Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategies
2.2. Eligibility Criteria and Study Selection
2.3. Study Extraction
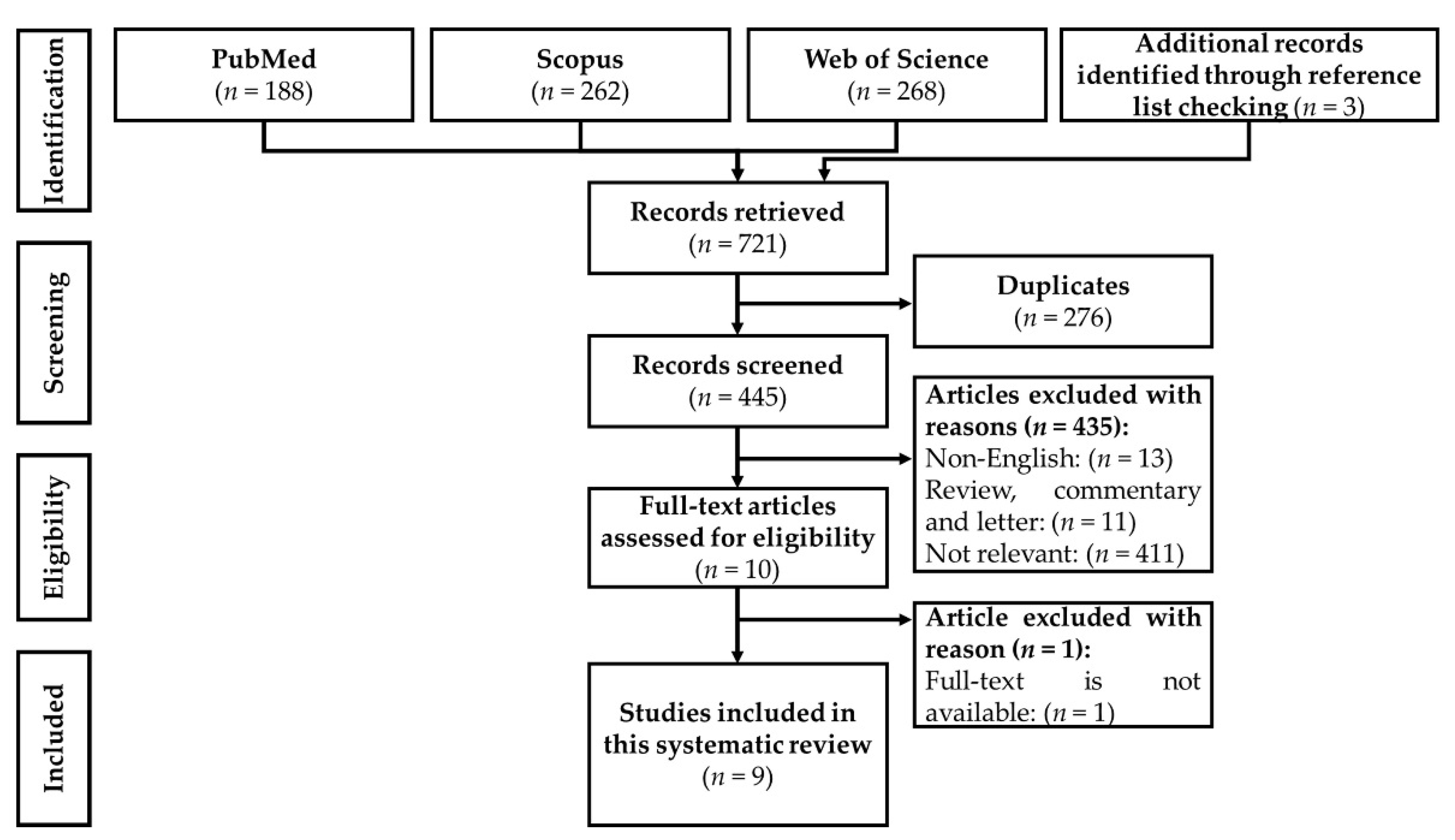
3. Results
3.1. Selection of Articles
3.2. Study Characteristics
3.3. Increased Thyroid Hormones in Euthyroid Animals
3.4. Improve Thyroid Profiles in Animals with Hypothyroidism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gouvinhas, I.; Machado, N.; Sobreira, C.; Dominguez-Perles, R.; Gomes, S.; Rosa, E.; Barros, A. Critical Review on the Significance of Olive Phytochemicals in Plant Physiology and Human Health. Molecules 2017, 22, 1986. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Olive: Native of Mediterranean region and Health benefits. Pharmacogn. Rev. 2008, 2, 135–142. [Google Scholar]
- Clodoveo, M.L.; Hachicha Hbaieb, R. Beyond the traditional virgin olive oil extraction systems: Searching innovative and sustainable plant engineering solutions. Food Res. Int. 2013, 54, 1926–1933. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Alarcón de la Lastra, C.; Barranco, M.D.; Motilva, V.; Herrerías, J.M. Mediterranean diet and health: Biological importance of olive oil. Curr. Pharm. Des. 2001, 7, 933–950. [Google Scholar] [CrossRef]
- Berry, E.M.; Arnoni, Y.; Aviram, M. The Middle Eastern and biblical origins of the Mediterranean diet. Public Health Nutr. 2011, 14, 2288–2295. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Olives and Bone: A Green Osteoporosis Prevention Option. Int. J. Env. Res. Public Health 2016, 13, 755. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Martínez-González, M.A. Olive oil in the primary prevention of cardiovascular disease. Maturitas 2011, 68, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Kamil, K.; Kumar, J.; Yazid, M.D.; Hj Idrus, R. Olive and Its Phenolic Compound as the Promising Neuroprotective Agent. Sains Malays. 2018, 47, 2811–2820. [Google Scholar] [CrossRef]
- International Olive Oil Council. Designations and definitions of olive oils. In Trade Standard Applying to Table Olives; International Olive Oil Council: Madrid, Spain, 2015. [Google Scholar]
- United States Department of Agriculture. United States Standards for Grades of Olive Oil and Olive-Pomace Oil; Agricultural Marketing Service; United States Department of Agriculture: Washington, DC, USA, 2010; pp. 1–19.
- Veneziani, G.; Sordini, B.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Urbani, S.; Maio, I.; Servili, M. Improvement of Olive Oil Mechanical Extraction: New Technologies, Process Efficiency, and Extra Virgin Olive Oil Quality. In Products from Olive Tree; IntechOpen: London, UK, 2016. [Google Scholar]
- Antonopoulos, K.; Valet, N.; Spiratos, D.; Siragakis, G. Olive oil and pomace oil processing. Grasas Aceites 2006, 57. [Google Scholar] [CrossRef]
- Lucci, P.; Bertoz, V.; Pacetti, D.; Moret, S.; Conte, L. Effect of the Refining Process on Total Hydroxytyrosol, Tyrosol, and Tocopherol Contents of Olive Oil. Foods 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Peri, C. The olive oil refining process. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; Wiley Blackwell: Oxford, UK, 2014; pp. 201–210. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. 4—Olive Oil Composition. In Olive Oil, 2nd ed.; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2006; pp. 41–72. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Johnson, R.L.; Mitchell, A.E. Reducing Phenolics Related to Bitterness in Table Olives. J. Food Qual. 2018, 2018, 3193185. [Google Scholar] [CrossRef]
- Nicoli, F.; Negro, C.; Vergine, M.; Aprile, A.; Nutricati, E.; Sabella, E.; Miceli, A.; Luvisi, A.; De Bellis, L. Evaluation of Phytochemical and Antioxidant Properties of 15 Italian Olea europaea L. Cultivar Leaves. Molecules 2019, 24, 1998. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure Properties, Acquisition Protocols, and Biological Activities of Oleuropein Aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Bakhouche, A.; Lozano-Sánchez, J.; Fernández-Gutiérrez, A.; Segura Carretero, A. Trends in chemical characterization of virgin olive oil phenolic profile: An overview and new challenges. OLIVAE 2015, 122, 3–15. [Google Scholar]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Lee, A.; Thurnham, D.I.; Chopra, M. Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Radic. Biol. Med. 2000, 29, 1051–1055. [Google Scholar] [CrossRef]
- Wang, H.; Sit, W.-H.; Tipoe, G.L.; Wan, J.M.-F. Differential protective effects of extra virgin olive oil and corn oil in liver injury: A proteomic study. Food Chem. Toxicol. 2014, 74, 131–138. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Nestares, M.T.; Ros, E.; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 2004, 23, 673–681. [Google Scholar] [CrossRef]
- Frankel, E.N. Nutritional and Biological Properties of Extra Virgin Olive Oil. J. Agric. Food Chem. 2011, 59, 785–792. [Google Scholar] [CrossRef]
- Oliveras-López, M.J.; Molina, J.J.; Mir, M.V.; Rey, E.F.; Martín, F.; de la Serrana, H.L. Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalized elderly humans. Arch. Gerontol. Geriatr. 2013, 57, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Yoon, L.; Liu, Y.-N.; Park, H.; Kim, H.-S. Olive Leaf Extract Elevates Hepatic PPAR α mRNA Expression and Improves Serum Lipid Profiles in Ovariectomized Rats. J. Med. Food 2015, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Mujic, I.; Zivkovic, J.; Nikolić, G.; Vidovic, S.; Trutic, N.; Kosić, U.; Jokic, S.; Ruznić, A. Phenolic compounds in olive leaf extract as a source of useful antioxidants. Hrvat. Čas. Za Prehrambenu Teh. Biotehnol. Nutr. 2011, 6, 129–133. [Google Scholar]
- Abunab, H.; Dator, W.L.; Hawamdeh, S. Effect of olive leaf extract on glucose levels in diabetes-induced rats: A systematic review and meta-analysis. J. Diabetes 2017, 9, 947–957. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Liu, Y.N.; Jung, J.H.; Park, H.; Kim, H. Olive leaf extract suppresses messenger RNA expression of proinflammatory cytokines and enhances insulin receptor substrate 1 expression in the rats with streptozotocin and high-fat diet-induced diabetes. Nutr. Res. 2014, 34, 450–457. [Google Scholar] [CrossRef]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E.; Gerothanassis, I.P. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Hudomal, S.; Tadic, V.; Goran, M.; Arsić, I.; MitroviĆ, D. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serb. Chem. Soc. 2009, 74, 367–377. [Google Scholar] [CrossRef]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef] [PubMed]
- Topalović, D.; Živković, L.; Čabarkapa, A.; Djelić, N.; Bajić, V.; Dekanski, D.; Spremo-Potparević, B. Dry olive leaf extract counteracts L-thyroxine-induced genotoxicity in human peripheral blood leukocytes in vitro. Oxidative Med. Cell. Longev. 2015, 2015, 762192. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Al-Zamely, H.A.N.; Al-Tamemi, Z.S.M. Role of hydroxytyrosol in ameliorating effects of high fat diet on male rats CNS. J. Pharm. Sci. Res. 2018, 10, 2448–2453. [Google Scholar]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Zhu, X.X.; Du, L.F. P38 MAP kinase is involved in oleuropein-induced apoptosis in A549 cells by a mitochondrial apoptotic cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Fabiani, R. Oleuropein Prevents Azoxymethane-Induced Colon Crypt Dysplasia and Leukocytes DNA Damage in A/J Mice. J. Med. Food 2016, 19, 983–989. [Google Scholar] [CrossRef]
- Shimao, R.; Muroi, H.; Furukawa, K.; Toyomizu, M.; Kikusato, M. Effects of low-dose oleuropein diet supplementation on the oxidative status of skeletal muscles and plasma hormonal concentration of growing broiler chickens. Br. Poult. Sci. 2019, 60, 784–789. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Toteda, G.; Lupinacci, S.; Vizza, D.; Bonofiglio, R.; Perri, E.; Bonofiglio, M.; Lofaro, D.; La Russa, A.; Leone, F.; Gigliotti, P.; et al. High doses of hydroxytyrosol induce apoptosis in papillary and follicular thyroid cancer cells. J. Endocrinol. Investig. 2017, 40, 153–162. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F.; Watanabe, K.; Senbongi, R.; Iwai, K. Oleuropein supplementation increases urinary noradrenaline and testicular testosterone levels and decreases plasma corticosterone level in rats fed high-protein diet. J. Nutr. Biochem. 2013, 24, 887–893. [Google Scholar] [CrossRef]
- Derouiche, A.; Jafri, A.; Driouch, I.; El Khasmi, M.; Adlouni, A.; Benajiba, N.; Bamou, Y.; Saile, R.; Benouhoud, M. Effect of argan and olive oil consumption on the hormonal profile of androgens among healthy adult Moroccan men. Nat. Prod. Commun. 2013, 8, 51–53. [Google Scholar] [CrossRef]
- Rostamzadeh, A.; Amini-khoei, H.; Mardani Korani, M.J.; Rahimi-madiseh, M. Comparison effects of olive leaf extract and oleuropein compounds on male reproductive function in cyclophosphamide exposed mice. Heliyon 2020, 6, e03785. [Google Scholar] [CrossRef]
- Almeer, R.S.; Abdel Moneim, A.E. Evaluation of the Protective Effect of Olive Leaf Extract on Cisplatin-Induced Testicular Damage in Rats. Oxidative Med. Cell. Longev. 2018, 2018, 8487248. [Google Scholar] [CrossRef]
- Nassef, N.A.; Mohamad, M.I. Normalization of serum corticosterone, testosterone levels, and testicular estrogen receptor-α expression in Wistar rats subjected to restraint stress—Beneficial effects of olive oil supplementation. Gene Rep. 2018, 11, 150–153. [Google Scholar] [CrossRef]
- Mansour, S.W.; Sangi, S.; Harsha, S.; Khaleel, M.A.; Ibrahim, A.R.N. Sensibility of male rats fertility against olive oil, Nigella sativa oil and pomegranate extract. Asian Pac. J. Trop. Biomed. 2013, 3, 563–568. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Visser, T.J. Regulation of Thyroid Function, Synthesis and Function of Thyroid Hormones. In Thyroid Diseases: Pathogenesis, Diagnosis and Treatment; Vitti, P., Hegedus, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–30. [Google Scholar]
- Sheehan, M.T. Biochemical testing of the thyroid: TSH is the best and, oftentimes, only test needed–a review for primary care. Clin. Med. Res. 2016, 14, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.L.; Goemann, I.M.; Meyer, E.L.; Wajner, S.M. Deiodinases: The balance of thyroid hormone: Type 1 iodothyronine deiodinase in human physiology and disease. J. Endocrinol. 2011, 209, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.R.; Zavacki, A.M. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur. Thyroid J. 2012, 1, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.P.; Bean, N.G. The Relationship between Population T4/TSH Set Point Data and T4/TSH Physiology. J. Thyroid Res. 2016, 2016, 6351473. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A.; Al-Damegh, M.A.; ElMougy, S.A. Effect of freeze dried extract of Olea europaea on the pituitary-thyroid axis in rats. Phytother. Res. 2002, 16, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; El-Saadany, A.S.; Shreif, E.Y.; El-Barbary, A.M. Effect of dietary olive leaves extract (oleuropein) supplementation on productive, physiological and immunological parameters in bandarah chickens 2-during production period. Egypt. Poult. Sci. J. 2018, 37, 277–292. [Google Scholar]
- Quitete, F.T.; Lisboa, P.C.; de Moura, E.G.; de Oliveira, E. Different oils used as supplement during lactation causes endocrine-metabolic dysfunctions in male rats. J. Funct. Foods 2018, 48, 43–53. [Google Scholar] [CrossRef]
- Farooq, M.; Ali, S.; Zubair, M.; Ullah, Q.; Jamil, H.; Haroon, M.; Ghaffar, A. Effect of feed supplementation with olive oil on serum testosterone, triiodothyronine, thyroxine and some biochemical metabolites in teddy goat bucks. Asian J. Agric. Biol. 2019, 7, 116–121. [Google Scholar] [CrossRef][Green Version]
- Abd-Alla, O.A.M.; Abdel-Samee, A.M.; EL-Adawy, S.A.I. Effect of acacia saligna and olive pulp on growth, biochemical and hormonal status in lambs under heat stress in Sinai province. SCVMJ 2007, XII, 129–138. [Google Scholar]
- Abdalla, E.B.; El-Masry, K.A.; Khalil, F.A.; Teama, F.E.; Emara, S.S. Alleviation of oxidative stress by using olive pomace in crossbred (Brown Swiss X Baladi) calves under hot environmental conditions. Arab J. Nucl. Sci. Appl. 2015, 48, 88–99. [Google Scholar]
- Oke, O.E.; Emeshili, U.K.; Iyasere, O.S.; Abioja, M.O.; Daramola, J.O.; Ladokun, A.O.; Abiona, J.A.; Williams, T.J.; Rahman, S.A.; Rotimi, S.O.; et al. Physiological responses and performance of broiler chickens offered olive leaf extract under a hot humid tropical climate. J. Appl. Poult. Res. 2017, 26, 376–382. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Ghorbel, H.; Feki, I.; Bouallagui, Z.; Guermazi, F.; Ayadi, L.; Sayadi, S. Oleuropein and hydroxytyrosol protect rats’ pups against bisphenol A induced hypothyroidism. J. Anat. 2018, 103, 1115–1126. [Google Scholar] [CrossRef]
- Mekircha, F.; Chebab, S.; Gabbianelli, R.; Leghouchi, E. The possible ameliorative effect of Olea europaea L. oil against deltamethrin-induced oxidative stress and alterations of serum concentrations of thyroid and reproductive hormones in adult female rats. Ecotoxicol. Environ. Saf. 2018, 161, 374–382. [Google Scholar] [CrossRef]
- Linazasoro, J.M.; Sanchez Martin, J.A. Effect of several oils and fats on iodine and thyroxine metabolism. Rev. Clin. Esp. 1977, 146, 311–313. [Google Scholar]
- Iwen, K.A.; Oelkrug, R.; Brabant, G. Effects of thyroid hormones on thermogenesis and energy partitioning. J. Mol. Endocrinol. 2018, 60, R157–R170. [Google Scholar] [CrossRef]
- Helmreich, D.L.; Parfitt, D.B.; Lu, X.Y.; Akil, H.; Watson, S.J. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology 2005, 81, 183–192. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Celi, P.; Leury, B.J.; Dunshea, F.R. High dietary selenium and vitamin E supplementation ameliorates the impacts of heat load on oxidative status and acid-base balance in sheep. J. Anim. Sci. 2015, 93, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S.; Mohamed, I.N.; Aminuddin, A.; Johari, M.H.; Ngah, W.Z.W. The relationships between thyroid hormones and thyroid-stimulating hormone with lipid profile in euthyroid men. Int. J. Med. Sci. 2014, 11, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Alkalby, J. Effect of Bisphenol A on thyroid, liver and testicular functions in adult male rats Bas. J. Vet. Res. 2015, 14, 187–206. [Google Scholar] [CrossRef]
- Silva, M.M.D.; Xavier, L.L.F.; Gonçalves, C.F.L.; Santos-Silva, A.P.; Paiva-Melo, F.D.; Freitas, M.L.; Fortunato, R.S.; Alves, L.M.; Ferreira, A.C.F. Bisphenol A increases hydrogen peroxide generation by thyrocytes both in vivo and in vitro. Endocr. Connect. 2018, 7, 1196–1207. [Google Scholar] [CrossRef]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.-B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Sekeroglu, V.; Sekeroglu, Z.A.; Demirhan, E. Effects of commercial formulations of deltamethrin and/or thiacloprid on thyroid hormone levels in rat serum. Toxicol. Ind. Health 2014, 30, 40–46. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, Y.J. Bisphenols and Thyroid Hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Bansal, R.; Parris, C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 2005, 146, 607–612. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Du, G.; Shen, O.; Sun, H.; Fei, J.; Lu, C.; Song, L.; Xia, Y.; Wang, S.; Wang, X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Ali, M.; Atif, F.; Kaur, M.; Bhatia, K.; Raisuddin, S. The modulatory effect of deltamethrin on antioxidants in mice. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 369, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Di Segni, C.; Raimondo, S.; Olivieri, G.; Silvestrini, A.; Meucci, E.; Currò, D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediat. Inflamm. 2016, 2016, 6757154. [Google Scholar] [CrossRef]
- Abdul-Hamid, M.; Salah, M. Lycopene reduces deltamethrin effects induced thyroid toxicity and DNA damage in albino rats. J. Basic Appl. Zool. 2013, 66, 155–163. [Google Scholar] [CrossRef]
- Gules, O.; Kum, S.; Yildiz, M.; Boyacioglu, M.; Ahmad, E.; Naseer, Z.; Eren, U. Protective effect of coenzyme Q10 against bisphenol-A-induced toxicity in the rat testes. Toxicol. Ind. Health 2019, 35, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kang, K.-H.; Arifuzzaman, S.; Pang, W.-K.; Ryu, D.-Y.; Song, W.-H.; Park, Y.-J.; Pang, M.-G. Effect of antioxidants on BPA-induced stress on sperm function in a mouse model. Sci. Rep. 2019, 9, 10584. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative Stress and BPA Toxicity: An Antioxidant Approach for Male and Female Reproductive Dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef]
- Çoban, J.; Öztezcan, S.; Doğru-Abbasoğlu, S.; Bingül, I.; Yeşil-Mizrak, K.; Uysal, M. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr. Gerontol. Int. 2014, 14, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Mihailovic-Stanojevic, N.; Milanovic, G.; Jovovic, D.; Miloradović, Z. Effects of high dose olive leaf extract on haemodynamic and oxidative stress parameters in normotensive and spontaneously hypertensive rats. J. Serb. Chem. Soc. 2014, 79, 1085–1097. [Google Scholar] [CrossRef]
- Schneider, M.J.; Fiering, S.N.; Thai, B.; Wu, S.Y.; St Germain, E.; Parlow, A.F.; St Germain, D.L.; Galton, V.A. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 2006, 147, 580–589. [Google Scholar] [CrossRef]
- Maia, A.L.; Kim, B.W.; Huang, S.A.; Harney, J.W.; Larsen, P.R. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J. Clin. Investig. 2005, 115, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Galton, V.A.; Martinez, E.; Hernandez, A.; St. Germain, E.A.; Bates, J.M.; St. Germain, D.L. The Type 2 Iodothyronine Deiodinase Is Expressed in the Rat Uterus and Induced During Pregnancy*. Endocrinology 2001, 142, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Chin, K.Y. Emerging Anticancer Potentials of Selenium on Osteosarcoma. Int. J. Mol. Sci. 2019, 20, 5318. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeöld, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef] [PubMed]
- Skřivan, M.; Marounek, M.; Englmaierová, M.; Skřivanová, V. Influence of dietary vitamin C and selenium, alone and in combination, on the performance of laying hens and quality of eggs. Czech. J. Anim. Sci. 2013, 58, 91–97. [Google Scholar] [CrossRef]
- Martin, R.F.; Young, V.R.; Blumberg, J.; Janghorbani, M. Ascorbic acid-selenite interactions in humans studied with an oral dose of 74SeO3(2-). Am. J. Clin. Nutr. 1989, 49, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J. The Effect of Soy and Fructooligosaccarides on the Selenium Status of Postmenopausal Women. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2005. [Google Scholar]
- Chin, K.-Y.; Pang, K.-L. Skeletal Effects of Early-Life Exposure to Soy Isoflavones—A Review of Evidence From Rodent Models. Front. Pediatrics 2020, 8, 563. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Shiau, S.-Y. Mutual sparing of dietary requirements for alpha-tocopherol and selenium in grouper, Epinephelus malabaricus. Aquaculture 2009, 294, 242–245. [Google Scholar] [CrossRef]
| References | Olive Derivative | Animal Model | Treatment Conditions | Findings |
|---|---|---|---|---|
| Euthyroid animal studies | ||||
| Al-Qarawi et al., 2002 [67] | Aqueous extract of Olea europaea leaves | Mature male Wistar rats (125–150 g; unknown sample size) | Control: distilled water (control) Treatment: 100, 250, and 500 µg/day of lyophilized olive leaf aqueous extract; oral gavage; 14 days | ↑ serum T3 and ↓ TSH level dose-dependentlyNS for the serum T4 level. |
| Ahmed et al., 2017 [68] | Oleuropein-rich olive leaf extract | Bandarah chickens (24 weeks old, N = 144) | Control: basal diet (control) Treatment: olive leaf extract in the basal diet, standardized to 50, 100, and 150 mg/kg of oleuropein; 18 weeks | All doses significantly and dose-dependently ↑ plasma T3, ↑ plasma SOD and total antioxidant capacity with ↓ MDA |
| Quitete et al., 2018 [69] | EVOO from Santa Cruz Biotechnology, Inc., TX, USA; Cat. No.: sc-215631A; Lot No.: #D1814) | Wistar rat dams (N = 40) (measured up to 21 days during lactation) and the male pups (N = 240) (measured up to 180 days postnatal) | Control: soybean oil (control) Treatment: EVOO, fish oil or coconut oil at 0.5 g/kg body weight during the 21-day lactation period. | EVOO ↓ plasma free T4 levels in lactating dams significantly more than in the control (NS for plasma free T3, TSH, liver TRβ1 protein levels and liver Dio1 mRNA expression). No alteration of the breast milk total T3 levels in EVOO group vs. control. NS for plasma free T3, T4, TSH, and liver TRβ1 levels, and liver Dio1 mRNA expression in 21-day breastfed pups in EVOO group vs. control. ↑ plasma free T3 level of mature offspring in the EVOO group vs. control (NS for plasma free T4, TSH, liver TRβ1 protein level, and liver Dio1 mRNA expression). |
| Farooq et al., 2019 [70] | Commercially available olive oil (unknown specification) | Adult male teddy goats (N = 9) | Control: basal diet Treatment: 15 and 30 mL olive oil (probably in diet); 8 weeks | ↑ serum T3 (both 15 and 30 mL) vs. control. ↑ serum T4 (only 30 mL) vs. control. |
| Hypothyroid animal studies | ||||
| Abd-Alla et al., 2007 [71] | Solid olive pulp from North Sinai, Egypt | Chronic heat-stressed growing lambs (N = 15) | Control: basal diet with rice straw/green acacia leaves Treatment: basal diet with 300 g of olive pulp solid; 3 months | Restored chronic heat-downregulated plasma T3 and T4 levels vs. control. |
| Abdalla et al., 2015 [72] | Solid olive pomace from new EL-Salheya olive mill factory—Sharkia Governorate, Egypt | Heat-stressed female crossbred (Brown Swiss x Baladi) calves (N = 10; 8-10 months old; mean body weight 112 kg) | Control: basal diet Treatment: olive pomace (15% of the diet); 2 months | ↑ serum T3 levels, ↓ MDA level, ↑ total antioxidant capacity and ↑ CAT levels significantly vs. control. |
| Oke et al., 2017 [73] | Olive leaf extract manufactured by Olive Leaf Australia (Coominya, Australia) with at least 4.4 mg/mL oleuropein | Chronic heat-stressed Arbor Acre broiler chickens (N = 240) | Control: drinking water without extract. Treatment: drinking water with 5, 10, and 15 mL/L leaf extract; 8 weeks. | ↑ plasma T3 level with ↑ plasma SOD level (only 15 mL/L extract). ↓ plasma MDA level (only 15 mL/L extract). Leaf extract (5–15 mL/L) is non-hematotoxic. |
| Mahmoudi et al., 2018 [74] | Oleuropein- and hydroxytyrosol-rich Olea europaea leaves extracts | Lactating adult female Swiss strain rats (N = 16; ~200 g) with female (N = 64) and male pups (N = 64). Outcomes were observed in the breastfed pups until 20 days of age. | BPA control: 250 mg/kg BPA; intramuscular injection of dams Treatment: BPA + oleuropein-rich extract (500 mg/kg body weight) or hydroxytyrosol-rich extract (150 mg/kg body weight) in drinking water for dams | Both extracts restored the total antioxidant capacity of BPA-reduced mature breast milk from lactating dams. Both extracts prevented BPA-induced upregulation of the TSH level and the reduction of plasma free T3 and T4 levels in breastfed pups. Both extracts prevented BPA-mediated thyroid gland mass loss and pathological changes (follicular cell hypertrophy, follicular cell dysfunction and calcitonin-positive cell number and area reduction) in pups. Both extracts improved BPA-suppressed body growth and bone health in pups. |
| Mekircha et al., 2018 [75] | Extra virgin Oleo europaea L. oil by traditional first cold pressure extraction on healthy Olea europaea L. fruits | Adult female Wistar albino rats (N = 30; 150–200 g) | Deltamethrin control: deltamethrin (2.56 mg/kg; oral) Treatment: EVOO (0.6 g/kg, oral) with or without deltamethrin for 28 days | Abrogated deltamethrin-induced body weight loss and the reduction of the absolute and relative thyroid weight. Restored deltamethrin-downregulated TSH and T4 levels to the baseline. ↓ deltamethrin-mediated oxidative stress in thyroid tissues with ↓ MDA, ↓ protein carbonyls, ↑ GSH, ↑ CAT, ↑ glutathione peroxidase, ↑ SOD and ↑ glutathione S-transferase levels. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.-L.; Lumintang, J.N.; Chin, K.-Y. Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients 2021, 13, 529. https://doi.org/10.3390/nu13020529
Pang K-L, Lumintang JN, Chin K-Y. Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients. 2021; 13(2):529. https://doi.org/10.3390/nu13020529
Chicago/Turabian StylePang, Kok-Lun, Johanna Nathania Lumintang, and Kok-Yong Chin. 2021. "Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review" Nutrients 13, no. 2: 529. https://doi.org/10.3390/nu13020529
APA StylePang, K.-L., Lumintang, J. N., & Chin, K.-Y. (2021). Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients, 13(2), 529. https://doi.org/10.3390/nu13020529








