "Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density" Nutrients 13, no. 3: 1012. https://doi.org/10.3390/nu13031012
APA StyleRondanelli, M., Peroni, G., Fossari, F., Vecchio, V., Faliva, M. A., Naso, M., Perna, S., Di Paolo, E., Riva, A., Petrangolini, G., Nichetti, M., & Tartara, A. (2021). Evidence of a Positive Link between Consumption and Supplementation of Ascorbic Acid and Bone Mineral Density. Nutrients, 13(3), 1012. https://doi.org/10.3390/nu13031012