Comparison of 24-Hour Recall and 3-Day Food Records during the Complementary Feeding Period in Thai Infants and Evaluation of Plasma Amino Acids as Markers of Protein Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Dietary Assessment Tools
2.3. Food Composition Programme
2.4. Plasma Amino Acids Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Relative Validation at Group Level
3.3. Relative Validation at Individual Level
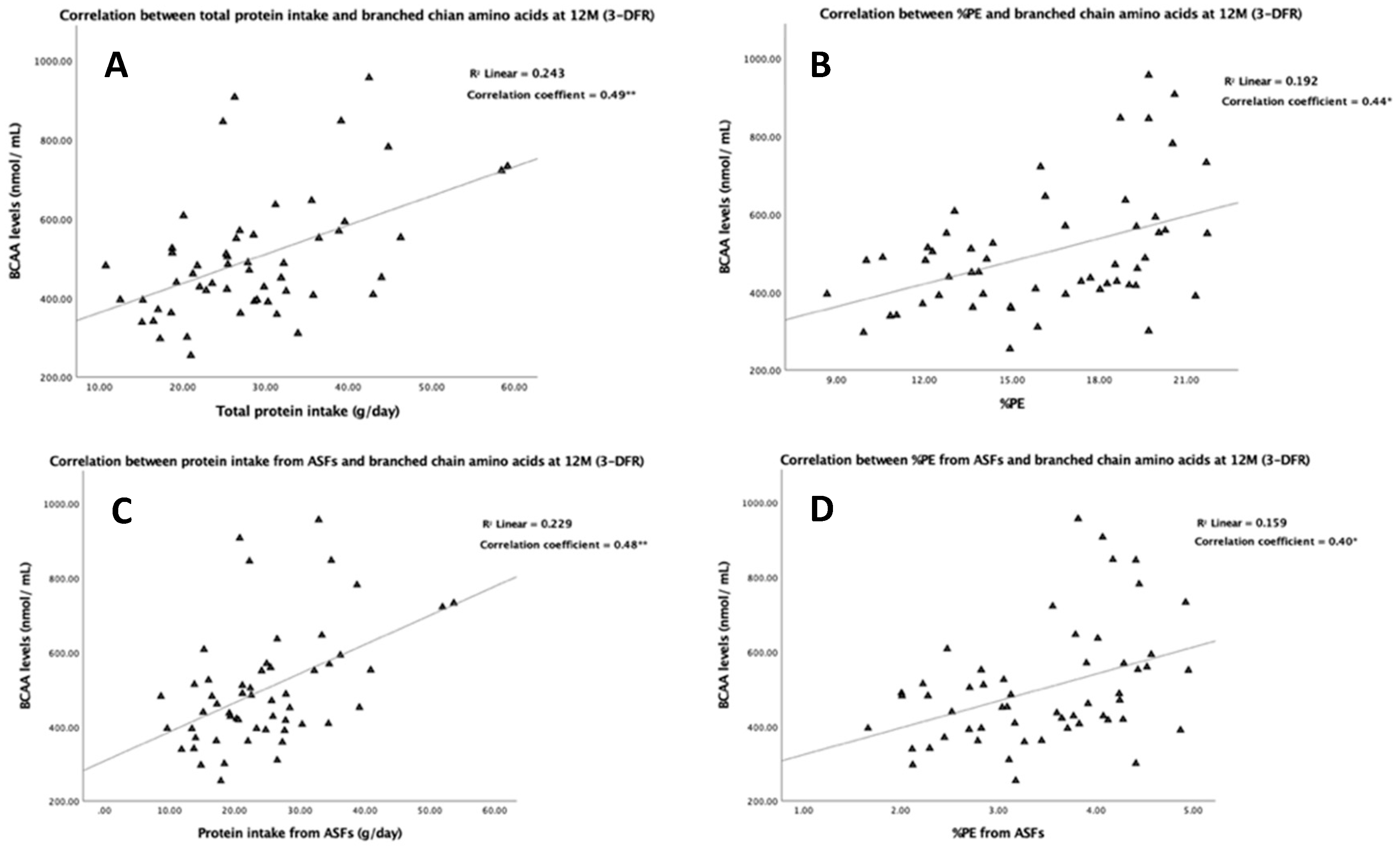
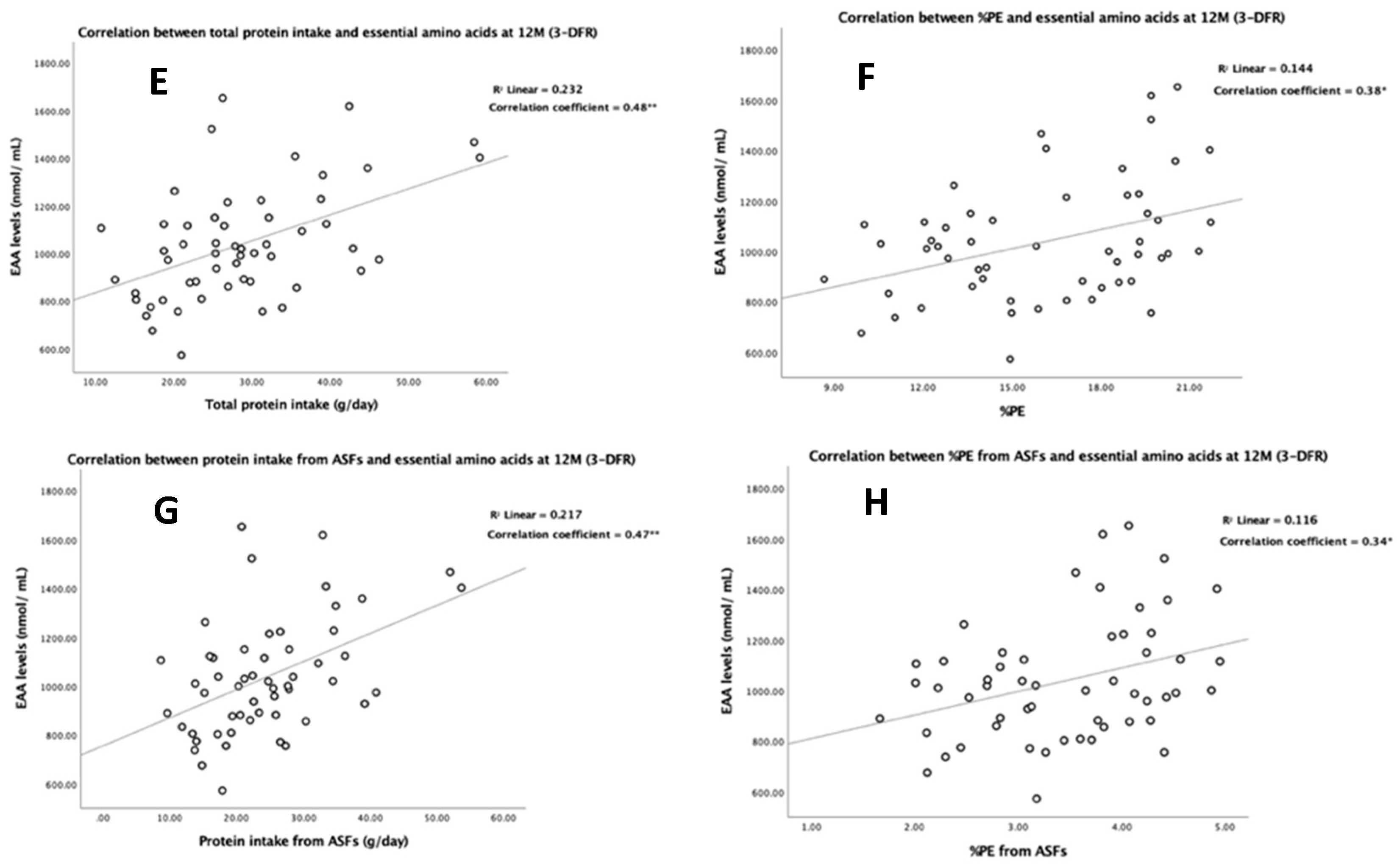
3.4. Association of Protein Intake with Plasma Amino Acids
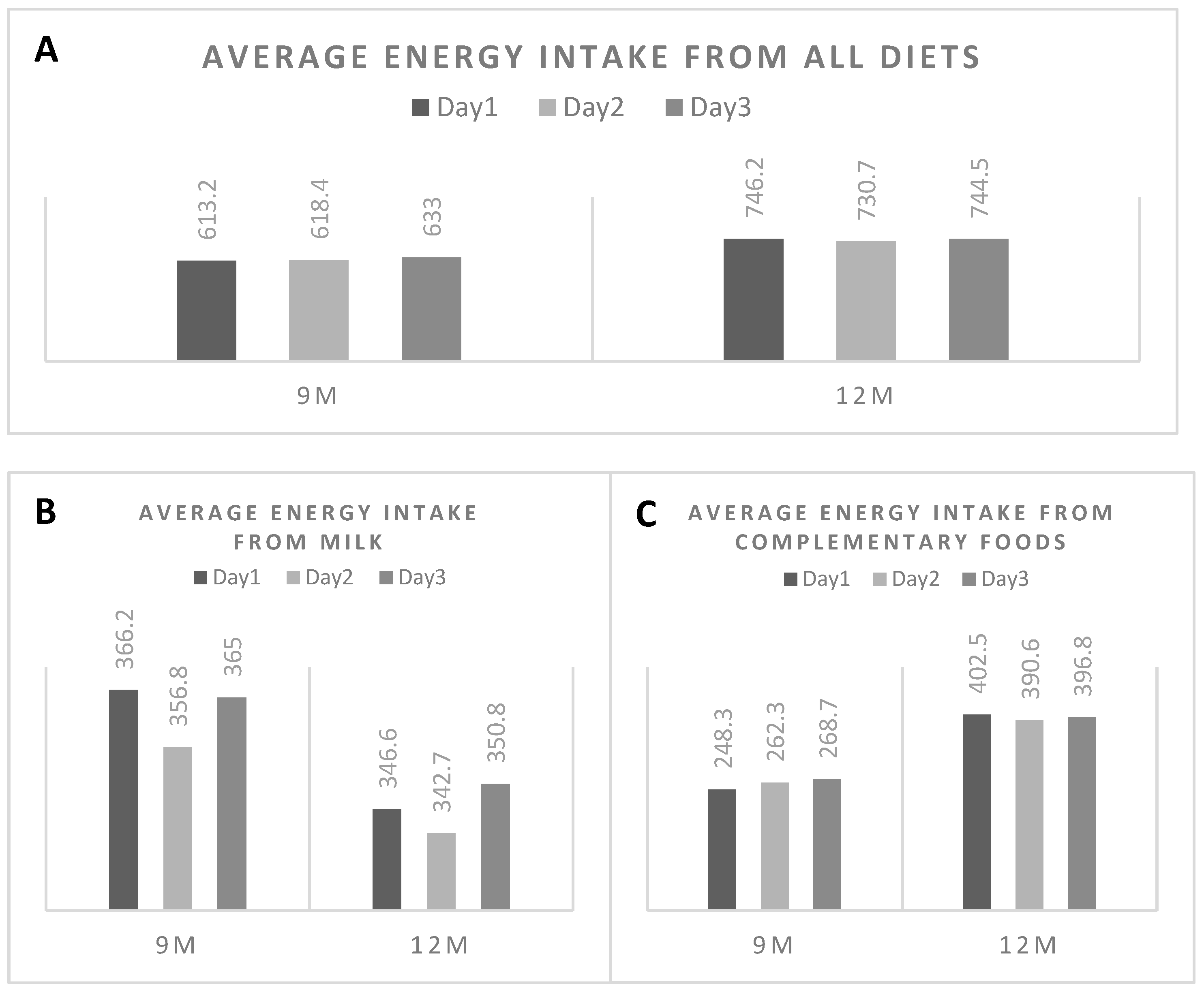
3.5. Daily Variation of Energy Consumption from the 3-DFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Batal, M.; Steinhouse, L.; Delisle, H. The nutrition transition and the double burden of malnutrition. Med. Sante Trop. 2018, 28, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- MacIntyre, U.E.; Wenhold, F.A.M. Measuring dietary intake. In Introduction to Human Nutrition, 3rd ed.; Lanham-New, S.A., Hill, T.R., Gallagher, A.M., Vorster, H.H., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 2020; pp. 74–84. [Google Scholar]
- Klohe, D.M.; Clarke, K.K.; George, G.C.; Milani, T.J.; Hanss-Nuss, H.; Freeland-Graves, J. Relative validity and reliability of a food frequency questionnaire for a triethnic population of 1-year-old to 3-year-old children from low-income families. J. Am. Diet Assoc. 2005, 105, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.J. Dietary Intake Research in Asian Children: Significance and Challenges. J. Nutr. Sci. Vitam. (Tokyo) 2015, 61, S189–S191. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, J.; Arimond, M.; Kumwenda, C.; Rehman, A.M.; Maleta, K.; Ashorn, U.; Keogh, R.; Ferguson, E.L. Comparison of an interactive 24-h recall and weighed food record for measuring energy and nutrient intakes from complementary foods among 9-10-month-old Malawian infants consuming lipid-based nutrient supplements. Br J. Nutr. 2018, 120, 1262–1271. [Google Scholar] [CrossRef]
- Gewa, C.A.; Murphy, S.P.; Neumann, C.G. A comparison of weighed and recalled intakes for schoolchildren and mothers in rural Kenya. Public Health Nutr. 2009, 12, 1197–1204. [Google Scholar] [CrossRef]
- Thakwalakwa, C.M.; Kuusipalo, H.M.; Maleta, K.M.; Phuka, J.C.; Ashorn, P.; Cheung, Y.B. The validity of a structured interactive 24-hour recall in estimating energy and nutrient intakes in 15-month-old rural Malawian children. Matern. Child. Nutr. 2012, 8, 380–389. [Google Scholar] [CrossRef]
- Saloheimo, T.J. Relative validility of an interactive 24-hour recall to assess the intake of energy and nutrients among 15-month old Malawian children. Master’s Thesis, University of Helsinki, Helsinki, Finland, 2012. [Google Scholar]
- Lanigan, J.A.; Wells, J.C.; Lawson, M.S.; Lucas, A. Validation of food diary method for assessment of dietary energy and macronutrient intake in infants and children aged 6–24 months. Eur. J. Clin. Nutr. 2001, 55, 124–129. [Google Scholar] [CrossRef]
- Horst, C.H.; Obermann-De Boer, G.L.; Kromhout, D. Validity of the 24-hour recall method in infancy: The Leiden Pre-School Children Study. Int. J. Epidemiol. 1988, 17, 217–221. [Google Scholar] [CrossRef]
- Verwied-Jorky, S.; Schiess, S.; Luque, V.; Grote, V.; Scaglioni, S.; Vecchi, F.; Martin, F.; Stolarczyk, A.; Koletzko, B.; European Childhood Obesity, P. Methodology for longitudinal assessment of nutrient intake and dietary habits in early childhood in a transnational multicenter study. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 96–102. [Google Scholar] [CrossRef]
- Cheng, G.; Hilbig, A.; Drossard, C.; Alexy, U.; Kersting, M. Relative validity of a 3 d estimated food record in German toddlers. Public Health Nutr. 2013, 16, 645–652. [Google Scholar] [CrossRef]
- Freisling, H.; Ocke, M.C.; Casagrande, C.; Nicolas, G.; Crispim, S.P.; Niekerk, M.; van der Laan, J.; de Boer, E.; Vandevijvere, S.; de Maeyer, M.; et al. Comparison of two food record-based dietary assessment methods for a pan-European food consumption survey among infants, toddlers, and children using data quality indicators. Eur. J. Nutr. 2015, 54, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.O.; Heath, A.L.; Taylor, R.W.; Mills, V.C.; Barris, A.C.; Skidmore, P.M. Relative validity and reproducibility of an FFQ to determine nutrient intakes of New Zealand toddlers aged 12–24 months. Public Health Nutr. 2015, 18, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, V.E.; Dietrich, A.M.; Estabrooks, P.A.; Savla, J.; Serrano, E.; Davy, B.M. Dietary biomarkers: Advances, limitations and future directions. Nutr. J. 2012, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Hooson Jzh, J.; Hutchinson Jyh, J.; Warthon-Medina, M.; Hancock, N.; Greathead, K.; Knowles, B.; Vargas-Garcia, E.; Gibson, L.E.; Bush, L.A.; Margetts, B.; et al. A systematic review of reviews identifying UK validated dietary assessment tools for inclusion on an interactive guided website for researchers: www.nutritools.org. Crit. Rev. Food Sci. Nutr. 2020, 60, 1265–1289. [Google Scholar] [CrossRef]
- Ansari, M.R.; Agustina, R.; Khusun, H.; Prafiantini, E.; Cahyaningrum, F.; Permadhi, I. Development and evaluation of a semiquantitative food frequency questionnaire for estimating omega-3 and omega-6 fatty acid intakes in Indonesian children. Asia Pac J. Clin Nutr. 2016, 25 (Suppl. S1), S20–S29. [Google Scholar] [PubMed]
- Orton, H.D.; Szabo, N.J.; Clare-Salzler, M.; Norris, J.M. Comparison between omega-3 and omega-6 polyunsaturated fatty acid intakes as assessed by a food frequency questionnaire and erythrocyte membrane fatty acid composition in young children. Eur. J. Clin. Nutr. 2008, 62, 733–738. [Google Scholar] [CrossRef][Green Version]
- Brouwer-Brolsma, E.M.; Brennan, L.; Drevon, C.A.; van Kranen, H.; Manach, C.; Dragsted, L.O.; Roche, H.M.; Andres-Lacueva, C.; Bakker, S.J.L.; Bouwman, J.; et al. Combining traditional dietary assessment methods with novel metabolomics techniques: Present efforts by the Food Biomarker Alliance. Proc. Nutr. Soc. 2017, 76, 619–627. [Google Scholar] [CrossRef]
- Hernandez-Alonso, P.; Becerra-Tomas, N.; Papandreou, C.; Bullo, M.; Guasch-Ferre, M.; Toledo, E.; Ruiz-Canela, M.; Clish, C.B.; Corella, D.; Dennis, C.; et al. Plasma Metabolomics Profiles are Associated with the Amount and Source of Protein Intake: A Metabolomics Approach within the PREDIMED Study. Mol. Nutr. Food Res. 2020, 64, e2000178. [Google Scholar] [CrossRef]
- Radjursoga, M.; Lindqvist, H.M.; Pedersen, A.; Karlsson, B.G.; Malmodin, D.; Ellegard, L.; Winkvist, A. Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients 2018, 10, 1063. [Google Scholar] [CrossRef]
- Socha, P.; Grote, V.; Gruszfeld, D.; Janas, R.; Demmelmair, H.; Closa-Monasterolo, R.; Subias, J.E.; Scaglioni, S.; Verduci, E.; Dain, E.; et al. Milk protein intake, the metabolic-endocrine response, and growth in infancy: Data from a randomized clinical trial. Am. J. Clin. Nutr. 2011, 94 (Suppl. S6), 1776S–1784S. [Google Scholar] [PubMed]
- Rousseau, M.; Guenard, F.; Garneau, V.; Allam-Ndoul, B.; Lemieux, S.; Perusse, L.; Vohl, M.C. Associations between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults. Nutrients 2019, 11, 173. [Google Scholar] [CrossRef]
- Kittisakmontri, K.; Lanigan, J.; Wells, J.C.K.; Fewtrell, M. The Impact of Dietary Protein in Complementary Foods on Infant Growth and Body Composition in a Population Facing the Double Burden of Malnutrition: Protocol for a Multicenter, Prospective Cohort Study. Jmir. Res. Protoc. 2020, 9, e18112. [Google Scholar] [CrossRef] [PubMed]
- Olaya, G.A.; Lawson, M.; Fewtrell, M.S. Efficacy and safety of new complementary feeding guidelines with an emphasis on red meat consumption: A randomized trial in Bogota, Colombia. Am. J. Clin. Nutr. 2013, 98, 983–993. [Google Scholar] [CrossRef]
- INMUCAL-Nutrients, 4.0; Institute of Nutrition, Mahidol University: Bangkok, Thailand, 2018.
- Lanigan, J.A.; Wells, J.C.; Lawson, M.S.; Cole, T.J.; Lucas, A. Number of days needed to assess energy and nutrient intake in infants and young children between 6 months and 2 years of age. Eur. J. Clin. Nutr. 2004, 58, 745–750. [Google Scholar] [CrossRef]
- Shapira, E.; Blitzer, M.G.; Miller, J.B. Biochemical Genetics: A Laboratory Manual, 1st ed.; Oxford University Press Inc.: New York, NY, USA, 1989. [Google Scholar]
- Lombard, M.J.; Steyn, N.P.; Charlton, K.E.; Senekal, M. Application and interpretation of multiple statistical tests to evaluate validity of dietary intake assessment methods. Nutr. J. 2015, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Standard Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Beaton, E.; Wright, J.; Devenish, G.; Do, L.; Scott, J. Relative Validity of a 24-h Recall in Assessing Intake of Key Nutrients in a Cohort of Australian Toddlers. Nutrients 2018, 10, 80. [Google Scholar] [CrossRef]
- Mejia-Rodriguez, F.; Neufeld, L.M.; Garcia-Guerra, A.; Quezada-Sanchez, A.D.; Orjuela, M.A. Validation of a food frequency questionnaire for retrospective estimation of diet during the first 2 years of life. Matern. Child. Health J. 2014, 18, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Rivas-Tumanyan, S.; Santiago-Rodriguez, E.J.; Sinigaglia, O.; Rios, E.M.; Campos, M.; Diaz, B.; Willett, W. A Semi-Quantitative Food Frequency Questionnaire Validated in Hispanic Infants and Toddlers Aged 0 to 24 Months. J. Acad. Nutr. Diet 2017, 117, 526–535 e9. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Gill, C.; Welch, A.; Day, K.; Cassidy, A.; Khaw, K.T.; Sneyd, M.J.; Key, T.J.; Roe, L.; Day, N.E. Comparison of dietary assessment methods in nutritional epidemiology: Weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br. J. Nutr. 1994, 72, 619–643. [Google Scholar] [CrossRef]
- Chinnock, A. Validation of an estimated food record. Public Health Nutr. 2006, 9, 934–941. [Google Scholar] [CrossRef]
- Erkkola, M.; Kyttala, P.; Takkinen, H.M.; Kronberg-Kippila, C.; Nevalainen, J.; Simell, O.; Ilonen, J.; Veijola, R.; Knip, M.; Virtanen, S.M. Nutrient intake variability and number of days needed to assess intake in preschool children. Br. J. Nutr. 2011, 106, 130–140. [Google Scholar] [CrossRef]
- Small, L.; Lane, H.; Vaughan, L.; Melnyk, B.; McBurnett, D. A systematic review of the evidence: The effects of portion size manipulation with children and portion education/training interventions on dietary intake with adults. Worldviews Evid. Based Nurs. 2013, 10, 69–81. [Google Scholar] [CrossRef]
- Michaelsen, K.F.; Grummer-Strawn, L.; Begin, F. Emerging issues in complementary feeding: Global aspects. Matern. Child. Nutr. 2017, 13 (Suppl. S2), e12444. [Google Scholar] [CrossRef]
- Altorf-van der Kuil, W.; Brink, E.J.; Boetje, M.; Siebelink, E.; Bijlsma, S.; Engberink, M.F.; van ‘t Veer, P.; Tome, D.; Bakker, S.J.; van Baak, M.A.; et al. Identification of biomarkers for intake of protein from meat, dairy products and grains: A controlled dietary intervention study. Br. J. Nutr. 2013, 110, 810–822. [Google Scholar] [CrossRef]
- Esko, T.; Hirschhorn, J.N.; Feldman, H.A.; Hsu, Y.H.; Deik, A.A.; Clish, C.B.; Ebbeling, C.B.; Ludwig, D.S. Metabolomic profiles as reliable biomarkers of dietary composition. Am. J. Clin. Nutr. 2017, 105, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Polberger, S.K.; Axelsson, I.E.; Raiha, N.C. Amino acid concentrations in plasma and urine in very low birth weight infants fed protein-unenriched or human milk protein-enriched human milk. Pediatrics 1990, 86, 909–915. [Google Scholar]
- Kirchberg, F.F.; Harder, U.; Weber, M.; Grote, V.; Demmelmair, H.; Peissner, W.; Rzehak, P.; Xhonneux, A.; Carlier, C.; Ferre, N.; et al. Dietary protein intake affects amino acid and acylcarnitine metabolism in infants aged 6 months. J. Clin. Endocrinol. Metab. 2015, 100, 149–158. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Results |
|---|---|
| Infant gender, n (%) | |
| Female | 71 (49.0) |
| Gestational age (weeks), means ± SD | 38.8 ± 1.0 |
| Type of milk feeding, n (%) | |
| 9 months | |
| - Breast milk only | 79 (54.5) |
| - Combined | 14 (9.6) |
| - Formula only | 52 (35.9) |
| 12 months | |
| - Breast milk only | 46 (31.7) |
| - Combined | 25 (17.2) |
| - Formula/Cow’s milk only | 74 (51.0) |
| Anthropometric measurements, means ± SD | |
| 9 months | |
| - WAZ | −0.4 ± 0.8 |
| - WLZ | −0.1 ± 0.8 |
| - LAZ | −0.4 ± 0.9 |
| 12 months | |
| - WAZ | −0.6 ± 0.9 |
| - WLZ | −0.2 ± 0.9 |
| - LAZ | −0.5 ± 0.9 |
| Parental BMI (kg/m2), means ± SD | |
| - Maternal BMI | 22.8 ± 4.0 |
| - Paternal BMI | 24.7 ± 3.6 |
| Maternal education, n (%) | |
| - Lower than bachelor’s degree | 76 (52.4) |
| - Bachelor’s degree or higher | 69 (47.6) |
| Paternal education, n (%) | |
| - Lower than bachelor’s degree | 87 (60.0) |
| - Bachelor’s degree or higher | 58 (40.0) |
| Family income per month * (Thai Bath), n (%) | |
| - <10,000 | 11 (7.6) |
| - 10,000–29,999 | 65 (44.8) |
| - 30,000–49,999 | 51 (35.2) |
| - ≥50,000 | 18 (12.4) |
| Completeness of dietary data, n (%) | |
| 24-h dietary recall | |
| - At 9 months | 142 (97.9) |
| - At 12 months | 144 (99.3) |
| 3-day food record | |
| - At 9 months | 129 (89.0) |
| - At 12 months | 125 (86.2) |
| 9 Months | 12 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| Energy and Macronutrients | 24-HR means ± SD | 3-DFR means ± SD | Mean difference (%) | p * | 24-HR means ± SD | 3-DFR means ± SD | Mean difference (%) | p * |
| Energy (kcal) | 624.5 ± 193.9 | 630.0 ± 191.4 | −5.6 (0.9) | 0.60 | 725.5 ± 236.9 | 747.0 ± 222.5 | −21.6 (2.9) | 0.14 |
| CHO (g) | 78.1 ± 25.0 | 79.7 ± 24.5 | −1.7 (2.1) | 0.28 | 86.7 ± 31.1 | 88.9 ± 28.5 | −2.2 (2.5) | 0.28 |
| Fat (g) | 25.6 ± 8.7 | 25.6 ± 8.7 | 0.04 (0.2) | 0.94 | 29.1 ± 12.0 | 30.4 ± 10.7 | −1.3 (4.3) | 0.14 |
| Protein (g) | 19.8 ± 8.4 | 20.2 ± 7.9 | −0.4 (2.0) | 0.41 | 28.4 ± 10.5 | 29.3 ± 10.1 | −0.8 (2.7) | 0.21 |
| % Caloric distribution - CHO - Fat - Protein | 50.4 ± 6.1 37.1 ± 5.7 12.4 ± 2.8 | 51.0 ± 5.3 36.3 ± 5.4 12.7 ± 2.6 | −0.6 (1.2) 0.8 (2.2) −0.2 (1.6) | 0.28 0.12 0.36 | 48.3 ± 8.0 35.8 ± 6.5 15.9 ± 3.7 | 47.8 ± 6.6 36.5 ± 5.5 15.7 ± 3.0 | 0.5 (1.0) −0.7 (1.9) 0.1 (0.6) | 0.48 0.27 0.57 |
| Micronutrients | 24-HR Median (IQR) | 3-DFR Median (IQR) | Mean difference (%) | p ** | 24-HR Median (IQR) | 3-DFR Median (IQR) | Mean difference (%) | p ** |
| Calcium (mg) | 234.0 (170.7, 539.2) | 231.0 (172.3, 415.4) | 20.4 (6.4) | 0.07 | 406.9 (209.3, 617.0) | 371.1 (194.7, 662.5) | 5.1 (1.2) | 0.78 |
| Phosphorous (mg) | 261.2 (174.5, 461.6) | 251.3 (195.4, 377.6) | 3.5 (1.1) | 0.91 | 415.8 (254.7, 583.6) | 406.4 (261.6, 627.9) | −8.8 (2.0) | 0.43 |
| Iron (mg) | 3.2 (1.9, 8.3) | 3.1 (2.2, 7.0) | 0.05 (1.0) | 0.83 | 5.1 (2.7, 8.9) | 4.4 (2.9, 9.0) | −0.3 (4.8) | 0.36 |
| Zinc (mg) | 2.3 (1.5, 5.0) | 2.3 (1.6, 4.3) | 0.1 (3.2) | 0.30 | 3.6 (2.2, 5.1) | 3.3 (2.2, 5.3) | −0.1 (2.5) | 0.97 |
| Vitamin A (RAE) | 563.8 (371.8, 1298.3) | 1105.0 (478.9, 1736.6) | −47.4 (3.7) | 0.03 | 474.3 (352.4, 781.3) | 610.8 (381.9, 1229.0) | −47.4 (4.0) | 0.05 |
| Vitamin B1 (mg) | 0.3 (0.2, 0.6) | 0.3 (0.2, 0.5) | −0.01 (2.5) | 0.11 | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.7) | 0.004 (0.8) | 0.70 |
| Vitamin B2 (mg) | 0.5 (0.3, 1.2) | 0.5 (0.4, 1.1) | 0.02 (2.9) | 0.74 | 0.9 (0.5, 1.3) | 0.9 (0.5, 1.3) | 0.003 (0.3) | 0.68 |
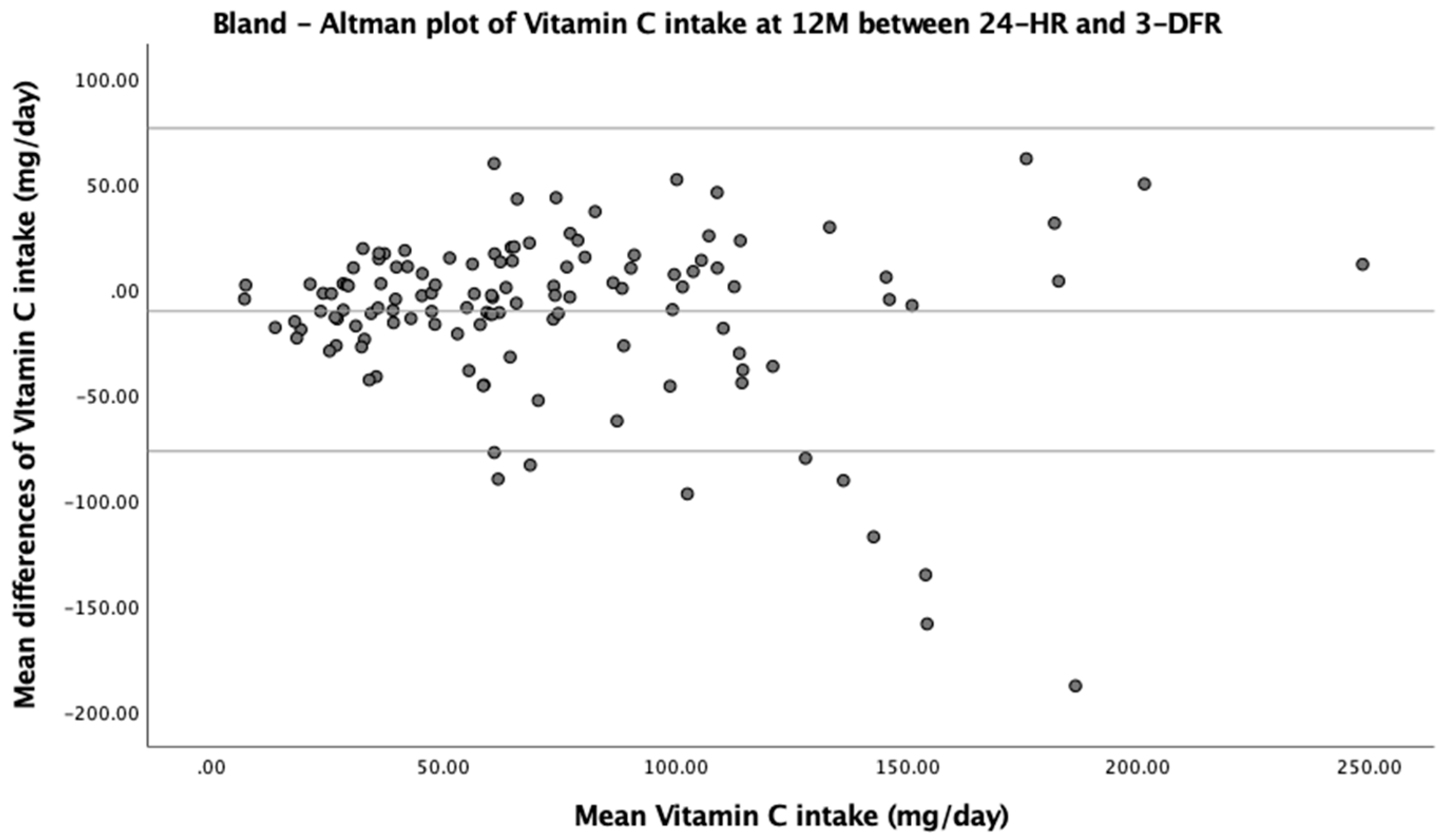
| Vitamin C (mg) | 60.5 (32.5, 95.4) | 59.5 (41.6, 96.0) | −5.8 (7.8) | 0.10 | 58.8 (35.7, 92.8) | 64.2 (40.9, 64.2) | −10.3 (13.2) | 0.04 |
| Nutrients | 9 Months | 12 Months | ||
|---|---|---|---|---|
| Kw | 95% CI | Kw | 95% CI | |
| Energy (kcal) | 0.326 | 0.323, 0.329 | 0.301 | 0.298, 0.304 |
| CHO (g) | 0.281 | 0.279, 0.284 | 0.337 | 0.334, 0.339 |
| Fat (g) | 0.281 | 0.279, 0.284 | 0.195 | 0.192, 0.198 |
| Protein (g) | 0.352 | 0.350, 0.355 | 0.275 | 0.272, 0.277 |
| Calcium (mg) | 0.352 | 0.350, 0.355 | 0.434 | 0.431, 0.437 |
| Phosphorous (mg) | 0.432 | 0.429, 0.435 | 0.372 | 0.369, 0.375 |
| Iron (mg) | 0.361 | 0.358, 0.364 | 0.372 | 0.369, 0.375 |
| Zinc (mg) | 0.485 | 0.483, 0.488 | 0.443 | 0.440, 0.445 |
| Vitamin A (RAE) | 0.148 | 0.146, 0.151 | 0.213 | 0.210, 0.215 |
| Vitamin B1 (mg) | 0.352 | 0.349, 0.355 | 0.336 | 0.334, 0.339 |
| Vitamin B2 (mg) | 0.361 | 0.358, 0.364 | 0.301 | 0.298, 0.304 |
| Vitamin C (mg) | 0.317 | 0.314, 0.320 | 0.275 | 0.272, 0.277 |
| Nutrients | 9 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Mean Differences | LOA ± 1.96 SD | Slope of Biases (p-Value) | Mean Differences | LOA ± 1.96 SD | Slope of Biases (p-Value) | |
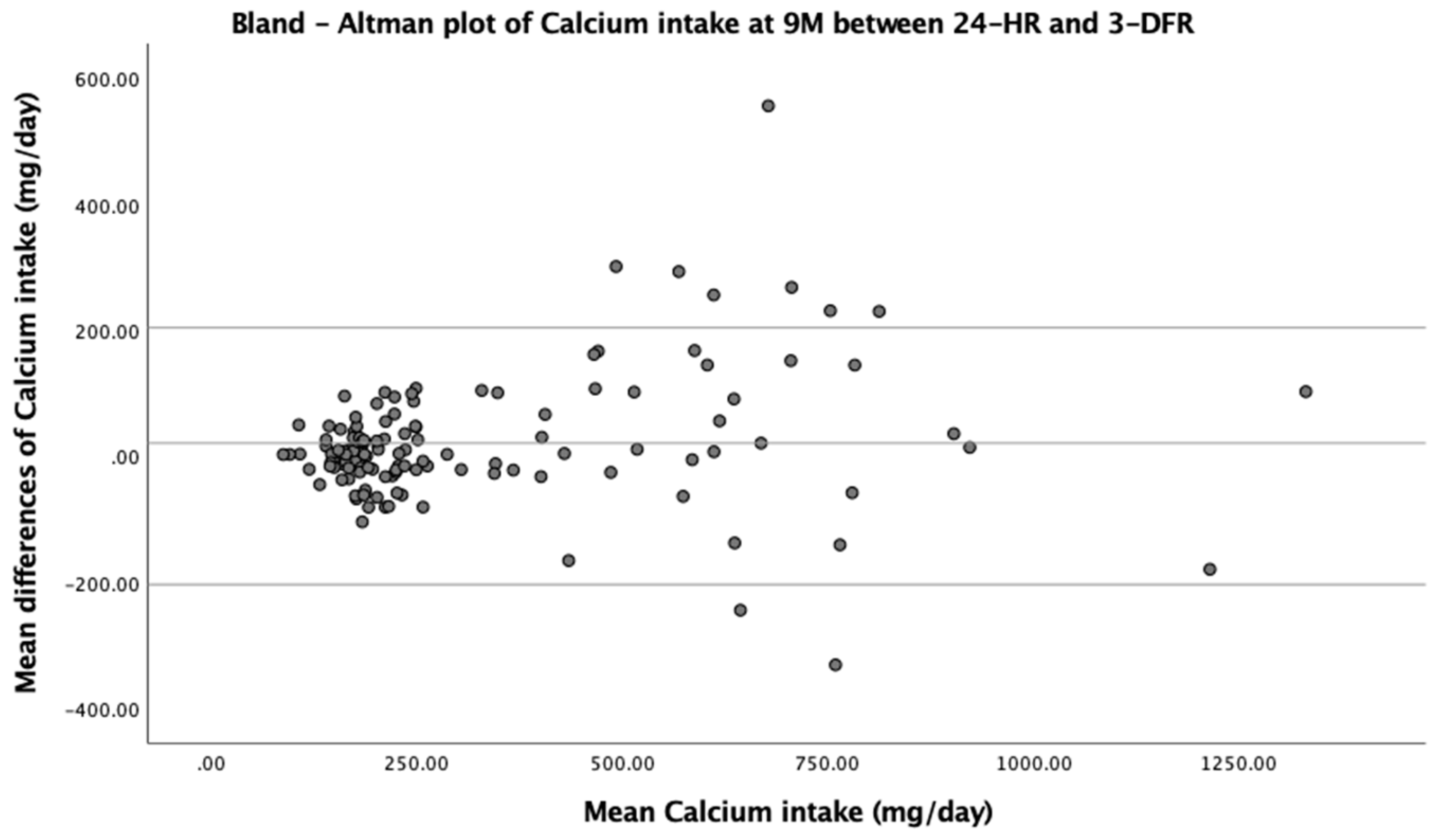
| Energy (kcal) | −5.6 | ±233.7 | −0.52 (0.60) | −21.6 | ±318.9 | −1.48 (0.14) |
| CHO (g) | −1.7 | ±33.8 | −1.09 (0.28) | −2.2 | ±44.3 | −1.08 (0.28) |
| Fat (g) | 0.04 | ±12.5 | 0.07 (0.94) | −1.3 | ±19.6 | −1.47 (0.14) |
| Protein (g) | −0.4 | ±10.7 | −0.83 (0.41) | −0.8 | ±14.5 | −1.26 (0.21) |
| Calcium (mg) | 20.4 | ±203.9 | 2.20 (0.03) | 5.1 | ±289.3 | 0.39 (0.70) |
| Phosphorous (mg) | 3.5 | ±177.5 | 0.43 (0.67) | −8.8 | ±263.4 | −0.73 (0.47) |
| Iron (mg) | 0.05 | ±3.5 | 0.30 (0.76) | −0.3 | ±4.7 | −1.46 (0.15) |
| Zinc (mg) | 0.1 | ±1.6 | 1.75 (0.08) | −0.1 | ±3.6 | −0.54 (0.59) |
| Vitamin A (RAE) | −47.4 | ±3466.3 | −1.01 (0.32) | −47.4 | ±4063.2 | −0.25 (0.80) |
| Vitamin B1 (mg) | −0.01 | ±0.2 | −0.93 (0.36) | 0.004 | ±0.4 | 0.24 (0.81) |
| Vitamin B2 (mg) | 0.02 | ±0.6 | 0.88 (0.38) | 0.003 | ±0.7 | 0.10 (0.92) |
| Vitamin C (mg) | −5.8 | ±66.5 | −1.91 (0.06) | −10.3 | ±76.7 | −2.92 (0.004) |
| Nutrients | 9 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| % Same Quartiles | % Opposite Quartiles | Correlation Coefficient * | % Same Quartiles | % Opposite Quartiles | Correlation Coefficient * | |
| Energy (kcal) | 54.8 | 0 | 0.79 | 53.2 | 1.6 | 0.72 |
| CHO (g) | 50.8 | 0 | 0.74 | 56.5 | 2.4 | 0.65 |
| Fat (g) | 50.8 | 0.8 | 0.75 | 43.5 | 4.8 | 0.59 |
| Protein (g) | 57.1 | 0.8 | 0.75 | 50.8 | 1.6 | 0.53 |
| Calcium (mg) | 57.1 | 0 | 0.85 | 65.3 | 0 | 0.86 |
| Phosphorous (mg) | 64.3 | 0 | 0.81 | 59.7 | 0 | 0.81 |
| Iron (mg) | 57.9 | 0 | 0.82 | 59.7 | 0.8 | 0.85 |
| Zinc (mg) | 69.0 | 0 | 0.87 | 66.1 | 0.8 | 0.80 |
| Vitamin A (RAE) | 38.9 | 4.8 | 0.37 | 45.2 | 4.8 | 0.52 |
| Vitamin B1 (mg) | 57.1 | 0 | 0.81 | 56.5 | 0 | 0.75 |
| Vitamin B2 (mg) | 57.9 | 0 | 0.78 | 53.2 | 0 | 0.79 |
| Vitamin C (mg) | 54.0 | 0.8 | 0.74 | 50.8 | 0 | 0.74 |
| Protein Intake (g/day) | BCAA Levels | EAA Levels | NEAA Levels | Total AA Levels | ||||
|---|---|---|---|---|---|---|---|---|
| Crude β | Adjusted 1 β | Crude β | Adjusted 1 β | Crude β | Adjusted 1 β | Crude β | Adjusted 1 β | |
| All sources | 7.4 ** | 11.2 * | 10.9 ** | 15.8 * | 4.2 | 3.8 | 15.0 * | 19.6 |
| ABP | 7.8 ** | 10.5 * | 11.5 ** | 14.8 * | 4.1 | 3.7 | 15.6 * | 18.5 |
| PBP | 31.3 * | 28.4 | 48.1 * | 39.1 | 25.8 | 6.5 | 73.8 * | 45.6 |
| Protein Intake (g/d) | BCAA | EAA | NEAA | Total AA | ||||
|---|---|---|---|---|---|---|---|---|
| % Same Quartile | % Opposite Quartile | % Same Quartile | % Opposite Quartile | % Same Quartile | % Opposite Quartile | % Same Quartile | % Opposite Quartile | |
| All sources | 44.4 | 1.9 | 38.9 | 3.7 | 27.8 | 7.4 | 24.1 | 5.6 |
| ABP | 42.6 | 1.9 | 37.0 | 3.7 | 29.6 | 9.3 | 29.6 | 7.4 |
| PBP | 33.3 | 7.4 | 29.6 | 9.3 | 27.8 | 11.1 | 38.9 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittisakmontri, K.; Lanigan, J.; Sangcakul, A.; Tim-Aroon, T.; Meemaew, P.; Wangaueattachon, K.; Fewtrell, M. Comparison of 24-Hour Recall and 3-Day Food Records during the Complementary Feeding Period in Thai Infants and Evaluation of Plasma Amino Acids as Markers of Protein Intake. Nutrients 2021, 13, 653. https://doi.org/10.3390/nu13020653
Kittisakmontri K, Lanigan J, Sangcakul A, Tim-Aroon T, Meemaew P, Wangaueattachon K, Fewtrell M. Comparison of 24-Hour Recall and 3-Day Food Records during the Complementary Feeding Period in Thai Infants and Evaluation of Plasma Amino Acids as Markers of Protein Intake. Nutrients. 2021; 13(2):653. https://doi.org/10.3390/nu13020653
Chicago/Turabian StyleKittisakmontri, Kulnipa, Julie Lanigan, Areeporn Sangcakul, Thipwimol Tim-Aroon, Pornchai Meemaew, Kanticha Wangaueattachon, and Mary Fewtrell. 2021. "Comparison of 24-Hour Recall and 3-Day Food Records during the Complementary Feeding Period in Thai Infants and Evaluation of Plasma Amino Acids as Markers of Protein Intake" Nutrients 13, no. 2: 653. https://doi.org/10.3390/nu13020653
APA StyleKittisakmontri, K., Lanigan, J., Sangcakul, A., Tim-Aroon, T., Meemaew, P., Wangaueattachon, K., & Fewtrell, M. (2021). Comparison of 24-Hour Recall and 3-Day Food Records during the Complementary Feeding Period in Thai Infants and Evaluation of Plasma Amino Acids as Markers of Protein Intake. Nutrients, 13(2), 653. https://doi.org/10.3390/nu13020653




