Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
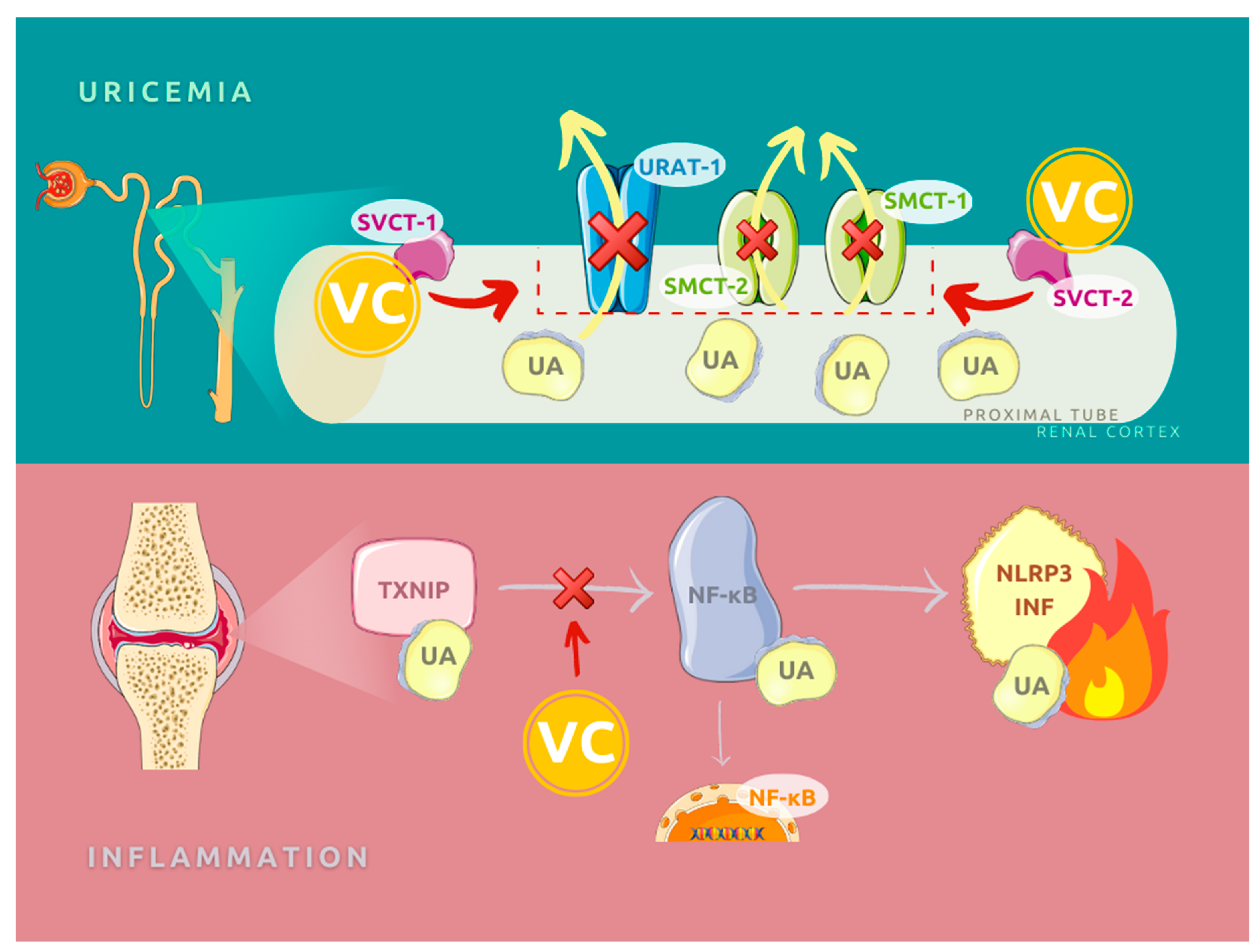
3.1. Molecular Function of Vitamin C in Gout Etiopathogenesis
3.2. Cross-Sectional Studies
| Study | Study Population | Dietary Assessment | Exclusion Criteria | Results |
|---|---|---|---|---|
| Bae et al. 2014 South Korea [53] | 9400 subjects: 3564 males 62.5 ± 9.6 years old 5836 females 61.6 ± 9.8 years old | 103-item food frequency questionnaire (FFQ) | Subjects with missing data, total energy intake <500, or >4000 kcal/day; ≥10 missing food items; or missing data on rice, (the staple food for most Koreans) | Mean UA concentration was significantly higher in males than females (5.8 ± 1.5 vs. 4.4 ± 1.1 mg/dL, p < 0.0001). In males and females with hyperuricemia, VC dietary intake was lower than in proper uric acid cases: respectively, 79.7 ± 54.1 vs. 86.0 ± 56.0 mg/day, p = 0.01 and 79.2 ± 60.9 vs. 86.0 ± 58.5 mg/day, p = 0.02. In total group, the difference was significant too (p < 0.001). The observed frequency of hyperuricemia decreased with increased dietary vitamin C intake in male and female subjects after multivariate adjustment (p for trend = 0.002 in males and p for trend = 0.02 in females). The relationship between total VC intake and the incidence of hyperuricemia was identified in females (p for trend = 0.04), but not males (p for trend = 0.06). |
| Ryu KA et al. 2014 South Korea [54] | 9010 subjects: 4869 males 4141 females 50.8 ± 9.65 years old | 3-days dietary recall (1 weekend and 2 weekdays) | No dietary record or less than 2 days of record Total energy intake <500 kcal or ≥3500 kcal Missing lab test results | Prevalence of hyperuricemia was 13.8% (27.1% men, 5.2% women). Hyperuricemia subjects had significantly lower intakes of VC than controls (57.5 ± 33.5 vs. 69.7 ± 39.8 mg/day, p < 0.001). |
| Zykova SN et al. 2015 Norway Australia [57] | 12,765 subjects: 4295 males 5439 females 25–91 years old Data from 2 study cohorts | 80-item FFQ/self-designed FFQ | Subjects younger than 25 years old Missing FFQ data or uric acid measurement Known or suspected myocardial infarction or ischemic stroke, pregnancy | In both cohorts mean UA concentration was significantly higher in males than females (5.73 ± 1.27 vs. 4.17 ± 1.0 mg/dL, p < 0.0 001, Australian Diabetes, Obesity and Lifestyle Study (AusDiab)) and 6.0 ± 1.42 vs. 4.48 ±1.1 mg/dL, p < 0.0001, Tromsø Study). Higher intake of vitamin C was associated with lower serum uric acid (SUA) levels only in females in the Australian cohort; in Norwegian study group, the difference was insignificant. The statistical difference was calculated for the lowest and the highest intake quartiles. |
| Sun Y et al. 2018 China [55] | 14,885 subjects: 7269 males 49.96 ± 17.77 years old 7516 females 49.28 ± 17.18 years old Pooled from three 2-yearcycles | 24 h dietary recall collected twice | Subjects younger than 20 years old Missing vitamin C (VC) daily intake data or blood sample Individuals whose total daily energy intake > mean + 3 standard deviations (4630 kcal) or < mean –3 standard deviations | Prevalence of hyperuricemia was 19.1% (21.5% men and 16.8% women). Both total and dietary VC intake was significantly lower in men with hyperuricemia (139 ± 227 vs. 170 ± 302 mg/day, p < 0.01 and 82.1 ± 84.9 vs. 93.0 ± 91.7 mg/d, p < 0.01) as well as in women subjects (142 ± 230 vs. 160 ± 227 mg/d, p = 0.03 and 70.0 ± 60.1 vs. 83.2 ± 72.3 mg/d, p < 0.01). Dietary vitamin C and total vitamin C intakes were negatively associated with the risk of hyperuricemia and the increase of OR was linear for successive quartiles. |
| Zheng Z et al. 2018 US [58] | 4576 subjects 1620 males 2956 females 55.5 ± 12.6 years old | 158-item FFQ | Missing VC daily intake data or blood sample Taking medication for gout | Prevalence of hyperuricemia was 25.6%. 9% were taking vitamin C supplements. 13% lower of hyperuricemia odds associated with a doubling of vitamin C intake (OR: 0.87, 95% CI: 0.78, 0.97). |
| So MW et al. 2020 South Korea [56] | 10,175 subjects 4200 males 5875 females ≥19 years old, mean 47 years old | single 24 h recall | Clinical history of hemophilia, anticoagulation therapy, chemotherapy in a month, poor vascular access, and age of 80 years or older | There was no statistically significant difference in daily VC intake between hyperuricemia and normouricemia for both men and women participants, respectively, 79 vs. 84 mg/day and 82 vs. 84 mg/day. In analysis of association between hyperuricemia and quartiles of VC intake the OR was significantly decreased with another increase in VC consumption. The highest quartile of dietary VC intake was negatively associated with the risk of hyperuricemia on men: OR = 0.79 (95% CI: 0.63–0.99). |
3.3. Case-Control Study
3.4. Longitudinal and Prospective Study
3.5. Interventional Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Pietrzak, K. Albert Szent-Györgyi (1893–1986): The scientist who discovered vitamin C. Clin. Dermatol. 2013, 31, 327–331. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Carpenter, K.J. The Discovery of Vitamin C. Ann. Nutr. Metab. 2012, 61, 259–264. [Google Scholar] [CrossRef]
- Maxfield, L.; Crane, J.S. Vitamin C Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef]
- Sauberlich, H.E. Pharmacology of Vitamin C. Annu. Rev. Nutr. 1994, 14, 371–391. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Namas, R.; Meysami, A.; Siegal, D.; Rubin, B. Gout and ultrasound: The disease of kings and the queen of imaging. Gout Hyperuricemia 2014, 1, 94–100. [Google Scholar] [CrossRef]
- Hansildaar, R.; Vedder, D.; Baniaamam, M.; Tausche, A.-K.; Gerritsen, M.; Nurmohamed, M.T. Cardiovascular risk in inflammatory arthritis: Rheumatoid arthritis and gout. Lancet Rheumatol. 2021, 3, e58–e70. [Google Scholar] [CrossRef]
- Rahimi-Sakak, F.; Maroofi, M.; Rahmani, J.; Bellissimo, N.; Hekmatdoost, A. Serum uric acid and risk of cardiovascular mortality: A systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc. Disord. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zeng, C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167–3181. [Google Scholar] [PubMed]
- Roman, Y.M.; Daniel, K. Inouye College of Pharmacy Scripts: Perspectives on the Epidemiology of Gout and Hyperuricemia. Hawaii J. Med. Public Health. 2019, 78, 71–76. [Google Scholar] [PubMed]
- Chen, L.; Han, S.; Liu, F.; Chen, S.; Chen, X.; Chen, H. Global prevalence of hyperuricemia in adolescents from 2000 to 2019: A meta-analysis. BMC Pediatrics 2020. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.; Cross, M.; Hill, C.; Smith, E.; Carson-Chahhoud, K.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Kaufman, J.; et al. Prevalence, deaths and disability adjusted life years (DALYs) due to musculoskeletal disorders for 195 countries and territories 1990-2017. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Chowalloor, P.V.; Keen, H.I. A systematic review of ultrasonography in gout and asymptomatic hyperuricaemia. Ann. Rheum. Dis. 2013, 72, 638–645. [Google Scholar] [CrossRef]
- Revaz, S.; Dudler, J. Clinical manifestations of gout. Rev. Med. Suisse 2007, 3, 728–730. (In French) [Google Scholar]
- Roddy, E.; Doherty, M. Gout. Epidemiology of gout. Arthritis Res. 2010, 12, 223. [Google Scholar] [CrossRef]
- Johnson, R.J.; Titte, S.; Cade, J.R.; Rideout, B.A.; Oliver, W.J. Uric acid, evolution and primitive cultures. Semin. Nephrol. 2005, 25, 3–8. [Google Scholar] [CrossRef]
- Wu, X.W.; Lee, C.C.; Muzny, D.M.; Caskey, C.T. Urate oxidase: Primary structure and evolutionary implications. Proc. Natl. Acad. Sci. USA 1989, 86, 9412–9416. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Muzny, D.M.; Lee, C.C.; Caskey, C.T. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J. Mol. Evol. 1992, 34, 78–84. [Google Scholar] [CrossRef]
- Oda, M.; Satta, Y.; Takenaka, O.; Takahata, N. Loss of Urate Oxidase Activity in Hominoids and its Evolutionary Implications. Mol. Biol. Evol. 2002, 19, 640–653. [Google Scholar] [CrossRef]
- Kratzer, J.T.; Lanaspa, M.A.; Murphy, M.N.; Cicerchi, C.; Graves, C.L.; Tipton, P.A.; Ortlund, E.A.; Johnson, R.J.; Gaucher, E. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc. Natl. Acad. Sci. USA 2014, 111, 3763–3768. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Sautin, Y.Y.; Oliver, W.J.; Roncal, C.; Mu, W.; Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Nakagawa, T.; Benner, S.A. Lessons from comparative physiology: Could uric acid represent a physiologic alarm signal gone awry in western society? J. Comp. Physiol. B 2008, 179, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. Potential of vitamin C in the prevention and treatment of gout. Nat. Rev. Rheumatol. 2011, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Shulten, P.; Thomas, J.; Miller, M.; Smith, M.; Ahern, M. The role of diet in the management of gout: A comparison of knowledge and attitudes to current evidence. J. Hum. Nutr. Diet. 2009, 22, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Liu, S.; Curhan, G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005, 52, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kobylecki, C.J.; Afzal, S.; Nordestgaard, B.G. Genetically high plasma vitamin C and urate: A Mendelian randomization study in 106 147 individuals from the general population. Rheumatol. 2018, 57, 1769–1776. [Google Scholar] [CrossRef]
- Kim, S.-K.; Choe, J.-Y.; Park, K.-Y. TXNIP-mediated nuclear factor-κB signaling pathway and intracellular shifting of TXNIP in uric acid-induced NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2019, 511, 725–731. [Google Scholar] [CrossRef]
- Choe, J.-Y.; Kim, S.-K. Quercetin and Ascorbic Acid Suppress Fructose-Induced NLRP3 Inflammasome Activation by Blocking Intracellular Shuttling of TXNIP in Human Macrophage Cell Lines. Inflammation 2017, 40, 980–994. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Yang, H. Conserved or Lost: Molecular Evolution of the Key Gene GULO in Vertebrate Vitamin C Biosynthesis. Biochem. Genet. 2013, 51, 413–425. [Google Scholar] [CrossRef]
- Shah, A.; Keenan, R.T. Gout, Hyperuricemia, and the Risk of Cardiovascular Disease: Cause and Effect? Curr. Rheumatol. Rep. 2010, 12, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Duplancic, D.; Kukoc-Modun, L.; Modun, D.; Radic, N. Simple and Rapid Method for the Determination of Uric Acid-Independent Antioxidant Capacity. Molecules 2011, 16, 7058–7067. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, B. Renal regulation of plasma total antioxidant capacity. Med. Hypotheses 2001, 56, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Fukae, J.; Fujioka, S.; Yanamoto, S.; Mori, A.; Nomi, T.; Hatano, T.; Fukuhara, K.; Ouma, S.; Hattori, N.; Tsuboi, Y. Serum uric acid level is linked to the disease progression rate in male patients with multiple system atrophy. Clin. Neurol. Neurosurg. 2017, 158, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Kim, H.R.; Song, J.S.; Choi, S.T. Low levels of serum urate are associated with a higher prevalence of depression in older adults: A nationwide cross-sectional study in Korea. Arthritis Res. 2020, 22, 104–114. [Google Scholar] [CrossRef]
- Keizman, D.; Ish-Shalom, M.; Berliner, S.; Maimon, N.; Vered, Y.; Artamonov, I.; Tsehori, J.; Nefussy, B.; Drory, V.E. Low uric acid levels in serum of patients with ALS: Further evidence for oxidative stress? J. Neurol. Sci. 2009, 285, 95–99. [Google Scholar] [CrossRef]
- Luo, X.L.J.J. A Double-edged Sword: Uric Acid and Neurological Disorders. Brain Disord. Ther. 2013, 2, 109. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Canas, J.-A.; Fanelli-Kuczmarski, M.T.; Tajuddin, S.M.; Evans, M.K.; Zonderman, A.B. Genetic risk scores, sex and dietary factors interact to alter serum uric acid trajectory among African-American urban adults. Br. J. Nutr. 2017, 117, 686–697. [Google Scholar] [CrossRef]
- Stein, H.B.; Hasan, A.; Fox, I.H. Ascorbic Acid-Induced Uricosuria. A Consequency of Megavitamin Therapy. Ann. Intern. Med. 1976, 84, 385–388. [Google Scholar] [CrossRef]
- Berger, L.; Gerson, C.D.; Yü, T.-F. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am. J. Med. 1977, 62, 71–76. [Google Scholar] [CrossRef]
- Mitch, W.E.; Johnson, M.W.; Kirshenbaum, J.M.; Lopez, R.E. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin. Pharmacol. Ther. 1981, 29, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.L.; Basu, T.K.; Dickerson, J.W. Effect of large doses of ascorbic acid in man on some nitrogenous components of urine. Hum. Nutr. Appl. Nutr. 1983, 37. [Google Scholar]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nat. Cell Biol. 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Torralba, K.D.; De Jesus, E.; Rachabattula, S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: Current and future directions. Int. J. Rheum. Dis. 2012, 15, 499–506. [Google Scholar] [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of Gout. Ann. Intern. Med. 2005, 143, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Ananth, S.; Martin, P.M.; Roon, P.; Smith, S.B.; Sterneck, E.; Prasad, P.D.; Ganapathy, V. c/ebpδ Null Mouse as a Model for the Double Knock-out of slc5a8 and slc5a12 in Kidney. J. Biol. Chem. 2006, 281, 26769–26773. [Google Scholar] [CrossRef]
- Nakagawa, T.; A Lanaspa, M.; Johnson, R.J. The effects of fruit consumption in patients with hyperuricaemia or gout. Rheumatol. 2019, 58, 1133–1141. [Google Scholar] [CrossRef]
- Bae, J.; Shin, D.H.; Chun, B.-Y.; Choi, B.Y.; Kim, M.K.; Shin, M.-H.; Lee, Y.-H.; Park, P.S.; Kim, S.-K. The effect of vitamin C intake on the risk of hyperuricemia and serum uric acid level in Korean Multi-Rural Communities Cohort. Jt. Bone Spine 2014, 81, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.A.; Kang, H.H.; Kim, S.Y.; Yoo, M.K.; Kim, J.S.; Lee, C.H.; Wie, G.A. Comparison of Nutrient Intake and Diet Quality Between Hyperuricemia Subjects and Controls in Korea. Clin. Nutr. Res. 2014, 3, 56–63. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Wang, J.; Gao, T.; Zhang, H.; Ma, A. Association between vitamin C intake and risk of hyperuricemia in US adults. Asia Pac. J. Clin. Nutr. 2018, 27, 1271–1276. [Google Scholar]
- So, M.W.; Lim, D.H.; Kim, S.H.; Lee, S. Dietary and nutritional factors associated with hyperuricemia: The seventh Korean National Health and Nutrition Examination Survey. Asia Pac. J. Clin. Nutr. 2020, 29, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Zykova, S.N.; Storhaug, H.M.; Toft, I.; Chadban, S.J.; Jenssen, T.G.; White, S.L. Cross-sectional analysis of nutrition and serum uric acid in two Caucasian cohorts: The AusDiab Study and the Tromsø study. Nutr. J. 2015, 14, 1–11. [Google Scholar] [CrossRef]
- Zheng, Z.; Harman, J.L.; Coresh, J.; Köttgen, A.; McAdams-DeMarco, M.A.; Correa, A.; Young, B.A.; Katz, R.; Rebholz, C.M. The Dietary Fructose:Vitamin C Intake Ratio Is Associated with Hyperuricemia in African-American Adults. J. Nutr. 2018, 148, 419–426. [Google Scholar] [CrossRef]
- Beck, K.L.; Houston, Z.L.; McNaughton, S.A.; Kruger, R. Development and evaluation of a food frequency questionnaire to assess nutrient intakes of adult women in New Zealand. Nutr. Diet. 2018, 77, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Poletto, J.; Harima, H.A.; Ferreira, S.R.G.; Gimeno, S.G.A. Hyperuricemia and associated factors: A cross-sectional study of Japanese-Brazilians. Cadernos de Saúde Pública 2011, 27, 369–378. [Google Scholar] [CrossRef]
- Choi, H.K.; Willett, W.; Curhan, G. Fructose-Rich Beverages and Risk of Gout in Women. JAMA 2010, 304, 2270–2278. [Google Scholar] [CrossRef]
- Lyu, L.-C.; Hsu, C.-Y.; Yeh, C.-Y.; Lee, M.-S.; Huang, S.-H.; Chen, C.-L. A case-control study of the association of diet and obesity with gout in Taiwan. Am. J. Clin. Nutr. 2003, 78, 690–701. [Google Scholar] [CrossRef]
- Gao, X.; Curhan, G.; Forman, J.P.; Ascherio, A.; Choi, H.K. Vitamin C intake and serum uric acid concentration in men. J. Rheumatol. 2008, 35, 1853–1858. [Google Scholar]
- Choi, H.K.; Gao, X.; Curhan, G. Vitamin C Intake and the Risk of Gout in Men. Arch. Intern. Med. 2009, 169, 502–507. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Fanelli-Kuczmarski, M.T.; Canas, J.-A.; Beydoun, H.A.; Evans, M.K.; Zonderman, A.B. Dietary factors are associated with serum uric acid trajectory differentially by race among urban adults. Br. J. Nutr. 2018, 120, 935–945. [Google Scholar] [CrossRef]
- Beser, E. The effects of short-term vitamin C on plasma bun, uric acid, cholesterol and triglyceride levels. Acta medica Hung. 1991, 48, 73–78. [Google Scholar]
- Yanai, H.; Morimoto, M. Effect of ascorbate on serum lipids and urate metabolism during exhaustive training. Clin. Sci. 2004, 106, 107–109. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Appel, L.J.; Choi, M.J.; Gelber, A.C.; Charleston, J.; Norkus, E.P.; Miller, E.R., III. The effects of vitamin C supplementation on serum concentrations of uric acid: Results of a randomized controlled trial. Arthritis Rheum. 2005, 52, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Simsek, M.; Naziroğlu, M. Effects of hormone replacement therapy with vitamin C and E supplementation on plasma thyroid hormone levels in postmenopausal women with Type 2 diabetes. Biomed. Pharmacother. 2009, 63, 717–722. [Google Scholar] [CrossRef]
- Hunter, D.C.; Brown, R.; Green, T.; Thomson, C.; Skeaff, M.; Williams, S.; Todd, J.M.; Lister, C.E.; McGhie, T.; Zhang, J.; et al. Changes in markers of inflammation, antioxidant capacity and oxidative stress in smokers following consumption of milk, and milk supplemented with fruit and vegetable extracts and vitamin C. Int. J. Food Sci. Nutr. 2011, 63, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Biniaz, V.; Tayebi, A.; Ebadi, A.; Shermeh, M.S.; Einollahi, B. Effect of vitamin C supplementation on serum uric acid in patients undergoing hemodialysis: A randomized controlled trial. Iran. J. Kidney Dis. 2014, 8. [Google Scholar]
- El Mashad, G.; Elsayed, H.; Nosair, N. Effect of vitamin C supplementation on lipid profile, serum uric acid, and ascorbic acid in children on hemodialysis. Saudi J. Kidney Dis. Transplant. 2016, 27, 1148. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.R.; Huq, S.; A Saleh, A.; Hakim, F.; Azad, A.K. Efficacy of Vitamin C in Lowering Serum Uric Acid. Mymensingh Med. J. 2016, 25, 681–685. [Google Scholar]
- Peng, H.; Feng, D.; Wang, Y.; Dong, Z.; Chen, Q.; Zhang, L.; Luo, R.; Chen, J.; Wang, A.; Ma, S. Effect of Oral Vitamin C Supplementation on High-Altitude Hyperuricemia in Young Men Initially Migrating to High Altitude: A Pilot Study. High Alt. Med. Biol. 2018, 19, 373–381. [Google Scholar] [CrossRef]
- Stamp, L.K.; O’Donnell, J.L.; Frampton, C.; Drake, J.M.; Chapman, P.T.; Zhang, M. Clinically Insignificant Effect of Supplemental Vitamin C on Serum Urate in Patients with Gout: A Pilot Randomized Controlled Trial. Arthritis Rheum. 2013, 65, 1636–1642. [Google Scholar] [CrossRef]
- Azzeh, F.S.; Al-Hebshi, A.H.; Al-Essimii, H.D.; Alarjah, M.A. Vitamin C supplementation and serum uric acid: A reaction to hyperuricemia and gout disease. PharmaNutrition 2017, 5, 47–51. [Google Scholar] [CrossRef]
- Brites, F.D.; Evelson, P.A.; Christiansen, M.G.; Nicol, M.F.; Basílico, M.J.; Wikinski, R.W.; Llesuy, S.F. Soccer players under regular training show oxidative stress but an improved plasma antioxidant status. Clin. Sci. 1999, 96, 381–385. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Miller, E.R.; Gelber, A.C. Effect of oral vitamin C supplementation on serum uric acid: A meta-analysis of randomized controlled trials. Arthritis Rheum. 2011, 63, 1295–1306. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Population | Follow-Up Duration | Dietary Assessment | Exclusion Criteria and End Point | Results |
|---|---|---|---|---|---|
| Gao X et al. 2008 US [63] | 1387 men Age 40–75 years old | FFQ was collected every two years for 8 years before blood sample collection 8 years | 131-item FFQ | BMI ≥ 30kg/m2, hypertension, blood sample drawn after fasting < 8 h at the beginning of the study | Greater VC supplement intake was significantly associated with a lower serum uric acid (p for trend < 0.001). Although higher dietary VC intake categories tended to have lower serum uric acid levels than the lowest category, the linear trend was not significant (p for trend = 0.10). The multivariate ORs for hyperuricemia across total VC intake categories were 1 (reference), 0.58, 0.57, 0.38, and 0.34 (95% CI: 0.20–0.58; p for trend < 0.001). The multivariate OR for the highest versus lowest categories of total VC intake was 0.31 (95% CI: 0.17–0.56, p for trend = 0.009). |
| Choi HK et al. 2009 US [64] | 46,994 men Age 40–75 years old | 20 years vitamin C intake was verified every 4 years | >130-item FFQ | Gout in history End point–case of gout | During the 20 years of follow-up, 1317 cases of gout incidents were confirmed Relative risk of gout in cases of VC intake < 250mg/day was 0.83 (95% confidence interval CI: 0.71 to 0.97) for total VC intake 500–999 mg/day; 0.66 (0.52 to 0.86) for 1000–1499 mg/day, and 0.55 (0.38 to 0.80) for ≥1500 mg/day (p for trend < 0.001). |
| Beydoun MA et al. 2018 US [65] | 2138 participants 1238 African American, 900 white urban adults 973 males 1165 females aged 30–64 years old 1208 women 930 men | 1–8 years Mean 4.64 ± 0.93 years | 24 h dietary recall collected twice and dietary supplements questionnaire adapted from NHANES 2007–08 | x | Supplemental vitamin C may have putative protective effects among both whites and African Americans. |
| Study | Study Population | Follow-Up Duration | Intervention | Exclusion Criteria | Results |
|---|---|---|---|---|---|
| Stein HB et al. 1976 Canada [44] | 14 subjects 10 males and 4 females 5 with gout, 3 hyperuricemia, 6 controls | 8 h/ 3–7 days | Diet: 2600 kcal, 70 g of protein, purine free
48 h brake between experiments 3 subjects administrated 8.0 g of VC by 3–7 days | Drug known to interfere with uric acid metabolism or excretion. | 4.0 g VC intake caused maximum increase to 202 ± 41% uric acid clearance. The peak effect varied between 6 to 8 h (p < 0.01). 2.0 g VC intake increase to 152 ± 24% UA clearance (p > 0.05). 0.5 g VC intake increase to 128 ± 6% UA clearance (p > 0.05). ASA intake decreased UA clearance. In prolonged VC administration, the UA clearance was increased to 174% ± 24% (p < 0.01) of the control values, and the effect was maintained for 1 to 2 days thereafter. In this group the serum uric acid decreased by 1.2 to 3.1 mg/dL because of a sustained uricosuria. |
| Mitch WE et al. 1981 US [46] | 6 healthy subjects 4 males 2 females Age 22–42 years old | 1month | 4 or 12 g of VC in 4 separate doses/ day | History of gout, hypertension, renal disease, or other diseases associated with abnormal uric acid metabolism No drugs or vitamins were taken for at least 1 week before study | There was no effect on serum uric acid concentration or uric acid excretion and clearance by the kidney. |
| Sutton JL et al. 1983 * UK [47] | 16 healthy subjects | 7 days | 1 g of VC 3 times/day | Data not available | Transient increase in uric acid excretion was observed. |
| Baser E* 1991 Turkey [66] | 152 healthy subjects 20.0 ± 0.38 years old | 1 month | 105 subjects: 500 mg of VC once/day 47 subjects: placebo | Data not available | There was no significant effect of VC on plasma uric acid concentration (p > 0.05). |
| Yanai H et al. 2004 Japan [67] | 8 well-trained male athletes | 3 weeks | 4 subjects: 1 g VC 4 subjects: placebo unified diet and training program | After training in the placebo group, significant increase of UA serum concentration was observed (4.48 ± 0.83 mg/dL before training vs. 6.15 ± 0.47 mg/dL in the end of the study, p < 0.05). In VC supplementing group, the UA concentration stayed stable (5.98 ± 0.67 vs. 5.13 ± 0.52 mg/dL; p > 0.05). | |
| Huang HY et al. 2005 US [68] | 184 subjects 74 males 110 females Age 58.15 ± 13.65 years old | 2 months | 92 subjects: 500 mg of VC once/day 92 subjects: placebo | Regular exposure to passive tobacco smoke for ≥1 h/ day or consumption of ≥14 servings of alcoholic beverages/week. 2-month period of supplement abstinence before study beginning was required. | Baseline VC dietary intake was equal. At the end of the supplementation period, the serum UA concentration was significantly reduced in the active vitamin C group but not in the placebo group (mean change 0.09, 95% CI: −0.05 to 0.2 vs. −0.5, 95% CI: −0.6 to −0.3, p< 0.0001). Serum UA was inversely correlated with changes in serum ascorbic acid −0.32 (p < 0.0002) in the VC group. Among persons who were hyperuricemic at baseline (n = 21), VC supplementation reduced serum UA by a mean of 1.5 mg/dL (p = 0.0008). |
| Naziroğlu M et al. 2009 Turkey [49] | 120 subjects 40 non-diabetic postmenopausal women 40 postmenopausal women with DM2 Age 45–65 years old 40 young controls Age 19–28 years old | 1.5 months | 40 control subjects–without intervention 40 postmenopausal non-diabetic subjects–HRT 20 postmenopausal diabetic subjects-HRT 20 postmenopausal diabetic subjects–HRT + 1 g VC and 600 mg VE | Women taking insulin or lipid lowering therapy or antioxidant vitamins within the last 6 months or HRT within the last 3 months Control group had not been taking oral contraceptives for at least 6 months before the blood sample collection | The UA concentration in group of postmenopausal diabetic women was significantly higher than in control group (3.4 ± 0.1 vs. 2.4 ± 0.5 mg/dL, p < 0.01). In this group after 6 weeks of HRT together with vitamins C and E supplementation, a significant decrease of UA serum concentration compared to baseline was observed (3.4 ± 0.1 vs. 2.9 ± 0.8 mg/dL, p < 0.05). |
| Hunter DC et al. 2011 New Zeland [70] | 48 smokers 18 males 25 females Age 44.3 ± 8.1 years old | 1.5 months | Nonsupplemented milk Prototype supplemented milk (i.e., 200mg VC/serve) 200mL of milk twice a day | Younger than 30 years old Chronic disease (e.g., diabetes, cardiovascular disease) Taking medications known to interfere with the immune system used nutritional supplements | In supplemented milk group, serum UA concentration was significantly lower after 6 weeks than in baseline (p = 0.026), and fasting concentrations of plasma vitamin C were significantly higher (p < 0.001). |
| Stamp LK et al. 2013 New Zeland [75] | 40 patients with gout or hyperuricemia 36 males 4 females Mean age 58.1 years old | 2 months | 10 subjects: Start allopurinol 50–100 mg/d (after 8 weeks the dose range 100–300 mg/day) 10 subjects: VC 500 mg/d 10 subjects: increase previous allopurinol dose (final dosage 150–500 mg/day) 10 subjects: allopurinol in dose as before + VC 500 mg/d | Taking over-the-counter vitamin supplements | There was no significant reduction of UA concentration between baseline and final sampling in group of patients with supplementation of VC. The reduction in UA levels was significantly lower in the 20 patients receiving VC compared to those who started receiving or increased the dose of allopurinol (mean reduction 0.23 vs. 1.98 mg/dL; p < 0.001). Results showed that supplemental VC at a dosage of 500 mg/day did not lead to a clinically significant reduction in the UA level in patients with gout. |
| Binaz V et al. 2014 Iran [71] | 172 hemodialysis patients 102 males 63 females Age 61.54 ± 12.72 years old | 2 months | 59 subjects: 250 mg of VC 3 times/week 58 subjects: Placebo 55 subjects: No intervention | 7 were excluded from the study due to transition to other dialysis centers, being infected by active infections, cancer, death, or refusal to continue participation | 46.7% patients had hyperuricemia. At baseline, the UA concentration was similar in all study groups. After 8 weeks of study, UA serum concentration was significantly lower in VC supplemented group than in both controls (5.8 ± 1.3 vs. 6.4 ± 1.3 and 6.3 ± 1.1 mg/dL, p = 0.02). In patients with hyperuricemia (n = 80), the decrease of UA after 8 weeks of VC supplementation was significantly lower than in both controls (5.90 ± 1.30 vs. 6.80 ± 0.64 and 6.80 ± 1.01 mg/dL, p = 0.004). |
| El Mashad GM et al. 2016 [72] | 60 children with end-stage renal disease 29 males 31 females Age 8.85 ± 10.2 | 3 months | 30 subjects: 250 mg VC i.v. 3 times/week 30 subjects: placebo (saline i.v.) | Primary (nonuremic) cardiovascular disease Taking VC supplementation during the last three months Participants in another clinical trial | Serum mean uric acid levels were 8.06 ± 1.8 mg/dL and ascorbic acid levels were 8.90 ± 4.06 μmol/L (norm in children > 28.4 μmol/L). In the supplemented group, statistically significant increase of VC serum concentration was observed (8.97 ± 4.38 vs. 22.06 ± 9.59 μmol/L, p < 0.0001) as well as significant decrease of UA concentration (8.33 ± 1.61 vs. 5.95 ± 0.75 mg/dL, p < 0.0001) between baseline and final sampling. |
| Choudhury MR et al. 2016 Bangladesh [73] | 71 subjects–patients with musculoskeletal disorder except gout 25 males 46 females Age 34.13 ± 13.5 years old | 3 months | 37 subjects: VC 500 mg/d 34 subjects: placebo | Gout or any malignant disorder in clinical history Smokers and heavy drinkers | In VC supplementing group, mean decrease of serum UA was −0.32 mg/dL (95% CI: −0.73 to 0.77), while in the placebo group, the mean change was + 0.12 mg/dL (95% CI: −0.22 to 0.47). Subjects were split into subgroups uniform in terms of sex, BMI, and baseline UA concentration—patients with a higher serum UA level had greater benefit from VC supplementation. |
| Azzech FS et al. 2017 Saudi Arabia [76] | 30 subjects 15 with gout 52.85 ± 11.36 years old 15 with hyperuricemia 54.23 ± 12.26 years old 16 males 14 females Aged 24–75 years old | 2 months | All subjects: VC 500 mg/d | Patients less than 20 years, history of dialysis, alcohol consumption, pregnant or lactating women, multivitamin supplements during the last three months, and diuretic drug and/or any uricosuric agent | After 8 weeks in gout group, the mean UA concentration was nonsignificantly higher than at the baseline (8.4 ± 1.15 vs. 8.09 ± 1.09 mg/dL, p > 0.05), while in hyperuricemia group, a significant decrease was observed (7.16 ± 1.04 vs. 7.94 ± 0.93 mg/dL, p < 0.05). The reduction of UA was slightly higher for women than men in hyperuricemia group. |
| Peng H et al. 2018 China [74] | 66 male army recruits Age 19.25 ± 1.5 years old | 1 month | 33 subjects: VC 500 mg/d 33 subjects: placebo | Any chronic disease and long-term medication necessity Previously lived at an altitude > 2500 m Not completing follow-up | At 1 month, UA concentration was significantly higher than at baseline (7.33 ± 1.33 vs. 6.02 ± 1.34 mg/dL, p < 0.001); prevalence of hyperuricemia was also significantly higher (63.6 vs. 19.7%, p < 0.001). In VC supplemented group, both the level of serum UA (6.92 ± 1.25 vs. 7.75 ± 0.92 mg/dL, p = 0.003) and the prevalence of hyperuricemia (48.5 vs. 78.8%; p = 0.020) were lower. |
| Peng H et al. 2018 China [74] | 120 male army recruits | 1 month | 57 subjects: VC 500 mg/d 58 subjects: VE 75 IU/d | Any chronic disease and long-term medication necessity Previously lived at an altitude > 2500 m Not completing follow-up | At 1 month, UA concentration was significantly higher than at baseline (7.32 ± 1.37 vs. 6.85 ± 1.19 mg/dL, p = 0.053), and a higher prevalence of hyperuricemia (59.6 vs. 43.1%, p = 0.076) was observed. Both were higher in the vitamin C group relative to the vitamin E group, but the differences were not statistically significant. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, O.; Styrzyński, F.; Makowska, J.; Walczak, K. Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review. Nutrients 2021, 13, 701. https://doi.org/10.3390/nu13020701
Brzezińska O, Styrzyński F, Makowska J, Walczak K. Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review. Nutrients. 2021; 13(2):701. https://doi.org/10.3390/nu13020701
Chicago/Turabian StyleBrzezińska, Olga, Filip Styrzyński, Joanna Makowska, and Konrad Walczak. 2021. "Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review" Nutrients 13, no. 2: 701. https://doi.org/10.3390/nu13020701
APA StyleBrzezińska, O., Styrzyński, F., Makowska, J., & Walczak, K. (2021). Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review. Nutrients, 13(2), 701. https://doi.org/10.3390/nu13020701







