Home Artificial Nutrition in Polish Children: An Analysis of 9-Year National Healthcare Provider Data
Abstract
1. Introduction
2. Materials and Methods
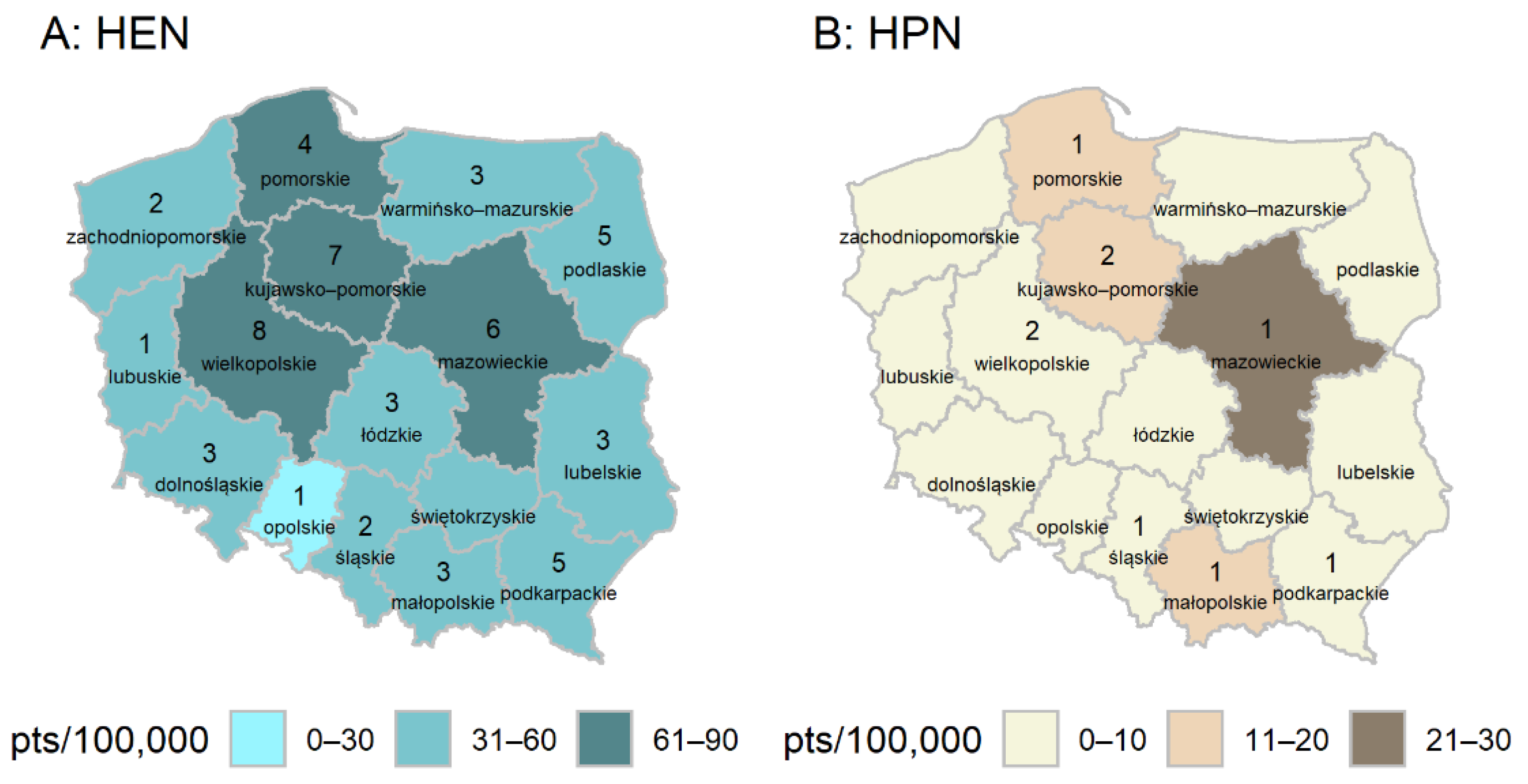
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ESPGHAN Committee on Nutrition. Practical Approach to Paediatric Enteral Nutrition: A Comment by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 110–122. [Google Scholar] [CrossRef]
- Toporowska-Kowalska, E.; Kierkuś, J.; Kudzin, J.; Wiernicka, A.; Matuszczyk, M.; Szczepański, M. Leczenie zywieniowe i zywienie dojelitowe. In Zywienie i leczenie zywieniowe dzieci i młodziezy, 1st ed.; Szajewska, H., Horvath, A., Eds.; Medycyna Praktyczna: Cracow, Poland, 2017; pp. 113–114. ISBN 978-83-7430-514-3. [Google Scholar]
- Yi, D.Y. Enteral Nutrition in Pediatric Patients. Pediatr. Gastroenterol. Hepatol. Nutr. 2018, 21, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Gawecka, A.; Ksiazyk, J. Zywienie pozajelitowe. In Zywienie i leczenie zywieniowe dzieci i młodziezy, 1st ed.; Szajewska, H., Horvath, A., Eds.; Medycyna Praktyczna: Cracow, Poland, 2017; p. 131. ISBN 978-83-7430-514-3. [Google Scholar]
- Hill, S.; Ksiazyk, J.; Prell, C.; Tabbers, M.; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Home parenteral nutrition. Clin. Nutr. 2018, 37, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, T.M.; Fairman, K.A. Pediatric Home Parenteral Nutrition: Indications and Short-Term Outcomes in a Large National Sample of Commercially Insured Children and Adolescents. Nutr. Clin. Pract. 2019, 34, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lezo, A.; Capriati, T.; Spagnuolo, M.I.; Lacitignola, L.; Goreva, I.; Di Leo, G.; Cecchi, N.; Gandullia, P.; Amarri, S.; Forchielli, M.L.; et al. Paediatric Home Artificial Nutrition in Italy: Report from 2016 Survey on Behalf of Artificial Nutrition Network of Italian Society for Gastroenterology, Hepatology and Nutrition (SIGENP). Nutrients 2018, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Pagano, M.C.; Pasanisi, F.; Contaldo, F. Home artificial nutrition: An update seven years after the regional regulation. Clin. Nutr. 2014, 33, 872–878. [Google Scholar] [CrossRef]
- Klek, S.; Hermanowicz, A.; Dziwiszek, G.; Matysiak, K.; Szczepanek, K.; Szybinski, P.; Galas, A. Home enteral nutrition reduces complications, length of stay, and health care costs: Results from a multicenter study. Am. J. Clin. Nutr. 2014, 100, 609–615. [Google Scholar] [CrossRef]
- Pedrón-Giner, C.; Navas-López, V.M.; Martínez-Zazo, A.B.; Martínez-Costa, C.; Sánchez-Valverde, F.; Blasco-Alonso, J.; Moreno-Villares, J.M.; Redecillas-Ferreiro, S.; Canals-Badía, M.J.; Rosell-Camps, A.; et al. Analysis of the Spanish national registry for pediatric home enteral nutrition (NEPAD): Implementation rates and observed trends during the past 8 years. Eur. J. Clin. Nutr. 2013, 67, 318–323. [Google Scholar] [CrossRef]
- Szlagatys-Sidorkiewicz, A.; Popińska, K.; Toporowska-Kowalska, E.; Borkowska, A.; Sibilska, M.; Gębora-Kowalska, B.; Kłęk, S.; Hapyn, E.; Kierkuś, J.; Grzybowska-Chlebowczyk, U.; et al. Home enteral nutrition in children--2010 nationwide survey of the Polish Society for Clinical Nutrition of Children. Eur. J. Pediatr. 2012, 171, 719–723. [Google Scholar] [CrossRef]
- Gómez-López, L.; Martínez-Costa, C.; Pedrón-Giner, C.; Calderón-Garrido, C.; Navas López, V.M.; Martínez Zazo, A.; Moreno Villares, J.M. Current status of pediatric home enteral nutrition in Spain: The importance of the NEPAD register. Nutr. Hosp. 2010, 25, 810–813. [Google Scholar] [CrossRef]
- Villar-Taibo, R.; Martínez-Olmos, M.Á.; Bellido Guerrero, D.; Peinó-García, R.; Martís-Sueiro, A.; Camarero-González, E.; Ríos-Barreiro, V.; Cao-Sánchez, P.; Durán Martínez, R.; Rodríguez Iglesias, M.J.; et al. Economic burden of home artificial nutrition in the health area of Santiago de Compostela. Nutr. Hosp. 2015, 32, 215–221. [Google Scholar] [CrossRef]
- Puntis, J.W. Nutritional support at home and in the community. Arch. Dis. Child. 2001, 84, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Daveluy, W.; Guimber, D.; Uhlen, S.; Lescut, D.; Michaud, L.; Turck, D.; Gottrand, F. Dramatic changes in home-based enteral nutrition practices in children during an 11-year period. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Shaffer, J.; Staun, M.; Hebuterne, X.; Bozzetti, F.; Pertkiewicz, M.; Thul, P.; Van Gossum, A. Home Artificial Nutrition Working Group—ESPEN. Survey on legislation and funding of home artificial nutrition in different European countries. Clin Nutr. 2001, 20, 117–123. [Google Scholar] [CrossRef]
- Castelló-Botía, I.; Wanden-Berghe, C.; Sanz-Valero, J. Artificial Nutritional Support Registries: Systematic review. Nutr. Hosp. 2009, 24, 711–716. [Google Scholar] [CrossRef]
- Annual BANS Report. Artificial Nutrition Support in the UK 2000–2010: A Report by the British Artificial Nutrition Survey (BANS), a Committee of BAPEN (The British Association for Parenteral and Enteral Nutrition). 2011. Available online: https://www.bapen.org.uk/pdfs/bans_reports/bans_report_11.pdf (accessed on 12 May 2012).
- The Ordinances of the President of the National Health Fund. Available online: https://www.nfz.gov.pl/zarzadzenia-prezesa/zarzadzenia-prezesa-nfz (accessed on 13 May 2009).
- Wanden-Berghe Lozano, C.; Cuerda Compes, C.; Maíz Jiménez, M.; Pereira Cunill, J.L.; Ramos Boluda, E.; Gómez Candela, C.; Virgili Casas, M.N.; Peláez, R.B.; de Luis Román, D.A.; Penacho Lázaro, M.Á.; et al. Nutrición parenteral domiciliaria en España 2018. Informe del Grupo de Nutrición Artificial Domiciliaria y Ambulatoria NADYA [Home and Ambulatory Artificial Nutrition (NADYA) Group Report. Home parenteral nutrition in Spain, 2018]. Nutr. Hosp. 2020, 37, 403–407. [Google Scholar] [CrossRef]
- Wanden-Berghe Lozano, C.; Pereira Cunill, J.L.; Cuerda Compes, C.; Ramos Boluda, E.; Maiz Jiménez, M.I.; Gómez Candela, C.; Virgili Casas, N.; Burgos Peláez, R.; Pérez de la Cruz, A.; Penacho Lázaro, M.Á.; et al. Nutrición parenteral domiciliaria en España 2017. Informe del Grupo de Nutrición Artificial Domiciliaria y Ambulatoria NADYA [Home and Ambulatory Artificial Nutrition (NADYA) Report. Home Parenteral Nutrition in Spain, 2017]. Nutr. Hosp. 2018, 35, 1491–1496. [Google Scholar] [CrossRef]
- Diamanti, A.; Di Ciommo, V.M.; Tentolini, A.; Lezo, A.; Spagnuolo, M.I.; Campanozzi, A.; Panetta, F.; Basso, M.S.; Elia, D.; Gambarara, M.; et al. Home enteral nutrition in children: A 14-year multicenter survey. Eur. J. Clin. Nutr. 2013, 67, 53–57. [Google Scholar] [CrossRef]
- Pedrón-Giner, C.; Calderón, C.; Martínez-Zazo, A.; Cañedo Villaroya, E.; Malillos González, P.; Sesmero-Lillo, M.Á. Home enteral nutrition in children: A 10 year experience with 304 pediatric patients. Nutr Hosp. 2012, 27, 1444–1450. [Google Scholar] [CrossRef]
- Mundi, M.S.; Pattinson, A.; McMahon, M.T.; Davidson, J.; Hurt, R.T. Prevalence of Home Parenteral and Enteral Nutrition in the United States. Nutr. Clin. Pract. 2017, 32, 799–805. [Google Scholar] [CrossRef]
- Beath, S.V.; Gowen, H.; Puntis, J.W. Trends in paediatric home parenteral nutrition and implications for service development. Clin. Nutr. 2011, 30, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O. 260.6: Pediatric Home Parenteral Nutrition (HPN) in France: A national survey on the behalf of the French Pediatric HPN network. Transplantation 2019, 103, S10. [Google Scholar] [CrossRef]
- Brooks, J.C.; Strauss, D.J.; Shavelle, R.M.; Tran, L.M.; Rosenbloom, L.; Wu, Y.W. Recent trends in cerebral palsy survival. Part II: Individual survival prognosis. Dev. Med. Child. Neurol. 2014, 56, 1065–1071. [Google Scholar] [CrossRef]
- Matuszczyk, M.; Rybak, A.; Szczepański, M.; Wiernicka, A.; Kierkuś, J. Home Enteral Nutrition in children—6 years of clinical experience. Postępy Nauk. Med. 2016, 4, 208–216. [Google Scholar] [CrossRef]
- Duro, D.; Kamin, D.; Duggan, C. Overview of pediatric short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Protheroe, S. Symposium 6: Young people, artificial nutrition and transitional care. Transition in young people on home parenteral nutrition. Proc. Nutr. Soc. 2009, 68, 441–445. [Google Scholar] [CrossRef]
- Allan, P.; Hogg, L.; Smith, M.; Howe, H.; Vokes, L.; Smith, A.; Hartley, J.; Vrakas, G.; Reddy, S.; Friend, P.; et al. 260.7: Effective transitioning of adolescents into adult intestinal transplant services—Survey of NITE members. Transplantation 2019, 103, S10. [Google Scholar] [CrossRef]
- Diamanti, A.; Capriati, T.; Lezo, A.; Spagnuolo, M.I.; Gandullia, P.; Norsa, L.; Lacitignola, L.; Santarpia, L.; Guglielmi, F.W.; De Francesco, A.; et al. Moving on: How to switch young people with chronic intestinal failure from pediatric to adult care. A position statement by italian society of gastroenterology and hepatology and nutrition (SIGENP) and italian society of artificial nutrition and metabolism (SINPE). Dig. Liver Dis. 2020, 52, 1131–1136. [Google Scholar] [CrossRef]
- Cuerda, C.; Planas, M.; Gómez Candela, C.; Luengo, L.M.; NADYA-SENPE group. Trends in home enteral nutrition in Spain: Analysis of the NADYA registry 1992–2007. Nutr. Hosp. 2009, 24, 347–353. [Google Scholar] [PubMed]

| Age Years | Enteral Nutrition (n = 3865) | Parenteral Nutrition (n = 626) |
|---|---|---|
| 0–2 | 1300 (33.6%) | 424 (67.7%) |
| 3–5 | 570 (14.7%) | 79 (12.6%) |
| 6–9 | 571 (14.8%) | 48 (7.7%) |
| 10–13 | 626 (16.2%) | 29 (4.6%) |
| 14–19 | 798 (20.6%) | 46 (7.3%) |
| Diagnosis Related Group | n (%) 1 |
|---|---|
| Symptoms, pathological features, and abnormal findings, not elsewhere classified | 914 (23.6%) |
| Symptoms and signs concerning food and fluid intake | 604 (15.6%) |
| Dysphagia | 147 (3.8%) |
| Lack of expected normal physical development | 129 (3.3%) |
| Unknown and unspecified causes of morbidity | 24 (0.6%) |
| Other | 10 (0.3%) |
| Diseases of the nervous system | 848 (21.9%) |
| Infantile cerebral palsy | 457 (11.8%) |
| Other disorders of brain | 130 (3.4%) |
| Spinal muscular atrophy and related syndromes | 87 (2.3%) |
| Paraplegia (paraparesis) and quadriplegia (quadriparesis) | 43 (1.1%) |
| Epilepsy | 39 (1.0%) |
| Primary disorders of muscles | 30 (0.8%) |
| Other | 62 (1.7%) |
| Endocrine, nutritional, and metabolic diseases | 845 (21.9%) |
| Cachexia | 348 (9.0%) |
| Protein-energy malnutrition of moderate and mild degree | 181 (4.7%) |
| Cystic fibrosis | 81 (2.1%) |
| Unspecified protein-calorie malnutrition | 77 (2.0%) |
| Disorders of sphingolipid metabolism and other lipid storage disorders | 52 (1.3%) |
| Unspecified severe protein-calorie malnutrition | 39 (1.0%) |
| Other metabolic disorders | 26 (0.7%) |
| Nutritional and metabolic disorders in diseases not elsewhere classified | 27 (0.7%) |
| Other | 14 (0.4%) |
| Factors influencing health status and contact with health services | 332 (8.6%) |
| Encounter for attention to artificial openings | 290 (7.5%) |
| Artificial opening status | 42 (1.1%) |
| Diseases of the digestive system | 302 (7.7%) |
| Crohn’s disease /regional enteritis | 187 (4.8%) |
| Gastro-esophageal reflux disease | 52 (1.3%) |
| Other | 63 (1.6%) |
| Congenital malformations, deformations, and chromosomal abnormalities | 177 (4.6%) |
| Other specified congenital malformation syndromes affecting multiple systems | 38 (1.0%) |
| Congenital malformations of esophagus | 26 (0.7%) |
| Other congenital malformations of brain | 23 (0.6%) |
| Edwards’ syndrome and Patau’s syndrome | 23 (0.6%) |
| Other congenital malformations, not elsewhere classified | 21 (0.5%) |
| Other | 46 (1.3%) |
| Diseases of the respiratory system | 32 (0.8%) |
| Respiratory failure, not elsewhere classified | 32 (0.8%) |
| Neoplasms | 25 (0.6%) |
| Malignant neoplasm of brain | 25 (0.6%) |
| Other * | 390 (10.1%) |
| Diagnosis Related Group | n (%) 1 |
|---|---|
| Diseases of the digestive system | 494 (78.9%) |
| Postprocedural disorders of digestive system, not elsewhere classified | 464 (74.1%) |
| Other functional intestinal disorders | 16 (2.6%) |
| Intestinal malabsorption | 14 (2.2%) |
| Endocrine, nutritional, and metabolic diseases | 21 (3.3%) |
| Protein-energy malnutrition of moderate and mild degree | 12 (1.9%) |
| Unspecified protein-calorie malnutrition | 9 (1.4%) |
| External causes of morbidity and mortality, and factors influencing health status and contact with health services | 18 (2.9%) |
| Acquired absence of organs, not elsewhere classified | 10 (1.6%) |
| Encounter for attention to artificial openings | 8 (1.3%) |
| Congenital malformations, deformations, and chromosomal abnormalities | 12 (1.9%) |
| Other congenital malformations of intestine | 12 (1.9%) |
| Other * | 81 (12.9%) |
| Duration of Treatment in Months | Enteral Nutrition (n = 743) | Parenteral Nutrition (n = 193) |
|---|---|---|
| 0–5 | 215 (28.9%) | 46 (23.8%) |
| 6–11 | 116 (15.6%) | 32 (16.6%) |
| 12–23 | 72 (9.7%) | 16 (8.3% |
| 24–35 | 48 (6.5%) | 14 (7.3%) |
| 36–47 | 35 (4.7%) | 5 (2.6%) |
| 48–59 | 38 (5.1%) | 12 (6.2%) |
| 60–107 | 219 (29.5%) | 68 (35.2%) |
| Diagnosis According to ICD 10 | Enteral Nutrition (n = 961) | Parenteral Nutrition (n = 60) |
|---|---|---|
| No data available | 503 (52.3%) | 19 (31.7%) |
| Cardiac arrest | 164 (17.1%) | 16 (26.7%) |
| Respiratory failure, not elsewhere classified | 74 (7.7%) | 9 (15.0%) |
| Other specified symptoms and signs involving the circulatory and respiratory systems | 61 (6.3%) | 0 (0.0%) |
| Heart failure | 57 (5.9%) | 8 (13.3%) |
| Childhood cerebral palsy | 15 (1.6%) | 0 (0.0%) |
| Other disorders of brain | 14 (1.5%) | 0 (0.0%) |
| Shock, not elsewhere classified | 13 (1.4%) | 0 (0.0%) |
| Other sepsis | 5 (0.5%) | 0 (0.0%) |
| Bronchopneumonia, unspecified organism | 8 (0.8%) | 0 (0.0%) |
| Other * | 47 (4.9%) | 8 (13.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszomirska, K.; Wyszomirski, A.; Brzeziński, M.; Borkowska, A.; Zagierski, M.; Kierkuś, J.; Książyk, J.; Romanowska, H.; Świder, M.; Toporowska-Kowalska, E.; et al. Home Artificial Nutrition in Polish Children: An Analysis of 9-Year National Healthcare Provider Data. Nutrients 2021, 13, 1007. https://doi.org/10.3390/nu13031007
Wyszomirska K, Wyszomirski A, Brzeziński M, Borkowska A, Zagierski M, Kierkuś J, Książyk J, Romanowska H, Świder M, Toporowska-Kowalska E, et al. Home Artificial Nutrition in Polish Children: An Analysis of 9-Year National Healthcare Provider Data. Nutrients. 2021; 13(3):1007. https://doi.org/10.3390/nu13031007
Chicago/Turabian StyleWyszomirska, Karolina, Adam Wyszomirski, Michał Brzeziński, Anna Borkowska, Maciej Zagierski, Jarosław Kierkuś, Janusz Książyk, Hanna Romanowska, Magdalena Świder, Ewa Toporowska-Kowalska, and et al. 2021. "Home Artificial Nutrition in Polish Children: An Analysis of 9-Year National Healthcare Provider Data" Nutrients 13, no. 3: 1007. https://doi.org/10.3390/nu13031007
APA StyleWyszomirska, K., Wyszomirski, A., Brzeziński, M., Borkowska, A., Zagierski, M., Kierkuś, J., Książyk, J., Romanowska, H., Świder, M., Toporowska-Kowalska, E., & Szlagatys-Sidorkiewicz, A. (2021). Home Artificial Nutrition in Polish Children: An Analysis of 9-Year National Healthcare Provider Data. Nutrients, 13(3), 1007. https://doi.org/10.3390/nu13031007






